Correlation of perinatal outcomes in preeclampsia with the dynamics of CD16+ monocyte content in peripheral blood
Tyutyunnik V.L., Mirzabekova D.D., Mikhailova O.I., Kan N.E., Krasnyi A.M.
Objective: To study the changes of CD16+ monocytes, the expression of CD86, CD152 in monocytes and CD28 in lymphocytes in the peripheral blood of pregnant women with preeclampsia in the course of therapy; to identify factors of prognostic value for determining the group of patients with complications in the neonatal period.
Materials and methods: The study included 26 patients with preeclampsia of various degrees of severity and time of manifestation. The expression of CD86 and CD152 in monocytes, CD28 in lymphocytes, and CD16+ in monocytes was determined using flow cytometry after diagnosis verification and before delivery. Two study groups were formed retrospectively: group 1 (main, n=10) included patients with a complicated course of the early neonatal period and group 2 (comparison group, n=16) included patients with an uncomplicated course of the early neonatal period. The change in the markers in response to therapy in both study groups was examined.
Results: There was a higher level of increase in the studied markers in the group of patients with a complicated course of the early neonatal period. There was a statistically significant increase in the CD16+ monocyte content in the group of patients with a complicated course (p<0.001). The median change in the content of CD16+ monocytes over five days was 13%. It was 0.95% in the group of patients with an uncomplicated course of the early neonatal period. The ROC analysis showed that the time change in the content of CD16+ in monocytes has a prognostic value for determining perinatal outcomes in preeclampsia, AUC=0.96.
Conclusion: The findings suggest the prospective prognostic value of determining the level of CD16+ monocytes in the peripheral blood of pregnant women in dynamics in order to predict perinatal outcomes. This can help optimize the planned delivery time.
Authors’ contributions: Tyutyunnik V.L., Mikhailova O.I., Kan N.E., Mirzabekova D.D., Krasnyi A.M. – developing the concept and design of the study, obtaining data for analysis, review of publications on the topic of the article, processing and analysis of the material, writing the text, editing the article.
Conflicts of interest: The authors declare no possible conflicts of interest.
Funding: The study was conducted without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: The patients provided an informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Tyutyunnik V.L., Mirzabekova D.D., Mikhailova O.I., Kan N.E., Krasnyi A.M. Correlation of perinatal outcomes in preeclampsia with the dynamics of CD16+ monocyte content in peripheral blood.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (9): 73-80 (in Russian)
https://dx.doi.org/10.18565/aig.2024.168
Keywords
Preeclampsia is a pregnancy-specific complication that is characterized by the presence of hypertension and proteinuria, and in some cases, multi-organ failure [1, 2]. Preeclampsia contributes significantly to maternal and neonatal morbidity and mortality worldwide [3].
Despite the long history of studying the etiology and pathogenesis of this pregnancy complication, the possibilities of predicting the increasing severity and perinatal outcomes remain unclear [4, 5]. The clinical criteria for diagnosing the condition and determining its severity, such as the presence of hypertension developed after 20 weeks of gestation and the level of proteinuria, are often nonspecific and ambiguous [1, 2, 6]. Given the heterogeneity of the pathogenesis, research to identify new markers is proceeding in several directions. Diagnostic markers such as soluble fms-like tyrosine kinase 1 (sFlt-1), placental growth factor (PlGF), and their ratio have already been widely used in practice and demonstrate high accuracy in diagnosing preeclampsia [7, 8]. According to Sachan R. et al, preeclampsia can be diagnosed on the basis of the level of proangiogenic protein endoglin (sEng) [9]. In addition, the studies of Karapetian A.O. et al. and Krasnyi A.M. et al. showed the possibility of predicting preeclampsia in early pregnancy using the level of extracellular fetal DNA [10, 11]. Boris D.A. investigated the possibility of verifying the severity of preeclampsia using the relative content of classical CD16-negative monocytes in the blood of pregnant women [12].
However, despite the impressive range of studies aimed at early prediction of preeclampsia, its complications and differentiation of its severity, there are still questions about the timing of delivery in cases with increasing severity of preeclampsia, assessment of treatment efficacy and the possibility of predicting perinatal outcomes [13–15]. In one of the recent studies devoted to these problems, it was shown that high risks of perinatal complications have a direct correlation with the increase in the level of total extracellular DNA during the treatment of preeclampsia, which appears to be an unfavorable prognostic criterion [11]. The research of Sadekova A.A. et al. describes the use of quantitative analysis of extracellular fetal DNA to identify the risk group for the development of fetal growth restriction in patients with preeclampsia in order to select the most optimal timing and modes of delivery for improving perinatal outcomes [16].
As the immunologic aspect of the development of preeclampsia presents the greatest interest, it is important to continue research in this direction. In previous studies, we analyzed the levels of surface markers expressed by peripheral blood mononuclear cells in patients with preeclampsia [17–20]. One study revealed that the expression levels of CD152, CD86, and CD28 in monocytes and CD16+ monocyte content were statistically significantly higher in patients with preeclampsia than in patients of the comparison group. This finding confirmed the potential of these biomarkers in the diagnosis of this complication [21].
Taking into account the high diagnostic potential of the above surface markers, one can use their study in the dynamics of the course of preeclampsia to predict perinatal outcomes and optimize the timing of delivery.
The objective of the study was to investigate the changes in the content of CD16+ monocytes, expression level of CD86, CD152 in monocytes and CD28 in lymphocytes in the peripheral blood during five days in pregnant women with preeclampsia while they were undergoing therapy. The study was also aimed at identifying factors that have prognostic value for detecting the group with complications in the neonatal period.
Materials and methods
The study was conducted at the clinical and laboratory base of the Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, Moscow. The study included 26 pregnant women with preeclampsia. The local research ethics committee approved this work. The diagnosis was made according to the clinical guidelines “Preeclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders during pregnancy, labor and postpartum” (2021). The patients were chosen on the basis of their referral to the specialized departments of the Center. The inclusion criteria for all study patients were as follows: singleton pregnancy complicated by preeclampsia at 22–40 weeks’ gestation, hospitalization for at least five days, and signed voluntary informed consent to participate in the study. There were the following non-inclusion criteria: multifetal gestation; pregnancy following in vitro fertilization (IVF); autoimmune, chromosomal, oncological, acute inflammatory and infectious diseases of the woman; severe extragenital pathology. The exclusion criteria were defined as delivery that occurred less or more than five days from the date of hospitalization.
Given that all patients were hospitalized for a period of between two and 14 days, a dynamic study of surface markers in the peripheral blood of pregnant women was conducted. The material was collected on days 1, 2, 4, 5, 7, 10, 12, and 14.
The material was obtained according to a standardized technique: the blood was collected from the patient into a tube with sodium-heparin, after which the tube was transported to the laboratory within 30 minutes. For the isolation of mononuclear cells, 5 μl of blood was taken into a tube and 45 μl of PBS solution was added. Subsequently, the specimens were treated with fluorescent tag conjugated antibodies according to the manufacturer’s protocol.
Leukocytes were analyzed using a flow cytometer (BD FACSCalibur, USA). The proportion (%) of monocytes and lymphocytes was determined by calculating the number of each cell type relative to the total number of monocytes and lymphocytes. The expression of the studied factors by immune cells was evaluated using the level of fluorescence of fluorescent tag conjugated antibodies to the studied antigens and was presented as fluorescence intensity, relative units (FI).
Statistical analysis
Statistical analysis was performed using the software program StatTech v. 4.3.3 (StatTech LLC, Russia). The quantitative comparisons between the two groups were performed using the Mann–Whitney U test, the quantitative data were described using median (Me) and lower and upper quartiles (Q1; Q3). The percentage comparisons were conducted using Fisher’s exact test, and the data are presented as percentages. ROC analysis was used to determine the predictive value and to determine the optimal threshold value of the studied factors. The differences among samples were considered statistically significant at p<0.05.
Results
The material was sampled from a total of 45 patients with preeclampsia. Most of the patients gave birth on the 5th day after hospitalization (n=26). Therefore, the study included 26 women whose mononuclear cell surface markers were examined on days 1 and 5. In addition, the choice of five days was justified by the fact that the duration of this period is sufficient for hypotensive, magnesium therapy for fetal neuroprotection, the effect of dexamethasone for the prevention of fetal respiratory distress, at gestational age up to 34 weeks. All patients underwent treatment according to the clinical guidelines “Preeclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders during pregnancy, labor and postpartum period” (2021) developed by the Russian Society of Obstetricians and Gynecologists.
Retrospectively, two study groups were formed on the basis of the course of the neonatal period: group 1 (main group, n=10) included patients with complications and group 2 (comparison group, n=16) included patients with uncomplicated course of the early neonatal period. The clinical characteristics and anamnestic data of the patients are presented in Table 1.
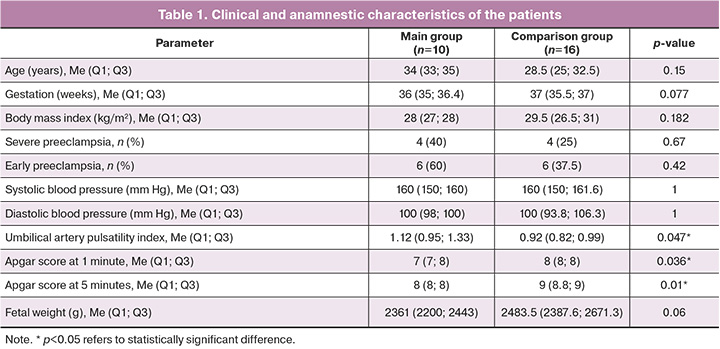
The gestational age of the patients included in the study was found to be 32–37 weeks. There were no statistically significant differences in age, gestational age at delivery, levels of systolic and diastolic blood pressure, and body mass index between the main and comparison groups. The groups did not demonstrate statistically significant differences in the severity and time of preeclampsia manifestations either.
All patients were treated with antihypertensive therapy in accordance with the severity of their condition. The analysis of therapy between the study groups was performed in order to level the intervention factor. Low-dose aspirin prophylaxis was prescribed to 3/10 women (30%) in the main group and 5/16 (31.3%) in the comparison group, p=0.64. In the main group, preventive treatment of fetal respiratory distress syndrome was performed in 4/10 patients (40.0%), in the comparison group it was performed in 6/16 (37.5%), p=0.56; therapy with low molecular weight heparin was administered to all 10/10 patients (100%) in the main group, and to 12/16 (75%) women in the comparison group, p=0.15. The analysis demonstrated homogeneity of the therapy in the groups, therefore the influence of this confounder was reduced on the final results of the study.
Hemodynamics in the umbilical arteries, namely, umbilical artery pulsatility index, which reflects the fetal condition, was analyzed. It was noted that the patients of the main group who had a complicated early neonatal period demonstrated a statistically significant higher pulsatility index than the patients of the comparison group (p=0.047).
Two women in the group with complicated course of the early neonatal period had spontaneous delivery, eight women had cesarean section; the indications were worsened fetal condition, severity of preeclampsia, and lack of effect from the therapy. Six women in the group with uncomplicated course of the early neonatal period had spontaneous vaginal delivery, and ten women underwent cesarean section; the indications were worsened fetal condition, unstable dynamics, and severe preeclampsia.
Twenty-six infants were born, and there were statistically significant differences in Apgar scores at the 1 and 5 minutes between the study groups. There were such complications as birth asphyxia, respiratory distress syndrome, congenital pneumonia, disseminated intravascular coagulation, hemorrhagic syndrome, and fetal central nervous system anomalies in the group with a complicated course of the early neonatal period. Neonatal jaundice was observed in one case in the comparison group.
The content of CD16+ monocytes, the expression levels of CD86, CD152 in monocytes and CD28 in lymphocytes in the peripheral blood of pregnant women at the first and second time points of the study were examined in each group; the dynamic change of the markers in the study groups was analyzed, and the difference between the final and baseline levels was calculated. The results of the study are shown in Figure 1, Tables 2 and 3.
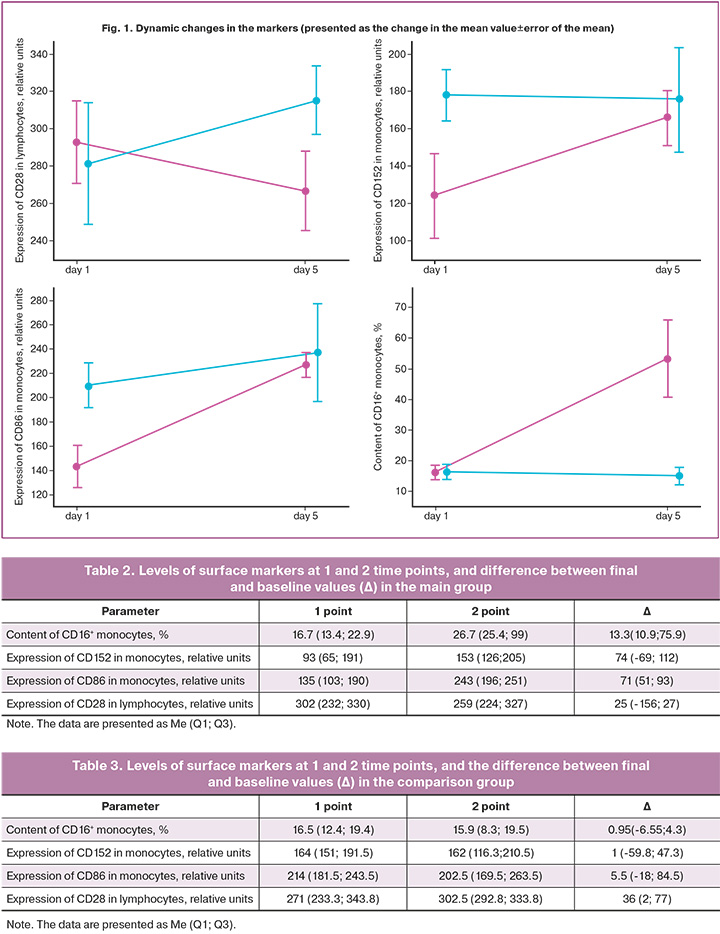
As can be seen in Figure 1, there is a tendency to increase in the levels of the markers in the main group, except for the expression of CD28 in lymphocytes. In the comparison group, all parameters, except CD28 expression in lymphocytes, tend to decrease in levels over the course of the study. It can be assumed that an increase in the studied factors, which are potential markers of preeclampsia, may be associated with worsened fetal condition.
The comparison of dynamic changes between the groups proved to be critical for identifying patients with complicated course of the early neonatal period. The comparison of the change in the dynamics (Δ) of the studied parameters between the groups is presented in Table 4 and Figure 2.
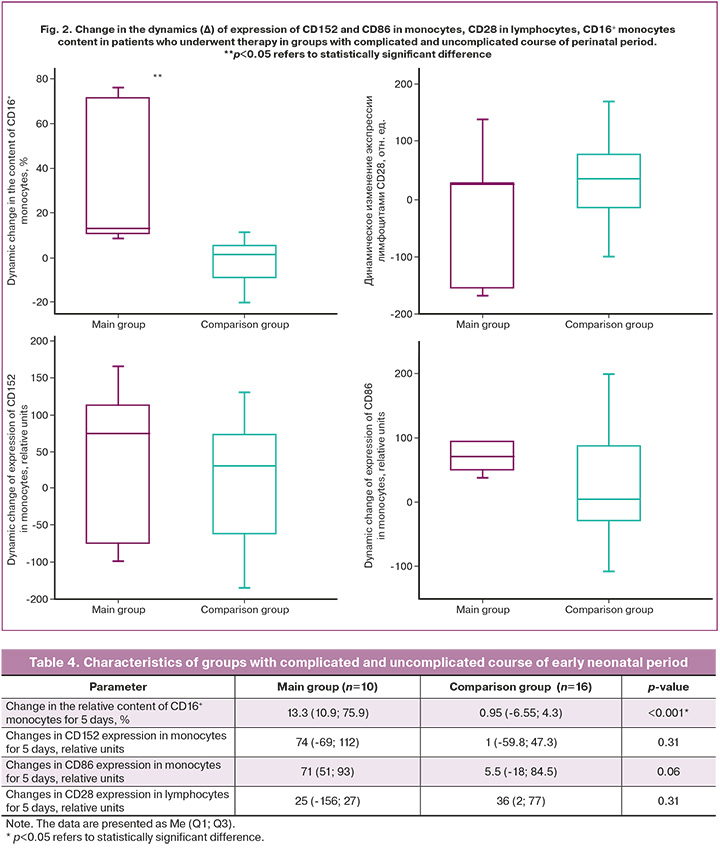
The statistically significant difference in Δ was observed in the content of CD16+ monocytes in peripheral blood: it was 13.3 (10.9; 75.9)% in the main group and 0.95 (-6.55; 4.3) % in the comparison group (p<0.001). There was no statistically significant difference in Δ for the expression of CD86 and CD152 in monocytes between the groups, but there was a tendency to increase. Therefore, it is necessary to continue investigating these factors.
In order to assess the prognostic value of determining pregnancy outcomes by Δ level of CD16+ monocytes in peripheral blood of patients who underwent therapy for 5 days, a ROC analysis was performed (Fig. 3).
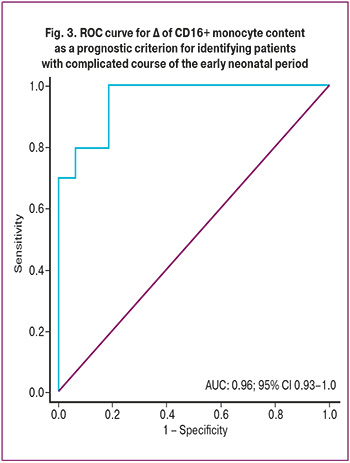
The area under the ROC curve was found to be 0.96 at the threshold value of the relative content of CD16+ monocytes equal to 5.7%. Sensitivity was 97%, specificity was 75%. The prognostic value of a positive result was 71% and the prognostic value of a negative result was 100%.
Based on the increase of CD16+ monocytes in the peripheral blood of pregnant women by ≥5.7% during 5 days of treatment, it is possible to predict the development of adverse perinatal outcomes with a sensitivity of 97% and specificity of 75%; moreover, this marker can be used as an additional criterion for early delivery.
The data obtained indicate the prospective prognostic use of Δ content of CD16+ monocytes in the peripheral blood of pregnant women. This can be used to assess the success of therapy, to choose the timing of delivery and to predict perinatal outcomes.
Discussion
It was assumed that the therapy aimed at stabilizing the patient’s condition should normalize hemodynamic parameters and contribute to the reduction of the immune response characterized by the expression of CD16, CD152, CD86 in monocytes and CD28 in lymphocytes, which contribute to the proinflammatory response in the body of a pregnant woman with preeclampsia [21]. However, we observed a decrease in the studied markers only in some patients who received the treatment. At the same time, their increase, in particular the increase in CD16+ monocytes, was an unfavorable prognostic criterion and associated with a high risk of perinatal complications, which may confirm the severe immune dysregulation in the body of the pregnant woman and excessive inflammatory response.
The results of this study show that the complicated course of the early neonatal period can be predicted with high sensitivity and specificity, and the identified marker can be used as an additional criterion for early delivery. There are limitations to be recognized in addition to strengths. Generalizability of the results of our study is limited by the small sample size. Furthermore, it is unclear whether the results would be statistically significant when the change in time is less or more than five days before delivery.
Conclusion
The study of CD16+ monocyte content and expression of CD152 and CD86 in monocytes and CD28 in lymphocytes in the peripheral blood of pregnant women with preeclampsia in the dynamics during the course of therapy is of great practical importance. During the course of the study, it was determined that the elevated levels of the analyzed markers associated with immune dysregulation in preeclampsia are indicative of complicated course of the early neonatal period in pregnant women who underwent treatment. The findings suggest the prospective prognostic value of determining the level of CD16+ monocytes in the peripheral blood of pregnant women in dynamics in order to predict perinatal outcomes. This can help optimize the planned delivery time.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде. М.; 2021. 81 с. [Ministry of Health of the Russian Federation. Preeclampsia. Eclampsia. Clinical guidelines. Edema, proteinuria and hypertensive disorders during pregnancy, childbirth and the postpartum period. Moscow; 2021. 81 p. (in Russian)].
- ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019; 133(1): 1. https://dx.doi.org/10.1097/AOG.0000000000003018.
- Jung E., Romero R., Yeo L., Gomez-Lopez N., Chaemsaithong P., Jaovisidha A. et al. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022; 226 (2S): S844-S866. https://dx.doi.org/10.1016/j.ajog.2021.11.1356.
- Turbeville H.R., Sasser J.M. Preeclampsia beyond pregnancy: long-term consequences for mother and child. Am. J. Physiol. Renal Physiol. 2020; 318(6): F1315-F1326. https://dx.doi.org/10.1152/ajprenal.00071.2020.
- Roberts J.M., Rich-Edwards J.W., McElrath T.F., Garmire L., Myatt L.; Global Pregnancy Collaboration. Subtypes of preeclampsia: recognition and determining clinical usefulness. Hypertension. 2021; 77(5): 1430-41. https://dx.doi.org/10.1161/HYPERTENSIONAHA.120.14781.
- Melchiorre K., Giorgione V., Thilaganathan B. The placenta and preeclampsia: villain or victim? Am. J. Obstet. Gynecol. 2022; 226 (2S): S954-S962. https://dx.doi.org/10.1016/j.ajog.2020.10.024.
- Duhig K.E., Myers J., Seed P.T., Sparkes J., Lowe J., Hunter R.M. et al.; PARROT trial group. Placental growth factor testing to assess women with suspected pre-eclampsia: a multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet. 2019; 393(10183): 1807-18. https://dx.doi.org/10.1016/S0140-6736(18)33212-4.
- Пылаева Н.Ю., Шифман Е.М., Пономарева Е.Г., Османова Э.С. Антитромбин III – эволюция от антикоагулянта к маркеру тяжелых форм преэклампсии. Анестезиология и реаниматология. 2020; (6): 57‑61. [Pylaeva N.Yu., Shifman E.M., Ponomareva E.G., Osmanova E.S. Antithrombin III – evolution from anticoagulant to a marker of severe preeclampsia. Russian Journal of Anesthesiology and Reanimatology. 2020; (6): 57‑61. (in Russian)]. https://dx.doi.org/10.17116/anaesthesiology202006157.
- Sachan R., Patel M.L., Dhiman S., Gupta P., Sachan P., Shyam R. Diagnostic and prognostic significance of serum soluble endoglin levels in preeclampsia and eclampsia. Adv. Biomed. Res. 2016; 5: 119. https://dx.doi.org/10.4103/2277-9175.186993.
- Karapetian А.О., Baev О.R., Sadekova А.А., Krasnyi А.М., Sukhikh G.T. Cell-free foetal DNA as a useful marker for preeclampsia prediction. Reprod. Sci. 2021; 28(5): 1563-9. https://dx.doi.org/10.1007/s43032-021-00466-w.
- Krasnyi A.M., Gracheva M.I., Sadekova A.A., Vtorushina V.V., Balashov I.S., Kan N.E. et al. Combined study of total, fetal DNA, cytokines in maternal blood plasma in preeclampsia Bull. Exp. Biol. Med. 2018; 164(6): 721-5. https://dx.doi.org/10.1007/s10517-018-4066-1.
- Борис Д.А., Волгина Н.Е., Красный А.М., Тютюнник В.Л., Кан Н.Е. Прогнозирование преэклампсии по содержанию CD16-негативных моноцитов. Акушерство и гинекология. 2019; 7: 49-55. [Boris D.A., Volgina N.E., Krasnyi A.M., Tyutyunnik V.L., Kan N.E. Prediction of preeclampsia on the couts of CD-16 negative monocytes. Obstetrics and Gynecology. 2019; (7): 49-55. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.7.49-55.
- MacDonald T.M., Walker S.P., Hannan N.J., Tong S., Kaitu'u-Lino T.J. Clinical tools and biomarkers to predict preeclampsia. EBioMedicine. 2022; 75: 103780. https://dx.doi.org/10.1016/j.ebiom.2021.103780.
- Staff A.C., Fjeldstad H.E., Fosheim I.K., Moe K., Turowski G., Johnsen G.M. et al. Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia. Am. J. Obstet. Gynecol. 2022; 226(2S): S895-S906. https://dx.doi.org/10.1016/j.ajog.2020.09.026.
- Chang K.J., Seow K.M., Chen K.H. Preeclampsia: Recent advances in predicting, preventing, and managing the maternal and fetal life-threatening condition. Int. J. Environ. Res. Public Health. 2023; 20(4): 2994. https://dx.doi.org/10.3390/ijerph20042994.
- Садекова А.А., Хачатрян З.В., Красный А.М., Кан Н.Е., Хачатурян А.А., Тютюнник В.Л. Диагностическая значимость определения уровня внеклеточной фетальной ДНК у беременных с преэклампсией и задержкой роста плода. Акушерство и гинекология. 2019; 8: 144-9. [Sadekova A.A., Khachatryan Z.V., Krasnyi A.M., Kan N.E., Khachaturian A.A., Tyutyunnik V.L. The diagnostic significance of determining the level of extracellular fetal DNA in pregnant women with preeclampsia and fetal growth retardation. Obstetrics and Gynecology. 2019; (8): 144-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.8.144-149.
- Uzunov A.V., Secara D.C., Mehedințu C., Cîrstoiu M.M. Preeclampsia and neonatal outcomes in adolescent and adult patients. J. Med. Life. 2022; 15(12): 1488-92. https://dx.doi.org/10.25122/jml-2022-0264.
- Overton E., Tobes D., Lee A. Preeclampsia diagnosis and management. Best Pract. Res. Clin. Anaesthesiol. 2022; 36(1): 107-21. https://dx.doi.org/10.1016/j.bpa.2022.02.003.
- Wang Y., Li B., Zhao Y. Inflammation in preeclampsia: genetic biomarkers, mechanisms, and therapeutic strategies. Front. Immunol. 2022; 13: 883404. https://dx.doi.org/10.3389/fimmu.2022.883404.
- Nirupama R., Divyashree S., Janhavi P., Muthukumar S.P., Ravindra P.V. Preeclampsia: Pathophysiology and management. J. Gynecol. Obstet. Hum. Reprod. 2021; 50(2): 101975. https://dx.doi.org/10.1016/j.jogoh.2020.101975.
- Красный А.М., Кан Н.Е., Мирзабекова Д.Д., Тютюнник В.Л., Панасенко Е.А., Садекова А.А. Фенотипический профиль мононуклеарных клеток периферической крови при преэклампсии. Акушерство и гинекология. 2023; 5: 68-74. [Krasnyi A.M., Kan N.E., Mirzabekova D.D., Tyutyunnik V.L., Panasenko E.A., Sadekova A.A. Phenotypic profile of peripheral blood mononuclear cells in preeclampsia. Obstetrics and Gynecology. 2023; (5): 68-74 (in Russian)]. https://dx.doi.org/0.18565/aig.2023.27.
Received 17.07.2024
Accepted 24.09.2024
About the Authors
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher of Center of Scientific and Clinical Researches, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, tioutiounnik@mail.ru, Researcher ID: B-2364-2015, SPIN-code: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099Dzhamilia D. Mirzabekova, graduate student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, Jamilya1705@yandex.ru, https://orcid.org/0000-0002-2391-3334
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN-code: 5378-8437,
Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Olga I. Mikhailova, PhD, Researcher at 2nd Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, omikhaylova@gmail.com, https://orcid.org/0000-0001-7569-8704
Aleksey M. Krasnyi, PhD, Head of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, alexred@list.ru, https://orcid.org/0000-0001-7883-2702



