Clinical and anamnestic risk factors and prediction models for the development of fetal growth restriction
Gasymova Sh.R., Tyutyunnik V.L., Kan N.E., Volochaeva M.V., Donnikov A.E.
Objective: To analyze clinical and anamnestic risk factors and develop prediction models for early- and late-onset fetal growth restriction.
Materials and methods: A cross-sectional study was conducted at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia, involving 382 pregnant women. Group 1 included 110 pregnant women with fetal growth restriction, whereas Group 2 included 272 women with pregnancies without fetal growth restriction. An analysis of the somatic and obstetric-gynecological history and course of the current pregnancy in both groups was performed. Prediction models for the likelihood of developing early- and late-onset fetal growth restriction were developed.
Results: In group 1, a history of arterial hypertension [10/110 (9.1%) vs. 6/272 (2.2%), p=0.045, OR=0.226 (95% CI 0.052–0.979)], chronic pyelonephritis [8/110 (7.3%) vs. 2/272 (0.7%), p=0.025, OR=0.094 (95% CI 0.010–0.865)], chronic endometritis [8/110 (7.3%) vs. 58/272 (21.3%), p=0.02, OR=0.82 (95% CI 0.015–10.353)], and a history of giving birth to children with growth restriction in previous pregnancies [8/110 (7.3%) vs. 2/272 (0.7%), p=0.025, OR=0.094 (95% CI 0.010–0.865)] were significantly more common than in group 2. During the current pregnancy, the following complications were significantly more prevalent in group 1 than in group 2: threatened miscarriage [35/110 (31.8%) vs. 64/272 (23.5%), p=0.04, RR=1.97 (95% CI 1.03–3.75)], preeclampsia [11/110 (10%) vs. 9/272 (3.3%), p=0.01, RR=3.53 (95% CI 1.37–9.08)], chronic arterial hypertension [11/110 (10%) vs. 11/272 (4.0%), p=0.02, RR=2.84 (95% CI 1.15–7.01)], and placenta previa [5/110 (4.5%) vs. 5/272 (1.8%), p=0.01, RR=0.84 (95% CI 0.42–1.68)]. Significant predictors of fetal growth restriction included a history of having a child with growth restriction, placenta previa, obesity, and preeclampsia. Based on the identified predictors, mathematical predictive models were developed to determine the likelihood of fetal growth restriction.
Conclusion: The developed predictive models for early- and late-onset fetal growth restriction can help reduce the incidence of pregnancy complications and improve perinatal outcomes.
Authors’ contributions: Gasymova Sh.R., Volochaeva M.V., Donnikov A.E. – conception and design of the study, data collection and analysis, review of relevant literature, material processing and analysis, statistical analysis; Gasymova Sh.R., Tyutyunnik V.L., Kan N.E. – drafting and editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Gasymova Sh.R., Tyutyunnik V.L., Kan N.E., Volochaeva M.V., Donnikov A.E.
Clinical and anamnestic risk factors and prediction models for the development of fetal growth restriction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (10): 52-59 (in Russian)
https://dx.doi.org/10.18565/aig.2024.166
Keywords
Fetal growth restriction (FGR) is a pressing issue in obstetrics and perinatology. FGR is one of the so-called “great obstetrical syndromes,” with consequences associated with a high incidence of perinatal morbidity and mortality [1]. The incidence of FGR varies from 5% to 23% [2, 3]. The causes of this complication include a variety of factors, including placental, fetal, maternal, and genetic factors as well as their combinations [4]. The pathophysiological basis of FGR related to the placenta is placental insufficiency, which manifests as decreased placental perfusion, reduced metabolic processes, and consequently impaired delivery of oxygen as well as various micro- and macronutrients to the fetus [5, 6]. Additionally, fetal malformations, chromosomal abnormalities, and congenital metabolic disorders can also contribute to FGR [7]. Thanks to advancements in molecular biology and genetics, variants in the genes of both the fetus and mother that may influence the development of FGR have been identified [4, 8].
The so-called "maternal factors" play a significant role in the development of FGR. These factors include advanced reproductive age, excess body weight, low socioeconomic status, teratogenic factors, and specific characteristics of obstetric and gynecological history [9–11]. Identifying predictors is crucial for developing methods to prevent pregnancy complications and improve and personalize the management of pregnant women affected by this pathology.
This study aimed to analyze the clinical and anamnestic risk factors and develop predictive models for early- and late-onset fetal growth restriction.
Materials and methods
This cross-sectional study was conducted at V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. This study included 382 pregnant women who were divided into two groups. The first group (study group) consisted of 110 pregnant women with FGR, of which 34 had early-onset (subgroup 1A) and 76 had late-onset (subgroup 1 B) FGR. The second group (control group), consisted of 272 women with physiological pregnancies without FGR.
The study population consisted of pregnant women aged 18–45 years with a singleton pregnancy at 22–40 weeks, who provided informed consent. The exclusion criteria were the presence of somatic diseases in the decompensated stage, fetal malformations, and maternal acute infectious diseases.
The baseline evaluation included the patient's history of somatic and obstetric-gynecological diseases, as well as a detailed examination of the current pregnancy, encompassing all aspects of both obstetric and extragenital complications.
Statistical analysis
Statistical analysis was performed using the SPSS 25.0, StatTech v. 4.2.7 (Stattech LLC, Russia). Data were analyzed using nonparametric statistical methods. Qualitative features were presented as both absolute and relative values. The binary variables were compared using contingency tables. During the development of the mathematical predictive model, variance and discriminant analyses, the binary logistic regression method and ROC analysis were used. To assess the presence of a relationship between a certain outcome (FGR) and risk factors, crude and adjusted odds ratios with 95% confidence intervals (95% CI) were calculated. During the ROC analysis, ROC curves were constructed, the area under them (AUROC) was determined, and the sensitivity and specificity of the model were also calculated. The value of a quantitative variable at the cut-off point was determined using the highest Youden index value. Statistical significance was set at p<0.05.
Results
The age of the study participants ranged from 18 to 42 years. No statistically significant differences in age or anthropometric parameters were found between the groups or within the subgroups of the study group. The median age in the study subgroup 1A was 31.0 (29.0; 35.0) years, in subgroup 1B – 33.0 (29.0; 35.0) years. In the control group, the median age was 31.0 (28.0; 34.0) years. The median body mass index (BMI) in subgroup 1A was 24.0 (22.0; 26.0) kg/m2, in subgroup 1B – 25.0 (23.0; 29.0) kg/m2, and 25.0 (23.0; 26.0) kg/m2 in the control group. A comparison of educational level, familial and social status, and presence or absence of unhealthy habits revealed no statistically significant differences between the study groups (p>0.05).
The two groups of patients were comparable in terms of age at menarche and nature of the menstrual cycle (p>0.05).
A comparative analysis of the structure of extragenital (somatic) pathology revealed that in group 1, the following were documented in the medical history significantly more often than in group 2: chronic arterial hypertension (10/110, 9.0%), chronic pyelonephritis (8/110, 7.3%), and chronic renal failure (5/110, 4.5%). The prevalence of chronic arterial hypertension was 1% in group 1 and 6/272 (2.2%) in group 2 (p=0.045, OR=0.226, 95% CI 0.052–0.979). Similarly, chronic pyelonephritis was observed in 8/110 (7.3%) patients in group 1 and 2/272 (0.7%) patients in group 2 (p=0.025, OR=0.094, 95% CI 0.010–0.865).
In terms of the frequency of history of gynecological pathology, the groups were comparable for most nosological forms, except for chronic endometritis [8/110 (7.3%) vs. 58/272 (21.3%), p=0.02, OR=0.82 (95% CI 0.015–10.353]) (by groups, respectively).
A comparative analysis of parity revealed that in group 2, there were significantly more women with a history of childbirth than in group 1 (p<0.05). There were no significant differences in the number of births (p=0.124) or pregnancy losses (p=0.308) between the study groups.
The diagnosis of FGR in previous pregnancies was significantly more often noted in group 1 than in group 2 [8/110 (7.3%) vs. 2/272 (0.7%), p=0.025, OR=0.094 (95% CI 0.010–0.865]). No statistically significant differences were found in the other complications of previous pregnancies.
The results of the analysis of the current pregnancy data in two subgroups (1A and 1B) of group 1 compared to group 2 are presented in Table 1. Statistically significant differences were found in the incidence of placenta previa for group 2 and subgroup 1A (p=0.003), as well as in the incidence of chronic arterial hypertension and preeclampsia for subgroup B and group 2 (p=0.023 and p=0.007, respectively).
The results of this analysis were used to develop mathematical models to predict the development of early- and late-onset FGRs in the pregnant mothers enrolled in the study.
The predictive model for the likelihood of developing early-onset FGR included the following indicators: pregnancy over 35 years of age, parity, smoking before pregnancy, FGR in previous pregnancies, obesity, threatened miscarriage, gestational diabetes mellitus, preeclampsia, placenta previa, and smoking during current pregnancy.
The analysis of the statistically significant dependence of the model predictors on the probability of early-onset FGR is presented in Table 2.
The obtained dependence can be described by the following equation:
P=1/(1+e-z) ×100%,
z=-2.565+0.455Xpregnancy over 35 years of age+0.299XObesity-1.416XNumber of births +2.821XFetal growth restriction in previous pregnancies+0.106XThreatened miscarriage+0.969XGestational diabetes mellitus+0.235XPreeclampsia+2.900XPlacenta previa+22.934XSmoking during pregnancy-20.590XSmoking before pregnancy,
where P is the probability of developing early-onset FGR.
The resulting regression model was statistically significant (p=0.015).
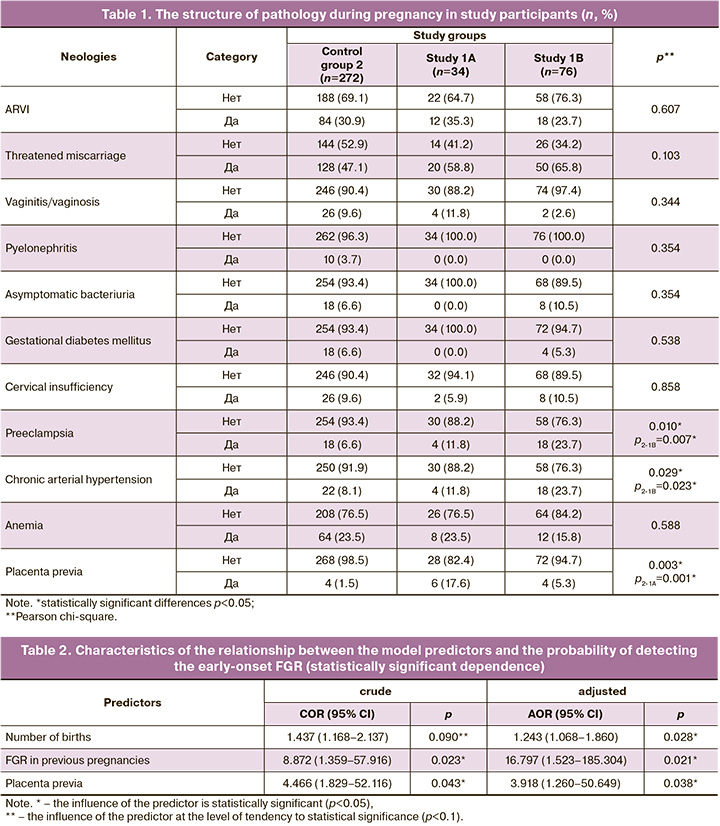
Assessment of the dependence of the probability of developing early-onset FGR on the value of the logistic function P by ROC analysis yielded the following curve (Fig. 1).
The area under the ROC curve was 0.823±0.067, 95% CI 0.692–0.955. The model was found to be statistically significant (p<0.001).
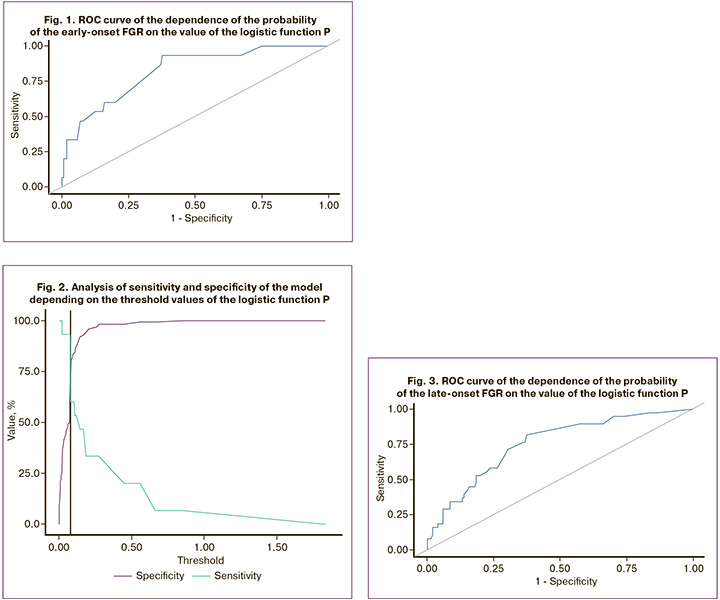
The threshold value of the logistic function P at the cutoff point, which corresponded to the highest value of the Youden index, was 0.074. The probability of developing the early-onset FGR was predicted at a function value of P higher than or equal to this value. The sensitivity and specificity of this model were 93.3 and 62.5%, respectively (Fig. 2). A predictive mathematical model was also developed to determine the probability of developing late-onset FGR depending on parity, age at pregnancy over 35 years, smoking before pregnancy, obesity, threatened termination of pregnancy, gestational diabetes mellitus, preeclampsia, and smoking during the current pregnancy.
The observed dependence was described by the equation:
P=1/(1+e-z) ×100%,
z=-2.494+0.136XNumber of births+0.209Xpregnancy over 35 years of age +0.977XObesity+0.769XThreatened miscarriage+0.029XGestational diabetes+1.283XPreeclampsia+0.845XSmoking during pregnancy+0.533XSmoking before pregnancy,
where P is the probability of developing the late-onset FGR.
The resulting regression model was statistically significant (p=0.004).
The ROC curve of the dependence of the probability of developing the late-onset FGR on the value of the logistic function P is shown in Figure 3; Table 3 presents the ROC analysis data.

The area under the ROC curve was 0.754±0.048, 95% CI 0.659–0.849. The resulting model was statistically significant (p<0.001).
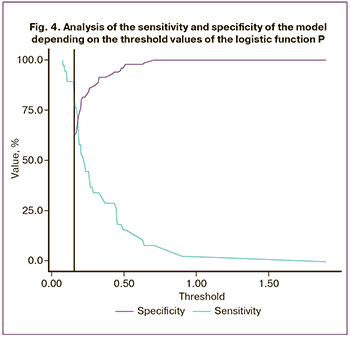
The threshold of the logistic function P value at the cut-off point, which corresponded to the highest value of the Youden index, was 0.155. The development of late-onset FGR was predicted by a P value greater than or equal to this value. The sensitivity and specificity of the model were 81.6 and 62.7% (Fig. 4).
Discussion
The results of this study align with data from both foreign and Russian studies. Specifically, studies by Lees et al. and others have reported a relationship between obesity and chronic maternal hypertension and the development of FGR [12, 13]. Arterial hypertension is a common complication during pregnancy. Zhang H.G. et al. examined 100 newborns in the intensive care unit of mothers whose pregnancies were complicated by preeclampsia (n=53) and gestational hypertension (n=47). A control group of 100 healthy infants from the physiology department, born to mothers with uncomplicated pregnancies, was also included. The findings indicated that chronic hypertension and preeclampsia were significantly associated with the development of FGR, along with complications, such as placental abruption and fetal distress (p<0.05) [14]. In the present study, a history of chronic endometritis was significantly more common in pregnant women with FGR. The existing literature on the impact of chronic endometritis before pregnancy on the subsequent development of FGR is relatively limited. A study by Pestrikova T.Yu. et al., placental bed biopsies were taken from pregnant women with FGR and those with normal pregnancies who underwent cesarean sections. The results revealed that one-third of the patients in the FGR group showed no inflammatory changes in the placenta, whereas over 70% of the patients in the normal pregnancy group did. Statistically significant differences were also found between the groups in terms of the number of inflammatory cells and the area of necrotic foci based on the morphological study of the placental bed (p<0.05). The pathological reactions identified in the placental bed of pregnant women with FGR were interpreted by the authors as signs of chronic endometritis, manifesting during pregnancy as basal deciduitis [15].
Analysis of the current pregnancy course in our study found that complications, such as preeclampsia, threatened miscarriage, and chronic arterial hypertension, were significantly more prevalent in the FGR group than in the control group. Numerous studies indicate that the pathophysiology of preeclampsia and FGR is linked to mechanisms involving impaired cytotrophoblast invasion and remodeling of spiral arteries, which subsequently leads to significant structural changes in the placenta [16–18]. Consequently, preeclampsia is often associated with FGR during pregnancy, particularly in cases of early-onset FGR. In the present study, significant differences in the incidence of preeclampsia were observed only in the late-onset FGR group compared with the control group, which may be attributed to the small sample size of subgroup 1A (early-onset FGR).
The relationship between threatened miscarriages and FGR has been explored in various studies. A systematic review published in 2010 indicated that the risk of having a low-birth-weight baby was higher in women experiencing threatened miscarriage in the first trimester (OR=1.83, 95% CI 1.48–2.28) compared than in women without this complication (p<0.0001). The overall risk ranged from 1.1 to 3.7 [19]. Additionally, the association between threatened miscarriage accompanied by vaginal bloody discharge and FGR has been investigated. It is presumed that bloody discharge during pregnancy may indicate placental dysfunction, which could manifest at a later stage [20–22].
Thus, both the results of the current study and the conclusions of foreign colleagues indicate that threatened miscarriage, especially in the presence of vaginal bloody discharge, should be considered as a risk factor for the development of FGR. Among the analyzed clinical and anamnestic factors, the mathematical model for determining the probability of developing early-onset FGR included pregnancy at age > 35 years, parity, smoking before pregnancy, FGR in previous pregnancies, obesity, threatened miscarriage, gestational diabetes, preeclampsia, placenta previa, and smoking during current pregnancy. Statistically significant predictors of early-onset FGR in our study were previous pregnancies and placenta previa during the current pregnancy. In the presence of placenta previa, the chance of developing early FGR increased by 3.9 times. With a history of FGR, the chance of developing early FGR increased by 16.8 times. The predictive model for calculating the probability of developing late-onset FGR included the following variables: parity, pregnancy over 35 years of age, obesity, threatened miscarriage, gestational diabetes, preeclampsia during the current pregnancy, and smoking during and before pregnancy. Statistically significant predictors of late-onset FGR in our study were maternal obesity and preeclampsia during current pregnancy. The presence of maternal obesity during pregnancy increased the chances of developing late FGR by 2.7 times, while the presence of preeclampsia increased the chances by 3.6 times. It should be noted that factors such as threatened miscarriage and smoking before and during pregnancy tended to be statistically significant in the development of late FGR, and with an increase in sample size, they may attain statistical significance for this pathology. These factors in the sample of pregnant women we studied should be considered significant predictors of FGR.
Conclusion
The results of our study showed that women with chronic arterial hypertension, pyelonephritis, and chronic endometritis could be classified as a risk group for the development of FGR. This syndrome in previous pregnancies and placenta previa during the current pregnancy are significant predictors of early-onset FGR, increasing the risk of developing this pathology by 16.8 and 3.9 times, respectively. Maternal obesity and preeclampsia are significant predictors of late-onset FGR, increasing the risk of developing this pathology by 2.7 and 3.6 times, respectively. Thus, predictive mathematical models for the development of early-onset (sensitivity, 93.3%; specificity, 62.5%) and late-onset (sensitivity, 81.6%; specificity, 62.7%) FGR, developed based on the identified and most significant clinical and anamnestic factors using the binary logistic regression method, make it possible to reduce the incidence of pregnancy complications and improve perinatal outcomes.
References
- Brosens I., Pijnenborg R., Vercruysse L., Romero R. The «Great Obstetrical Syndromes» are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011; 204(3): 193-201. https://dx.doi.org/110.1016/j.ajog.2010.08.009.
- Ганичкина М.Б., Мантрова Д.А., Кан Н.Е., Тютюнник В.Л., Хачатурян А.А., Зиганшина М.М. Ведение беременности при задержке роста плода. Акушерство и гинекология. 2017; 10: 5-11. [Ganichkina M.B., Mantrova D.A., Kan N.E., Tyutyunnik V.L., Khachaturyan A.A, Ziganshina M.M. Management of pregnancy during fetal growth retardation. Obstetrics and Gynecology. 2017; (10): 5-11. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.10.5-11.
- Priante E., Verlato G., Giordano G., Stocchero M., Visentin S., Mardegan V., Baraldi E. Intrauterine growth restriction: new insight from the metabolomic approach. Metabolites. 2019; 9(11): 267. https://dx.doi.org/10.3390/metabo9110267.
- Sharma D., Shastri S., Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin. Med. Insights Pediatr. 2016; 10: 67-83. https://dx.doi.org/10.4137/CMPed.S40070.
- Якубова Д.И., Игнатко И.В., Меграбян А.Д., Богомазова И.М. Особенности течения беременности и исходы родов при различных фенотипах задержки роста плода. Акушерство и гинекология. 2022; 8: 54-62. [Yakubova D.I., Ignatko I.V., Megrabian A.D., Bogomazova I.M. Features of pregnancy course and delivery outcomes in various phenotypes of fetal growth restriction. Obstetrics and Gynecology. 2022; (8): 54-62. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.8.54-62.
- Burton G.J, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018; 218(2S): S745-61. https://dx.doi.org/10.1016/j.ajog.2017.11.577.
- Hendrix N., Berghella V. Non-placental causes of intrauterine growth restriction. Semin. Perinatol. 2008; 32(3): 161-5. https://dx.doi.org/10.1053/j.semperi.2008.02.004.
- Serin S., Bakacak M., Ercan Ö., Köstü B., Avci F., Arıkan D., Kıran G. The evaluation of Nesfatin-1 levels in patients with and without intrauterine growth restriction. J. Matern. Fetal Neonatal Med. 2016; 29(9): 1409-13. https://dx.doi.org/10.3109/14767058.2015.1049524.
- Tao Z., Chen Y., He F., Tang J., Zhan L., Hu H. et al. Alterations in the gut microbiome and metabolisms in pegnancies with ffetal growth restriction. Microbiol. Spectr. 2023; 11(3): e0007623. https://dx.doi.org/10.1128/spectrum.00076-23.
- Tanner L.D., Brock A C., Chauhan S.P. Severity of fetal growth restriction stratified according to maternal obesity. J. Matern. Fetal Neonatal Med. 2022; 35(10): 1886-90. https://dx.doi.org/10.1080/14767058.2020.1773427.
- Salmeri N., Ca rbone I.F., Cavoretto P.I., Farina A., Morano D. Epigenetics beyond Fetal growth restriction: A comprehensive overview. Mol. Diagn. Ther. 2022; 26(6): 607-26. https://dx.doi.org/10.1007/s40291-022-00611-4.
- Lees C., Marlow N., Arabin B., Bilardo C.M., Brezinka C., Derks J.B. et al; TRUFFLE Group. Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet. Gynecol. 2013; 42 (4): 400-8. https://dx.doi.org/10.1002/uog.
- Allen V.M., Joseph K., Murphy K.E., Magee L.A., Ohlsson A. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: a population based study. BMC Pregnancy Childbirth. 2004; 4(1): 17. https://dx.doi.org/10.1186/1471-2393-4-17.
- Zhang H.G., Yang L., Qiao Z.X., Guo W. Effect of gestational hypertension on fetal growth restriction, endocrine and cardiovascular disorders. Asian J. Surg. 2022; 45 (4): 1048-9. https://dx.doi.org/10.1016/j.asjsur.2022.01.050.
- Пестрикова Т.Ю., Юрасова Е.А., Ткаченко В.А. Плацентарная недостаточность как базовая патология осложнений и исходов гестационного периода. Российский вестник акушера-гинеколога. 2020; 20(1): 5‑15. [Pestrikova T.Iu., Iurasova E.A., Tkachenko V.A. Placental insufficiency as the underlying condition of the complications an outcomes of the gestation period. Russian Bulletin of Obstetrician-Gynecologist. 2020; 20(1): 5‑15. (in Russian)]. https://dx.doi.org/10.17116/rosakush2020200115.
- Тапильская Н.И., Мельников К.Н., Кузнецова И.А., Глушаков Р.И. Плацентарная недостаточность и синдром задержки роста плода: этиология, профилактика, лечение. Медицинский Aлфавит. 2020; 4: 6-10. [Tapilskaya N.I., Mel’nikov K.N., Kuznetsova I.A., Glushakov R.I. Placental insufficiency and fetal growth restiction: etiology, prevention, and treatment. Medical Alphabet. 2020; (4): 6-10. (in Russian)]. https://dx.doi.org/10.33667/2078-5631-2020-4-6-10.
- Diguisto C., Le Gouge A., Marchand M.S., Megier P., Ville Y., Haddad G. et al; Groupe de Recherche en Obstétrique et Gynécologie (GROG). Low-dose aspirin to prevent preeclampsia and growth restriction in nulliparous women identified by uterine artery Doppler as at high risk of preeclampsia: A double blinded randomized placebo-controlled trial. PLoS One. 2022; 17(10): e0275129. https://dx.doi.org/10.1371/journal.pone.0275129.
- Lin L., Guo Y.N., Xu X., Huang L.P., Yang Q.P., Yan J.Y. Analysis of maternal and fetal outcomes and establishment of prediction model of vaginal delivery in pregnant women with pre-eclampsia complicated with fetal growth restriction. Eur. Rev. Med. Pharmacol. Sci. 2023; 27(20): 9947-954. https://dx.doi.org/10.26355/eurrev_202310_34173.
- Saraswat L., Bhattacharya S., Maheshwari A., Bhattacharya S. Maternal and perinatal outcome in women with threatened miscarriage in the first trimester: a systematic review. BJOG. 2010; 117(3): 245-57. https://dx.doi.org/10.1111/j.1471-0528.2009.02427.x.
- Wijesiriwardana A., Bhattacharya S., Shetty A., Smith N., Bhattacharya S. Obstetric outcome in women with threatened miscarriage in the first trimester. Obstet. Gynecol. 2006; 107(3): 557-62. https://dx.doi.org/10.1097/01.AOG.0000199952.82151.de.
- McLindon L.A., James G., Beckmann M.M., Bertolone J., Mahomed K., Vane M. et al. Progester.one for women with threate.ned miscarriage (STOP trial): a pla cebo-controlled randomized clinical trial. Hum. Reprod. 2023; 38(4): 560-8. https://dx.doi.org/10.1093/humrep/dead029.
- Волочаева М.В., Кан Н.Е., Тютюнник В.Л., Амирасланов Э.Ю., Леонова А.А., Солдатова Е.Е. Клинико-анамнестические факторы в прогнозировании и диагностике задержки роста плода. Акушерство и гинекология. 2023; 9: 82-90. [Volochaeva M.V., Kan N.E., Tyutyunnik V.L., Amiraslanov E.Yu, Leonova A.A., Soldatova E.E. Clinical and anamnestic factors in the prediction and diagnosis of fetal growth retardation. Obstetrics and Gynecology. 2023; (9): 82-90. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.9.82-90.
Received 12.07.2024
Accepted 27.09.2024
About the Authors
Shagane R. Gasymova, Junior Researcher, Department of Fetal Medicine of the Institute of Obstetrics; Diagnostic Medical Sonographer, Department of Ultrasound and Functional Diagnostics; Obstetrician-Gynecologist, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(916)542-22-99, shagane2501@mail.ru, https://orcid.org/0009-0001-2626-6670Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at the Center for Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, tioutiounnik@mail.ru,
Researcher ID: B-2364-2015, SPIN-code: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN-code: 5378-8437,
Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Maria V. Volochaeva, PhD, Senior Researcher at the Department of Regional Cooperation and Integration; Physician at the 1 Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4,
volochaeva.m@yandex.ru, https://orcid.org/0000-0001-8953-7952
Andrey E. Donnikov, PhD, Head of the Laboratory of Molecular Genetic Methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology
and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, donnikov@dna-technology.ru, https://orcid.org/0000-0003-3504-2406
Corresponding author: Shagane R. Gasymova, shagane2501@mail.ru



