The cluster approach to assessment of the subgingival microbiome composition in women with adverse pregnancy outcomes
Burduli A.G, Balmasova I.P., Tetruashvili N.K., Donnikov A.E., Krasheninnikova R.V., Arutyunov S.D.
Objective: To develop optimal approach to assessment of the clinical features of the gestational process and the role of associations of the members of the subgingival microbiome in the development of pregnancy complications associated with inflammatory changes in periodontium.
Materials and methods: The total number of patients in the study was 146 pregnant women, who were divided into 4 groups depending on the presence or absence of the concomitant inflammation of periodontal tissues (gingivitis) and/or local inflammation of the lower reproductive tract. The study design included obstetric and gynecological anamneses collection, assessment of the current pregnancy complications, determination of the composition of the subgingival microbiome and the vaginal microbiome using PCR. Statistical data processing included cluster analysis.
Results: The study found relationship between systemic manifestations of periodontal diseases and preterm birth, low birth weight. There was statistically significant relationship between these complications and gingivitis. Cluster analysis was used to identify 4 different associations of the microorganisms exhibiting periodontopathogenic properties, 3 of them were found in more than half of cases in the groups with concomitant gingivitis.
Conclusion: The cluster approach to assessment of the subgingival microbiome in pregnant women helped to identify associations of the microorganisms exhibiting periodontopathogenic properties. High frequency of their occurrence was accompanied by the presence of clinical signs of gingivitis and the development of systemic effects of pregnancy complications.
Authors' contributions: Burduli A.G., Balmasova I.P., Tetruashvili N.K. – the concept and design of the study; Burduli A.G., Donnikov A.E., Krasheninnikova R.V. – material collection and processing; Burduli A.G., Balmasova I.P. – data analysis; Burduli A.G. – article writing; Tetruashvili N.K., Donnikov A.E., Arutyunov S.D. – article editing.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was conducted without any sponsorship.
Ethical Approval: The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Burduli A.G., Balmasova I.P., Tetruashvili N.K., Donnikov A.E., Krasheninnikova R.V., Arutyunov S.D.
The cluster approach to assessment of the subgingival microbiome composition in women with adverse pregnancy outcomes.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2025; (9): 89-97 (in Russian)
https://dx.doi.org/10.18565/aig.2025.185
Keywords
In the studies of the last decade devoted to the issues of obstetrics and gynecology, special attention is given to relationship between inflammatory-destructive periodontal diseases and the outcomes of the gestational process.
Currently it has been found that the development of inflammatory periodontal diseases in pregnant women is determined by many factors arising in pregnancy, including changes in the immune and hormonal status of women, and bone metabolism [1, 2]. Depending on the geographical regions, approximately 33–100% of pregnant women have periodontal diseases [3]. Gingivitis is one of the most common pathological conditions of periodontal tissues in pregnant women due to their age category. Gingivitis is an inflammation of the gums without destruction of the dentogingival junction. During pregnancy, the incidence of gingivitis increases significantly and reaches 60–76% [4, 5].
Epidemiological studies have shown that periodontal diseases are also risk factors for the development of systemic diseases. In particular, systemic inflammation caused by focal periodontal infections is associated with immunopathological conditions, cardiovascular, cerebrovascular, respiratory, oncological and other diseases [6–8]. Systemic complications associated with periodontitis also include pregnancy complications, such as preterm labor and birth, fetal growth restriction, as well as less common premature rupture of membranes, preeclampsia, and gestational diabetes mellitus [9–11].
The search for the cause of this relationship has led to the discovery of microorganisms in the placenta in healthy pregnant women resembling the composition of the oral microbiome. According to researchers, this indicates that infants are exposed in utero to maternal microbiota-derived epitope through the bloodstream before birth [12]. Currently, two options are proposed to interpret the mechanisms of development of this phenomenon: direct penetration of oral microorganisms or their components through the blood into the fetal-placental complex, as well as invasion of inflammatory mediators produced in the oral cavity, with their subsequent impact on the functioning of the fetal-placental complex [13].
Thorough solution of this problem could help developing pathogenetically justified methods to prevent the above-mentioned pregnancy complications. However, the existing attempts to confirm each of these hypotheses failed, as the researchers faced conflicting data. One of the aspects of the problem is determination of the role of specific periodontopathogenic bacteria in induction of systemic effects leading to complications of the gestational process. In our study, the working hypothesis was based on the assumption that stable bacterial associations, but not individual bacteria influence the development of concomitant systemic pathology.
In this regard, the purpose of the study was to assess the clinical features of the gestational process and the role of associations of the members of the subgingival microbiome in the development of pregnancy complications associated with inflammatory changes in periodontium.
Мaterials and methods
The observational prospective cohort study was conducted at the 2nd Obstetric Department of Pregnancy Pathology. The study included 146 pregnant women. Their obstetric and gynecological anamneses were analyzed, and the course of pregnancy and perinatal outcomes were assessed. Multiple pregnancy complications in all women were registered both during current pregnancy and in the obstetric history. According to published data, these complications are classified in the category of pathological processes accompanying periodontal diseases. These include preterm birth, premature rupture of membranes, preeclampsia, fetal growth restriction, newborns’ low birthweight, gestational diabetes mellitus. Non-inclusion criteria in the study were the following: multiple pregnancy, congenital anomalies, chromosomal abnormalities, placenta previa, severe somatic pathology, oncological and autoimmune diseases. Inclusion criteria were patient’s refusal to participate in the study, acute inflammatory diseases or exacerbation of chronic diseases.
In accordance with the World Medical Association Declaration of Helsinki, informed consent to participate in the study was obtained from all women involved in the study. The study was approved by the Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, Ministry of Health of Russia.
All pregnant women at 10–14 weeks of gestation underwent consultation with the dentist to register and characterize their dental status due to the existing periodontal diseases. In parallel with this, all women underwent testing for the presence of local inflammation. Examination of the scrapings of the epithelial cells of the mucous membrane lining the cervical canal was performed using test system “ImmunoKvantex” (DNA-Technology, Moscow). Medical assessment report on the presence of local inflammatory reaction was based on calculation of the value of systemic immune-inflammation index (SII) using the software for automatic detecting amplifier. When the value of SII was more than 60%, the presence of a local inflammatory reaction in the lower reproductive tract was considered determined [14]. In addition, polymerase chain reaction (PCR) test was performed in all women to detect oral cavity biocenosis using Real Time PCR Detection Kit “ParodontoScreen” (DNA-Technology, Moscow) for detection of 7 microorganisms with the properties of periodontal pathogens and their associates, and “Femoflor-16” test system (DNA-Technology, Moscow) for assessment of the occurrence of the most frequently registered microorganisms within the vaginal mucosa.
The patients were divided into 4 groups. Group 1 included 25/146 pregnant women (17%) without the signs of gingivitis and without the signs of local inflammation in the scrapings from the epithelial cells of the mucous membrane lining the cervical canal showed no signs of local inflammation. Group 2 consisted of 17/146 pregnant women (12%) with concomitant catarrhal gingivitis at the time of examination, and the absence of local inflammatory reaction in the scrapings from the epithelial cells of the mucous membrane lining the cervical canal. Group 3 consisted of 78/146 pregnant women (53%) with local inflammatory reaction in the scrapings from the epithelial cells of the mucous membrane lining the cervical canal without the signs of gingivitis. Group 4 consisted of 26/146 pregnant women (18%), who were enrolled in the study, when two categories of local inflammatory changes were simultaneously found in oral cavity (gingivitis) and in the scrapings from the epithelial cells of the mucous membrane lining the cervical canal.
Statistical analysis
Statistical data processing was performed using SPSS statistical software, Version 23. Descriptive statistics were used for data analysis. The non-parametric Kruskal–Wallis test was used in the absence of normal distribution of quantitative data in all groups in the study. When the probability of differences between the groups were below the significance threshold of p<0.05, the posteriori analysis was performed (the groups were compared with the Bonferroni correction for multiple comparisons at p<0.0125), using the Mann–Whitney U test for quantitative data or Pearson’s chi-square (χ2) test for relative values. Cluster analysis of K-means measured as the number of genome equivalents was done to identify statistically significant associations of the tested microorganisms.
Results and discussion
The first stage of the study was analysis of obstetric and gynecological anamneses and the concomitant signs of local inflammation in different epitopes of biocenoses in periodontal tissues and/or in the scrapings from the epithelial cells of the mucous membrane lining the cervical canal. The results of analysis are shown in Table 1.
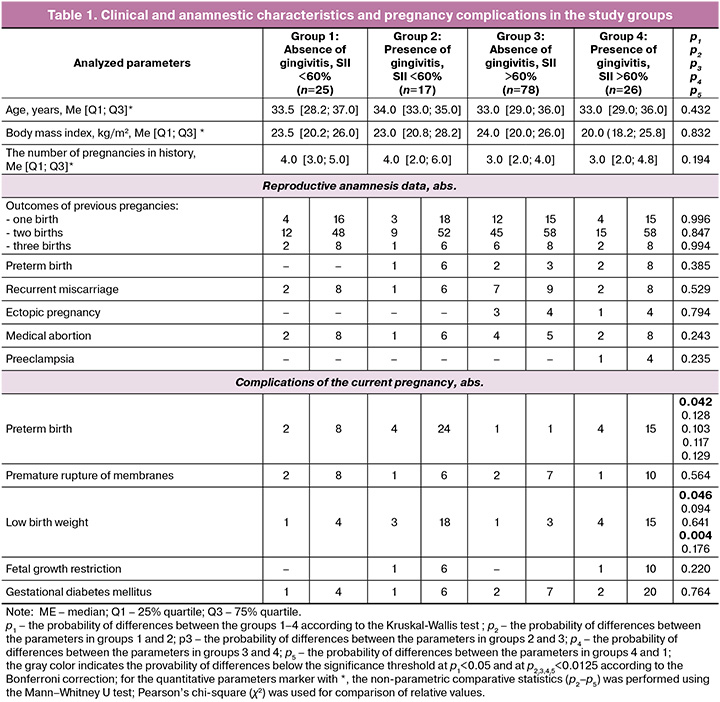
As follows from Table 1, the study groups were comparable for the parameters, such as the average age of women, the average body mass index, and the outcomes of previous pregnancies. This made it possible with good reason to assess the explored characteristics of anamnesis and the current gestational process.
In groups 2, 3, 4 the presence of inflammatory lesions was accompanied by a tendency for the increasing number of complications of different severity in current pregnancy. Thus, high frequency of occurrence of preterm birth and low birthweight was statistically significant only in the intergroup comparisons. Pairwise comparisons of means in the groups found statistical significance only for the parameter, such as low birthweight. High frequency of occurrence of low birthweight was found only in groups 3 and 4 in pregnant women with gingivitis and inflammation in the lower reproductive tract. Similar shifts in group 1 (without concomitant inflammation) and in group 3, where according to the test results using the ImmunoKvantex test-system, pregnancies were accompanied by inflammation involving epithelial cells from the cervical canal (SII >60%), were not so strong and did not reach the level of statistical significance.
The next stage was PCR data analysis of subgingival biocenosis using the Parodonoscreen test system and biocenosis of the vaginal mucosa from the posterior vaginal fornix using the Femoflor-16 test system.
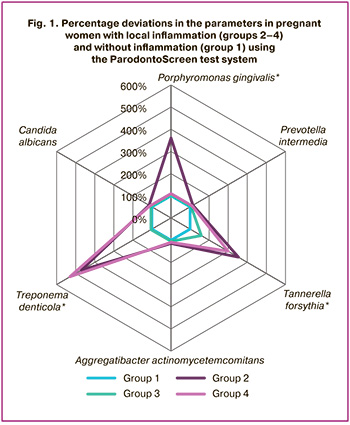
PCR test results are presented in Table 2 and Figure 1. The latter shows not the values of the content of each microorganism in the microbiome measured in genome equivalents, but the percentage of deviation of dataset in the groups 2, 3, 4 from the indicators in group 1, where inflammatory lesions in the periodontium or lower reproductive tract were not found.
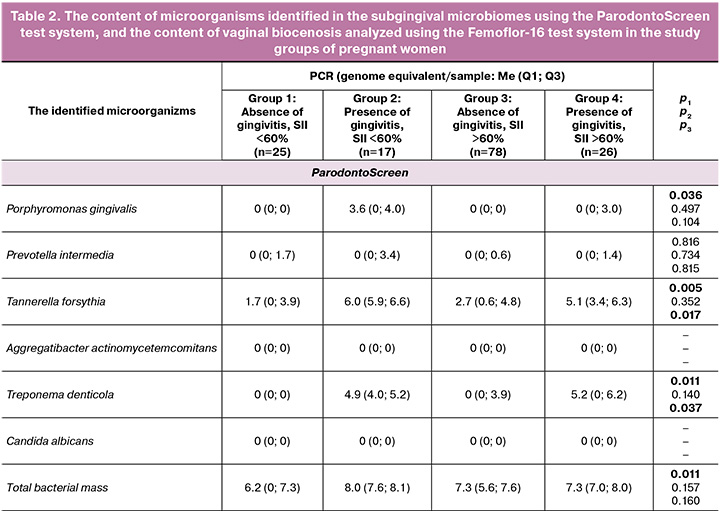

As follows from the presented results, the subgingival microbiome at the species level determined by the ParodontoScreen test system manifested itself differently in the study groups. The greatest shifts were found in group 2, where pregnancy was accompanied by gingivitis. In this group, such periodontopathogens as Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola induced responses, that influenced the total rate of bacterial growth.
In group 3, where pregnancy was accompanied by inflammation in the lower reproductive tract, there were no changes in the sugingival microbiome.
In group 4, a combination of inflammation in two sites contributed to the increased growth of two species of microorganisms – Tannerella forsythia and Treponema denticola. However, this was not accompanied by statistically significant total bacterial growth rate.
Examination of microbiome samples obtained by scrapings from the vaginal mucosa showed that none of 16 tested microorganisms, except for Candida albicans fungi in group 2, caused statistically significant shifts.
The obtained data indirectly confirm the possibility of direct influence of subgingival microbiome on the development of the systemic impact of periodontal disease involving female reproductive system. Moreover, the leading role given by many authors to focal inflammation raises doubt, since our study shows that not only the presence of local inflammation is of great importance, but also the localization of inflammation of, namely, association with periodontal tissues (gingivitis).
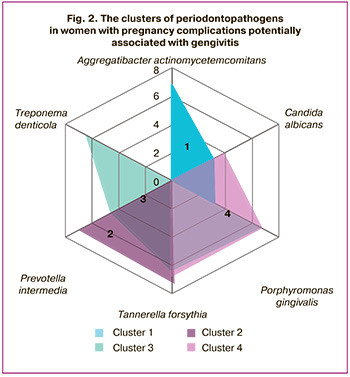
Cluster analysis was used to confirm that the subgingival microbiome plays a special role in the development of pregnancy complications. If the proposed working hypothesis would prove to be correct, it would help us to identify the associations of periodontopathogenic bacteria that could lead to pregnancy complications. The results of cluster analysis of microorganisms in each study group, which were identified using the Parodontoscreen test system, are shown in Figure 2 and Table 3.

As follows from the presented results, in pregnant women in group 1, who had no local inflammation affecting periodontal tissues or the lower reproductive tract, the clusters of the informative associations of periodontopathogens had no statistical significance. In pregnant women with concomitant gingivitis in group 2, two clusters composed of association of periodontopathogens Tannerella forsythia + Prevotella intermedia (cluster 2), as well as association of Porphyromonas gingivalis + Prevotella intermedia + Tannerella forsythia + Treponema denticola (cluster 3) were informative. At the same time, both clusters included Tannerella forsythia and Prevotella intermedia. In women with inflammation in the lower reproductive tract in group 3, no statistically significant clusters were identified. In group 4 characterized by local inflammation in both epitopes, only one informative cluster composed of Tannerella forsythia + Treponema denticola + Candida albicans (cluster 4) was identified.
Thus, the identified clusters represented informativeness only in two groups, where pregnancies were accompanied by gingivitis. At the same time, the composition of microbial associations in these groups was different. The presence of local inflammatory reaction in the scrapings from the epithelial cells of the mucous membrane lining the cervical canal showed no relationship with formation of certain associations of microorganisms, although their influence on the composition of the microbial association in combination with gingivitis in this case cannot be excluded.
Conclusion
In pregnant women, the presence of concomitant gingivitis is generally associated with a number of sings (preterm birth, low birthweight of newborns) characteristic of systemic manifestations of periodontal diseases. However, in the framework of our study the influence of inflammatory diseases of the lower reproductive tract cannot be fully excluded.
The obtained results support the hypothesis that the microbiome of periodontal tissues plays one of the leading roles in the development of systemic effects in pregnant women.
The cluster approach to evaluate formation of different associations of periodontopathogens and concomitant microorganisms makes it possible to confirm their special pathogenic role in the development of systemic effects associated with periodontal diseases (gingivitis), as well as to suggest that two microbial associations (Tannerella forsythia + Prevotella intermedia and Tannerella forsythia + Treponema denticola + Candida albicans) have informative significance in involvement in the systemic reaction in the reproductive system of pregnant women.
The obtained results will be useful for obstetricians and gynecologists in managing pregnant women at risk for complications associated with periodontal diseases, and can be a perspective for further research in this area.
References
- Макеева И.М., Игнатко А.А., Чурганова А.А., Лебедев В.А., Mакеева M.K. Болезни пародонта и осложненное течение беременности. Стоматология. 2019; 98(1): 70-3. [Makeeva I.M., Ignatko A.A., Churganova A.A., Lebedev V.A., Makeeva M.K. Periodontal diseases and complicated pregnancy. Stomatology. 2019; 98(1): 70-3 (in Russian)]. https://dx.doi.org/10.17116/stomat20199801170
- Oliveira L.J.C., Cademartori M.G., Sfreddo C.S., Silveira M.F.D., Barros F.C., Correa M.B. et al. Factors associated with periodontal diseases in pregnancy: findings of the 2015 Pelotas birth cohort study. Braz. Oral. Res. 2023; 37: e110. https://dx.doi.org/10.1590/1807-3107bor-2023.vol37.0110
- Gesase N., Miranda-Rius J., Brunet-Llobet L., Lahor-Soler E., Mahande M.J., Masenga G. The association between periodontal disease and adverse pregnancy outcomes in Northern Tanzania: a cross-sectional study. Afr. Health Sci. 2018; 18(3): 601-11. https://dx.doi.org/10.4314/ahs.v18i3.18
- Gallardo Chávez L.M., Rodríguez Díaz J.M., Juárez Medel C.A., Hernández Clemente J., Herrera Santos A.U. Prevalence of gingivitis and risk factors among pregnant women from Acapulco, Guerrero: a cross-sectional study. Rev. Cient. Odontol. (Lima). 2022; 10(1): e094. https://dx.doi.org/10.21142/2523-2754-1001-2022-094
- Shrestha R., Pradhan S., Baral G. Prevalence of gingivitis in second trimester of pregnancy. Kathmandu Univ. Med. J. (KUMJ). 2022; 20(79): 301-6.
- Балмасова И.П., Царёв В.Н., Янушевич О.О., Маев И.В., Мкртумян М.А., Арутюнов С.Д. Микроэкология пародонта. Взаимосвязь локальных и системных эффектов. М.: Практическая медицина; 2021. 264 с. [Balmasova I.P., Tsarev V.N., Yanushevich O.O., Maev I.V., Mkrtumyan M.A., Arutyunov S.D. Periodontal microecology. Interrelation of local and systemic effects. Moscow: Practical medicine; 2021. 264 p. (in Russian)].
- Sanz M., Marco Del Castillo A., Jepsen S., Gonzalez-Juanatey J.R., D'Aiuto F., Bouchard P. et al. Periodontitis and cardiovascular diseases: consensus report. J. Clin. Periodontol. 2020; 47(3): 268-88. https://dx.doi.org/10.1111/jcpe.13189
- Walther K.A., Gröger S., Vogler J.A.H., Wöstmann B., Meyle J. Inflammation indices in association with periodontitis and cancer. Periodontol. 2000. 2024; 96(1): 281-315. https://dx.doi.org/10.1111/prd.12612
- Le Q.A., Akhter R., Coulton K.M., Vo N.T.N., Duong L.T.Y., Nong H.V. et al. Periodontitis and preeclampsia in pregnancy: a systematic review and meta-analysis. Matern. Child Health. J. 2022; 26(12): 2419-43. https://dx.doi.org/10.1007/s10995-022-03556-6
- Saadaoui M., Singh P., Khodor S.A. Oral microbiome and pregnancy: a bidirectional relationship. J. Reprod. Immunol. 2021; 145: 103293. https://dx.doi.org/10.1016/j.jri.2021.103293
- Гажва С.И., Сулима А.Н., Гажва Ю.В., Тетерин А.И., Кашин Ю.А. Микробиом полости рта и его влияние на течение беременности. Акушерство и гинекология. 2025; 5: 14-9. [Gazhva S.I., Sulima A.N., Gazhva Yu.V., Teterin A.I., Kashin Yu.A. Oral microbiome and its impact on pregnancy. Obstetrics and Gynecology. 2025; (5): 14-9 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.332
- Stupak A., Kwaśniewski W. Evaluating current molecular techniques and evidence in assessing microbiome in placenta-related health and disorders in pregnancy. Biomolecules. 2023; 13(6): 911. https://dx.doi.org/10.3390/biom13060911
- Xu B., Han Y.W. Oral bacteria, oral health, and adverse pregnancy outcomes. Periodontol. 2000. 2022; 89(1): 181-9. https://dx.doi.org/10.1111/prd.12436
- Регистрационное удостоверение на медицинское изделие от 04.04.2016 г. № РЗН 2016/3903. Инструкция по применению набора реагентов для определения профиля экспрессии мРНК генов врожденного иммунитета с целью оценки локального воспаления нижних отделов женского репродуктивного тракта методом ОТ-ПЦР в режиме реального времени (ИммуноКвантэкс С/V). М.: НПО ДНК-Технология; 2015. 24 c. [Registration certificate for a medical device dated 04.04.2016, No. RZN 2016/3903. Instructions for use of a set of reagents for determining the mRNA expression profile of innate immunity genes in order to assess local inflammation of the lower parts of the female reproductive tract using real-time RT-PCR (ImmunoQuantex C/V). Moscow: NPO DNA-Technology; 2015. 24 p. (in Russian)].
Received 03.07.2025
Accepted 22.08.2025
About the Authors
Anna G. Burduli, PhD, Senior Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)531-44-44, +7(985)120-36-14, burdulianna@gmail.com, https://orcid.org/0000-0002-2849-5426Irina P. Balmasova, Dr. Med. Sci., Professor, Leading Researcher at the Laboratory of Molecular Biological Research, Scientific Research Medical Dentistry Institution, Scientific and Educational Institution of Basic Medicine named after V.I. Pokrovsky, Russian University of Medicine, Ministry of Health of Russia, 127006, Russia, Moscow, Dolgorukovskaya str. 4, +7(910)468-97-72, iri.balm@mail.ru, https://orcid.org/0000-0001-8194-2419
Nana K. Tetruashvili, Dr. Med. Sci., Associate Professor, Head of the Obstetric Department of Pregnancy Pathology No. 2, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)438-14-77,
n_tetruashvili@oparina4.ru, https://orcid.org/0000-0002-9201-2281
Andrey E. Donnikov, PhD, Head of the Laboratory of Molecular Genetic Methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)438-49-51, a_donnikov@oparina4.ru, https://orcid.org/0000-0003-3504-2406
Regina V. Krasheninnikova, Geneticist at the Laboratory of Molecular Genetic Methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4, +7(495)438-49-51, ex. 3101, r_krasheninnikova@oparina4.ru,
https://orcid.org/0009-0002-2903-1225
Sergey D. Arutyunov, Dr. Med. Sci., Professor, Head of Department of Orthopedic Dentistry and Digital Technologies, Russian University of Medicine, Ministry of Health of Russia, 127473, Russia, Moscow, Delegatskaya str., 20-1, sd.arutyunov@mail.ru, https://orcid.org/0000-0001-6512-8724
Corresponding author: Anna G. Burduli, burdulianna@gmail.com



