Differential diagnosis of early-stage ovarian cancer based on the bioinformatic analysis of the blood metabolome
Iurova M.V., Tokareva A.O., Chagovets V.V., Starodubtseva N.L., Frankevich V.E.
The early diagnosis of ovarian cancer (OC) represents an important step in reducing the number of fatal outcomes associated with the disease. Timely treatment initiation, minimizing the risk of recurrence and side effects of therapy, largely depend on early diagnosis.
Objective: To create stable panels of blood metabolites for differentiating healthy patients, patients with early-stage high-grade OC from other ovarian tumors.
Materials and methods. A search for markers for clustering of blood samples obtained from patients with verified stage I–II high-grade OC (n=10) and other proliferative processes (cystadenoma (n=30), endometrioid cyst (n=56), teratoma (n=21), borderline tumor (n=28), low-grade OC (n=16), stage III–IV high-grade (n=49)) and control volunteers (n=19) was performed. The study was carried out at the V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow, Russia. The data were analyzed using recursive variable removal from the support vector machine and other statistical tools. There was a search for markers of malignant neoplasms (MNPs), followed by the analysis of their involvement in metabolic pathways. Models based on neural networks were built.
Results: The panels of metabolite-markers of early stages of MNPs were identified. The overlap of the obtained panels with metabolic pathway databases was studied. Artificial neural network models were developed to differentiate blood samples from I–II stage OC from controls with sensitivity and specificity of 90% and 89%, and from I–II stage OC from other ovarian neoplasms with sensitivity and specificity of 80% and 71%, respectively.
Conclusion: The introduction of post-genomic research has the potential to increase the diagnostic value of the methods used to detect ovarian MNPs at an earlier stage and also to expand the available data on the processes of carcinogenesis in the ovaries.
Authors’ contributions: Iurova M.V. – developing the concept of the study, material collection, receiving funding, project management, verification, resource search, supervision, writing literature review, initial draft and editing; Tokareva A.O. – data curation, methodology, providing software, resource search, writing, editing; Chagovets V.V. – making formal analysis, methodology, providing software; Starodubtseva N.L. – methodology, making formal analysis, developing the concept of the study, supervising, writing, editing; Frankevich V.E. – making formal analysis, supervising, reviewing and editing.
Conflicts of interest: The authors declare that the study was conducted in the absence of any commercial or financial relationship that could be interpreted as a potential conflict of interest.
Funding: The study was carried out within the framework of the Russian Science Foundation Grant dated 29.12.2023 No. 24-25-00407 “New approaches of application of artificial intelligence capabilities to differential diagnosis of benign tumors and ovarian cancer on the basis of blood metabolome characteristics determined by physical methods”.
Ethical Approval: The study was approved by the Ethical Review Board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology Moscow, Russia (Protocol No. 10 dated December 05, 2019). The study was initiated after obtaining the approval of the mentioned committee and was carried out in accordance with the Russia’s Federal Law of 27 July 2006 No. 152-FZ on Personal Data (‘the Law on Personal Data’) , Federal Law of 21.11.2011 No. 323-FZ “On the basics of health protection of citizens in the Russian Federation”, the provisions of the Declaration of Helsinki with all subsequent additions and amendments regulating scientific research on biomaterials obtained from human subjects, as well as the International ethical guidelines for biomedical research involving human subjects of the Council for International Organizations of Medical Sciences (CIOMS).
Patients’ Consent for Publication: The patients signed informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Iurova M.V., Tokareva A.O., Chagovets V.V., Starodubtseva N.L., Frankevich V.E. Differential diagnosis of early-stage ovarian cancer based on the bioinformatic analysis of the blood metabolome.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (12): 118-126 (in Russian)
https://dx.doi.org/10.18565/aig.2024.283
Keywords
Early diagnosis of ovarian malignant neoplasms (MNPs) is one of the most urgent and challenging clinical problems. In the Russian Federation, ovarian tumors account for the highest incidence of cancer among women, with an annual prevalence rate of 82.2 cases per 100,000 individuals. This rate is lower than that of breast cancer (541.7 cases per 100,000 women), followed by skin, colorectal, uterine body and cervical cancers. In 2023, the rate of active detection of ovarian MNPs remained low, namely 17.9%; the detection rate of stage I–II cancer was 43.7%. The prevalence of ovarian cancer (OC) as a leading cause of gynecologic cancer mortality surpasses that of breast, uterine body and cervical cancer.
The five-year survival rate for high-grade malignancies is only 47%, compared to 85% for breast cancer, in highly developed countries such as the United States and Canada [2, 3]. This is due to the lack of effective measures aimed at the detection of ovarian MNPs at an early stage: 55–75% of cases are diagnosed at stage III-IV of the disease [1]. According to the United States Preventive Services Task Force [4], screening for ovarian neoplasia is considered to be inappropriate because of the high risk of unnecessary surgical intervention in asymptomatic women. The difficulties are due to the lack of pathognomonic manifestations of adnexal diseases, the low specificity and limited availability of diagnostic methods [5, 6].
Malignant ovarian epithelial tumors include the following histological types (WHO classification, 5th edition, 2020) [7]:
- serous carcinoma:
- low-grade serous carcinoma;
- high-grade serous carcinoma;
- endometrioid carcinoma;
- seromucinous carcinoma;
- mucinous carcinoma;
- clear cell carcinoma;
- Brenner tumor;
- undifferentiated carcinoma;
- mixed epithelial carcinoma.
The main task of a surgeon operating on a patient with OC is to perform a complete or optimal primary cytoreductive operation. The amount of work required is proportional to the extent of the tumor process, and the degree of feasibility correlates with the oncological prognosis.
Between 3 and 14% of patients diagnosed with ovarian MNPs are women of reproductive age [8, 9]. Organ preservation surgery (unilateral adnexectomy with resection of the second ovary, omentectomy, pelvic and lumbar lymphadenectomy and staging) is sometimes acceptable in young women who wish to preserve their fertility. The following conditions should be met for the performance of organ preservation surgery: the presence of highly differentiated serous, endometrioid or mucinous carcinomas of stages IA and IC1 with the possibility of careful follow-up and proven absence of hereditary nature of the disease [7]. In this context, early detection of the disease is essential, as well as a differentiated approach to patient management based on tumor histological type and stage.
Multiparametric metabolic profiling technologies are primarily based on nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry techniques. The quality of the analysis is improved by combining mass spectrometry with prior chromatographic separation of the sample compounds. Mass spectrometry is used for the identification of a compound on the basis of the mass-to-charge ratio of the ion and (optionally) the mass-to-charge ratio of fragment ions obtained by induced fragmentation of the ions of the compound. High-resolution NMR spectroscopy makes it possible to identify compounds based on the emission spectrum which is produced by the magnetic reorientation of the nuclei of the compound atoms. The analysis of multivariate data, which include the results of mass spectrometry and NMR analysis, is performed using chemometric and statistical methods. These methods are used to isolate sets of compounds that serve as biological markers, which are then used to construct classification models through machine learning methods.
The aim of the work was to create panels of blood metabolites for differentiating patients with early-stage OC from healthy women and from patients with other ovarian lesions.
Materials and methods
The study was carried out at the National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow, Russia. A search for markers for clustering of blood samples obtained from patients with verified stage I–II high-grade OC (n=10), cystadenoma (n=30), endometrioid cyst (n=56), teratoma (n=21), borderline tumor (n=28), low-grade OC (n=16), stage III–IV high-grade (n=49) and control volunteers (n=19) was performed. The research is based on the data on lipid profiles obtained earlier in the course of clinical studies conducted at the above-mentioned center. Blood samples were collected in a tube containing ethylenediaminetetraacetic acid (EDTA) before the patient took preoperative medications (antibiotics, analgesics, etc.). The collected samples were centrifuged at 300g for 20 minutes at 4°C. After centrifugation, the supernatant was withdrawn and centrifuged at 12000g for 10 minutes at 20°C. The plasma was then transferred into 0.5 ml tubes and stored at -80°C.
Lipid extraction and chromatography-mass spectrometry analysis as well as sample preparation followed by NMR analysis were performed as in previous studies [10].
Statistical analysis
R scripts, version 4.3.2, were written for the statistical analysis and implemented in the RStudio environment. The following groups were compared in pairs: control group/group with ovarian tumors, control group/group with early-stage high grade OC, group with ovarian tumors/group with early-stage high grade OC. This involved the use of the following statistical tools: the Mann–Whitney test and the Welch’s t-test with a statistical significance threshold of p<0.05, discriminant analysis of orthogonal projections to latent structures with a variable importance of projection (VIP) threshold greater than 1, the method of random forest with 1000 trees and selection of the first n0.5 connections out of the n connections with the maximum value of the Gini index, the method of recursive elimination of variables by means of Support vector machine-recursive feature elimination (SVM-RFE) as long as it leads to an increase in the accuracy of the model determined by means of cross-validation with 10-fold partitioning, the Least Absolute Shrinkage and Selection Operator (LASSO), Boruta algorithm. The stability of each method for variable selection was assessed based on the value of the Koch Index of biotal dispersity calculated for 100 panels obtained on 100 occasions, with 80% of the samples chosen at random. The final marker panels for each pair of comparisons included compounds common to the 100 panels generated for each pair of comparisons.
For the panels with the highest Koch Index of biotal dispersity, the quality of clustering of principal component in coordinate space was analyzed using the following metrics: the Davies–Bouldin index, the Hubert–Levin index. The pattern of variation in levels between groups was assessed as follows: 100 subsets of samples were created by randomly selecting 80% of the samples in the classification of interest, and the ratios of the median compound values were calculated for them. After confirming the normality of the distribution of the calculated median ratios using the Shapiro-Wilk test (p>0.05), the probability of the median value being equal to one (p) was determined by means of the t-test.
The generated optimal panels were used to create classification models based on artificial neural networks with cross-validation via tenfold partitioning. Optimal sensitivity and specificity were calculated by maximizing the sum of sensitivity and specificity.
The ConsensusPath database-human web resource with access to databases KEGG, Reactome, BioCyc, Pathway interaction database, Biocarta, NetPath, INOH, EHMN, PharmGkb, WikiPathways, SMPDB, was also used to analyze pathway saturation of compounds included in the marker panel. Pathways exhibiting a false discovery probability of less than 0.05, as determined by the Benjamin-Hochberg correction method, were considered statistically significantly enriched.
Results
The best stability of the panels was found for the variable selection method via OPLS and SVM-RFE (Table 1); therefore, further data and interpretations are given in this paper for these two methods.
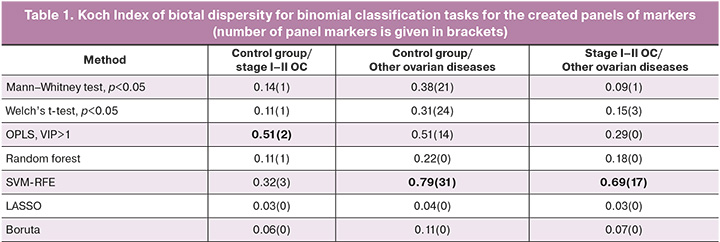
The most stable panels of markers for differentiation tasks included the compounds shown in Table 2.
In a panel of markers to discriminate the control group from the group with ovarian tumor obtained by OPLS, the levels of phosphatidylcholine PC 16:0_20:4 and triacylglycerols TG 16:0_18:1_18:2, TG 16:0_18:1_18:1 increased with the development of tumour; the level of the first two markers increased more than one and a half times. In contrast, the levels of lysophosphatidylcholines LPC 18:0, LPC 16:0, LPC 18:2, LPC 20:5, phosphatidylcholines PC 16:0_18:2, PC 16:0_20:2, PC 16:0_20:2, PC 16:0_20:3, PC 18:0_18:1, PC 18:0_18:2, monogalactosyldiacylglycerol MGDG 18: 0_18:0, oxidised phosphatidylcholine OxPC 20:4_14:0(COOH) were reduced in patients with tumors in comparison with the control group (Fig. 1A). The level of sphingomyelin SM d18:1/16:0, defined as a marker by OPLS, was elevated in patients with stage I-II OC, while glucose levels were reduced (Fig. 1B).
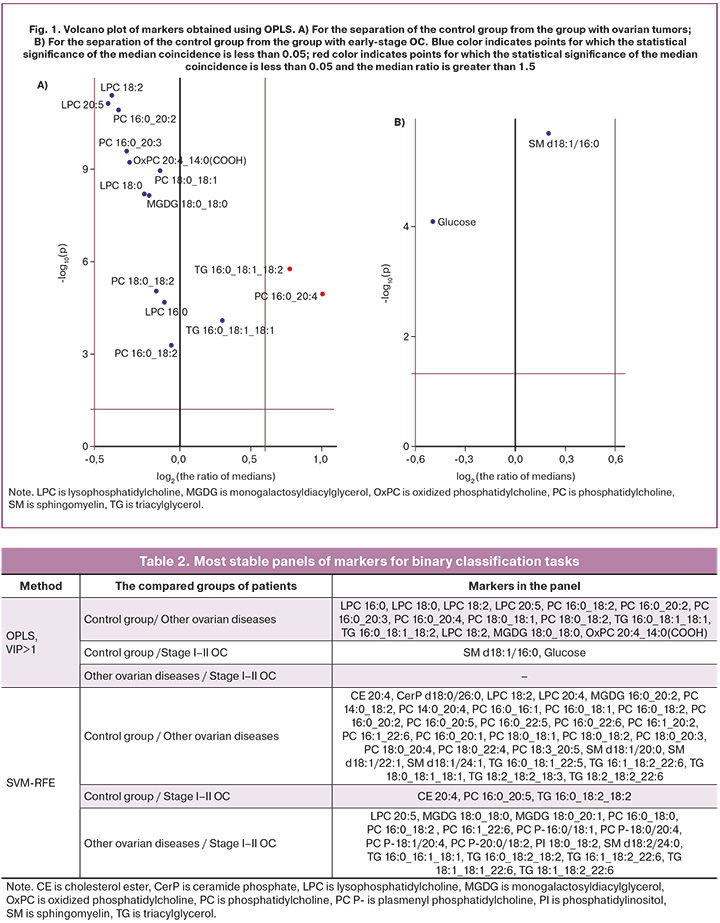
In a panel of markers to discriminate the control group from the group with ovarian tumors obtained by SVM-RFE, the levels of phosphatidylcholines PC 16:1_20:2, PC 16:0_18:1, PC 16:0_22:5, PC 16:0_22:6, PC 18:0_20:3, PC 18:0_20: 4, triacylglycerols TG 18:0_18:1_18:1_18:1, TG 18:2_18:2_18:3, monogalactosyls MGDG 16:0_20:2 increased with the development of tumors, with the ratio of median levels of phosphatidylcholine PC 16:1_20:2 in patients with ovarian tumor and in the control group exceeding 1.5. The levels of cholesterol ester CE 20:4, lysophosphatidylcholines LPC 18:2, LPC 20:4, phosphatidylcholines PC 14: 0_18:2, PC 14:0_20:4, PC 16:0_16:1, PC 16:0_18:2, PC 16:0_20:1, PC 16:0_20:1, PC 16:0_20:2, PC 16:0_20:5, PC 16:1_22:6, PC 18:0_22:4, PC 18:0_18:1, PC 18:0_18:2, PC 18:3_20: 5, sphingomyelins SM d18:1/20:0, SM d18:1/22:1, SM d18:1/24:1, TG 16:0_18:1_22:5, ceramide phosphate CerP 16:0/22:6 were decreased during the development of tumors, and the level of phosphatidylcholine PC 14:0_18:2 was decreased by more than one and a half times (Fig. 2A).
When a panel of markers was created to discriminate the control group from the group with early-stage OC, an increase in triacylglycerol TG 16:0_18:2_18:2 and a decrease in cholesterol ester CE 20:4, phosphatidylcholine PC 16:0_20:5 were observed (Fig. 2B). In a panel developed to discriminate patients with early-stage OC of high malignancy from other ovarian tumors, early OC showed an increase in lysophosphatidylcholine LPC 20:5, monogalactosyldiacylglycerol MGDG 18: 0_18:0, phosphatidylinositol PI 18:0_18:2, triacylglycerols TG 18:1_18:1_22:6, TG 18:1_18:2_22:6, TG 16:1_18:2_22:6, sphingomyelin SM d18:2/24: 0 and a drop in levels of triacylglycerol TG 16:0_16:1_18:1, phosphatidylcholines PC 16:1_22:6, plasmenyl phosphatidylcholines PC P-18:0/20:4, PC P-20:0/18:2, PC P-16:0/18:1.
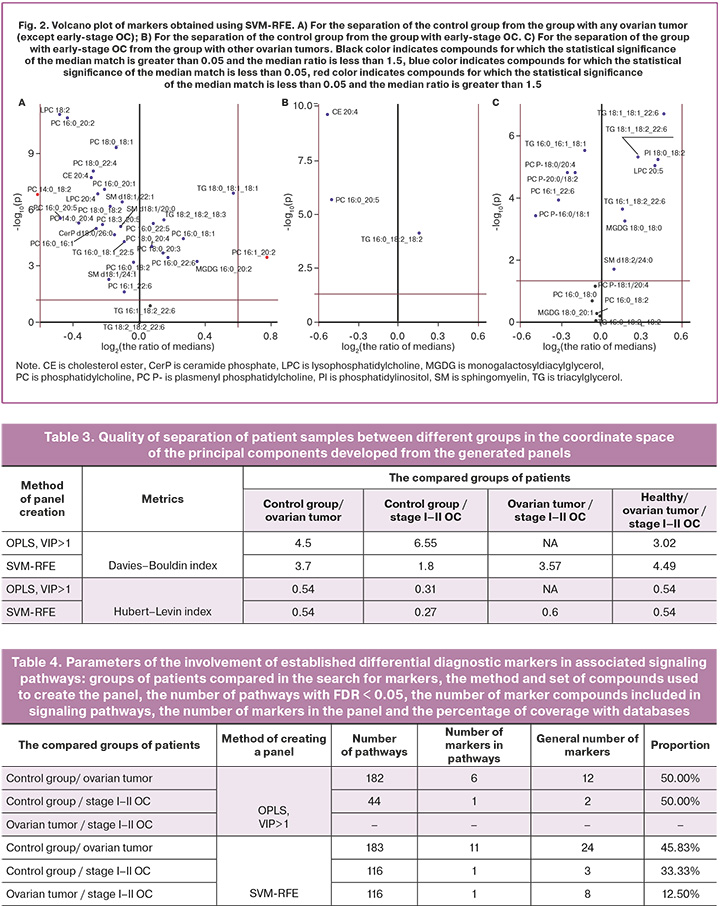
The variable selection method using SVM-RFE gave the best result for binary partitioning (Table 3). However, the proportion of database coverage in panels created using OPLS was found to exceed that of panels created using SVM-RFE (Table 4).
Phosphatidylcholines PC 16:0_20:3, PC 16:0_20:4, PC 18:0_18:1 and triacylglycerol TG 16:0_18:1_18:1 that entered into the panel to differentiate control group/ovarian tumor via OPLS VIP>1 are included in pathways related to the immune system. Lysophosphatidylcholines LPC 16:0, LPC 18:0, phosphatidylcholines PC 16:0_20:3, PC 16:0_20:4, PC 18:0_18:1 are part of the choline metabolism pathway in cancer and metabolism of many medications. Lysophosphatidylcholines LPC 16:0, LPC 18:0, phosphatidylcholines PC 16:0_20:3, PC 16:0_20:4, PC 18:0_18:1, triacylglycerol TG 16:0_18:1_18:1 are included in pathways related to lipoprotein metabolism. Glucose from a panel created for differentiating healthy patients from early-stage OC patients obtained via OPLS VIP>1 is included in the TCA Cycle Nutrient Utilization and Invasiveness of Ovarian Cancer pathway as well as the pathways of glycolysis and glycogenesis.
Phosphatidylcholines PC 14:0_20:4, PC 16:0_20:5, PC 16:0_22:5, PC 18:0_18:1, PC 18:0_20:4, PC 16:0_18:1 and triacylglycerol TG 18:0_18:1_18:1 from the panel for the control group/ovarian tumor obtained via SVM-RFE are included in pathways related to the immune system. Lysophosphatidylcholine LPC 20:4, phosphatidylcholines PC 14:0_20:4, PC 16:0_18:1, PC 16:0_20:5, PC 16:0_22:5, PC 18:0_18:1, PC 18:0_20:4 and triacylglycerol TG 18:0_18:1_18:1 are included in pathways related to lipoprotein metabolism. Sphingomyelins SM d18:1/20:0, SM d18:1/22:1, SM d18:1/24:1 are associated with the pathways of necroptosis and apoptosis.
Phosphatidylcholine PC 16:0_20:5 from a panel aimed at differentiating healthy patients from early-stage OC patients generated using SVM-RFE is included in the choline metabolism pathways of cancer and multidrug metabolism. Plasmenyl phosphatidylcholine PC P-16:0/18:1 may be considered for differentiating patients with early-stage OC from patients with other ovarian tumors.
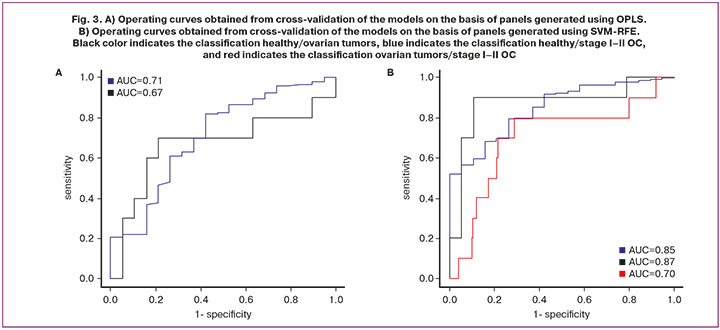
The panels created by means of OPLS made it possible to develop classification models with a sensitivity of 82% and a specificity of 58% to discriminate the control group from the group of patients with ovarian tumors and a sensitivity of 70% and a specificity of 79% to discriminate the control group from the group with early-stage OC (Fig. 3A). The panels created by means of SVM-RFE made it possible to develop classification models with a sensitivity of 80% and specificity of 74% to discriminate the control group from the group of patients with ovarian tumors, a sensitivity of 90% and specificity of 89% to discriminate the control group from the group with early-stage OC, and a sensitivity of 80% and specificity of 71% to discriminate the group with early-stage OC from other ovarian tumors (Fig. 3B). One can say that the panels generated by SVM-RFE have a higher discriminative power.
Discussion
Although previous metabolomic studies have successfully provided evidence of metabolic dysregulation associated with MNP OC, the biology of the disease and the underlying mechanisms associated with its progression remain largely unknown from a metabolomic perspective. The study of the lipidome provides an information-biological basis, the analysis of which and comparison with similar patterns in other disorders/other patients provides highly sensitive data to analyze the relationship between disease and associated biological processes. The confirmation of these associations on the basis of relevant databases on signaling pathways contributes to the body of knowledge on carcinogenesis.
Glycoconjugates (mixed biopolymers containing carbohydrates: glycopeptides and glycoproteins, glycolipids and lipopolysaccharides, glycolipoproteins, etc.) are the most important components of membranes, exhibiting their carbohydrate fragments on the cell surface; they perform structural and metabolic functions, and also participate in the processes of intercellular interaction. When elevated lipid content changes are detected, there is an increased biological and diagnostic significance of the panel as a source of the most familiar clinician’s tool, oncomarkers; their elevation could potentially indicate the presence of the suspected process (namely, an increase in lysophosphatidylcholine LPC 20: 5, monogalactosyldiacylglycerol MGDG 18:0_18:0, phosphatidylinositol PI 18:0_18:2, triacylglycerols TG 18:1_18:1_22:6, TG 18:1_18:2_22:6, TG 16:1_18:2_22:6, sphingomyelin SM d18:2/24:0). In this study, various methods were used to determine the increase of the following metabolites in stage I-II OC: sphingomyelin SM d18:1/16:0 (OPLS, SVM-RFE), monogalactosyldiacylglycerol MGDG 16: 0_20:2, phosphatidylcholines PC 16:0_18:1, PC 16:0_22:5, PC 16:0_22:6, PC 18:0_20:3, PC 18:0_20:4, PC 16:1_20:2, triacylglycerols TG 18: 0_18:1_18:1, TG 18:2_18:2_18:2_18:3, (SVM-RFE), triacylglycerol TG 16:0_18:2_18:2 (SVM-RFE), and others. Our findings of metabolome changes detected in earlier stages of the disease compared to advanced stages of OC are consistent with the findings of Ke C. et al. who demonstrated an increase in lysophosphatidylcholines in localized cancer in comparison with metastatic cancer in 448 plasma samples obtained from patients [11].
Metabolite panels generated with SVM-RFE have overlaps with compounds labelled in relevant databases; these compounds are part of pathways associated with cancer development.
Impaired regulation of phospholipid metabolism, particularly lysophosphatidylcholine and phosphatidylcholine, is a central concern in many cancers, including OC.
Conclusion
The analysis of the molecular profile of blood by mass spectrometry is therefore a minimally invasive, potentially effective diagnostic method. The introduction of post-genomic research has the potential to complete the existing data on the processes of carcinogenesis, as well as to improve the diagnostic value of methods for timely diagnosis of high grade OC at an earlier and potentially curable stage of the disease in order to achieve more prognostically favorable outcomes.
References
- Каприн А.Д., Старинский В.В., Шахзадова А.О., ред. Состояние онкологической помощи населению России в 2023 году. М.: МНИОИ им. П.А. Герцена – филиал ФГБУ «НМИЦ радиологии» Минздрава России; 2024. 262 с. [Kaprin A.D., Starinsky V.V., Shakhzadova A.O., eds. State of oncological care for the population of Russia in 2023. Moscow: P.A. Herzen MSROI - branch of the FSBI "National Medical Research Center of Radiology" of the Ministry of Health of Russia; 2024. 262 p. (in Russian)].
- Brenner D.R., Weir H.K., Demers A.A., Ellison L.F., Louzado C., Shaw A. et al.; Canadian Cancer Statistics Advisory Committee. Projected estimates of cancer in Canada in 2020. CMAJ. 2020; 192(9): E199-E205. https://dx.doi.org/10.1503/cmaj.191292.
- Юрова М.В., Хабас Г.Н., Павлович С.В. Оценка результатов комбинированного лечения пациентов с диссеминированным раком яичников. Гинекология. 2022; 24(2): 132-9. [Iurova M.V., Khabas G.N., Pavlovich S.V. Evaluation of results of combined treatment in patients with disseminated ovarian cancer. Gynecology. 2022; 24(2): 132-9. (in Russian)]. https://dx.doi.org/10.26442/20795696.2022.2.201438.
- Voelker R. Ovarian cancer screening tests don’t pass muster. JAMA. 2016; 316(15): 1538. https://dx.doi.org/10.1001/jama.2016.14614.
- Павлович С.В., Юрова М.В., Мелкумян А.Г., Франкевич В.Е., Хабас Г.Н., Чаговец В.В. Биомаркеры при новообразованиях яичников: возможности, ограничения и перспективы применения у женщин репродуктивного возраста. Акушерство и гинекология. 2019; 11: 65-73. [Pavlovich S.V., Yurova M.V., Melkumyan A.G., Frankevich V.E., Chagovets V.V., Khabas G.N. Biomarkers in ovarian neoplasms: opportunities, limitations, and prospects for using in reproductive-aged women. Obstetrics and Gynecology. 2019; (11): 65-73. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.11.65-73.
- Сенча А.Н, Поморцев А.В., Костюков К.В., Федоткина Е.П. Ультразвуковая диагностика заболеваний органов малого таза. Избранные вопросы. М.: МЕДпресс-информ; 2023. 260 с. [Sencha A.N., Pomortsev A.V., Kostyukov K.V., Fedotkina E.P. Ultrasound diagnosis of pelvic diseases. Selected questions. Moscow: MEDpress-inform; 2023. 260 p. (in Russian)].
- Тюляндина А.С., Коломиец Л.А., Морхов К.Ю., Нечушкина В.М., Покатаев И.А., Румянцев А.А., Тюляндин С.А., Урманчеева А.Ф., Хохлова С.В. Практические рекомендации по лекарственному лечению рака яичников, первичного рака брюшины и рака маточных труб. Практические рекомендации RUSSO, часть 1. Злокачественные опухоли. 2023; 13(3s2-1): 201-15. [Tyulyandina A.S., Kolomiets L.A.,Morkhov K.Yu., Nechushkina V.M., Pokataev I.A., Rumyantsev A.A., Tyulyandin S.A., Urmancheeva A.F., Khokhlova S.V. Practical guidelines for the drug treatment of ovarian cancer, primary peritoneal cancer and fallopian tube cancer. RUSSO Practical guidelines, part 1. Malignant Tumours. 2023; 13(3s2-1): 201-15. (in Russian)]. https://dx.doi.org/10.18027/2224-5057-2023-13-3s2-1-201-215.
- Назаренко Т.А., Ашрафян Л.А., Джанашвили Л.Г., Мартиросян Я.О. Сохранение репродуктивного материала у онкологических больных как медико-социальная и организационная проблема. Онкология. Журнал им. П.А. Герцена. 2020; 9(1): 60-5. [Nazarenko T.A., Ashrafian L.A., Dzhanashvili L.G., Martirosyan Y.O. Retention of reproductive material in cancer patients as a sociomedical and organizational problem. P.A. Herzen Journal of Oncology. 2020; 9(1): 60-5. (in Russian)]. https://dx.doi.org/10.17116/onkolog2020901160.
- Ashraf M.A., Dasari P. Outcome of fertility-preserving surgery for ovarian malignancy in young women. Case Rep. 2018; 1(1): 51-4.
- Чаговец В.В., Васильев В.Г., Юрова М.В., Хабас Г.Н., Павлович С.В., Стародубцева Н.Л., Майборода О.А. Метаболомная подпись свободных муцинов при онкологических заболеваниях: CA125 и рак яичников высокой степени злокачественности. Вестник РГМУ. 2021; 6: 10-5. [Chagovets V.V., Vasil’iev V.G., Iurova M.V., Khabas G.N., Pavlovich S.V., Starodubtseva N.L., Mayboroda O.A. Metabolic "footprints" of the circulating cancer mucins: CA125 in the high-grade ovarian cancer. Bulletin of RSMU. 2021; (6): 10-5. (in Russian)]. https://dx.doi.org/10.24075/vrgmu.2021.065.
- Ke C., Hou Y., Zhang H., Fan L., Ge T., Guo B. et al. Large-scale profiling of metabolic dysregulation in ovarian cancer. Int. J. Cancer. 2015; 136(3): 516-26. https://dx.doi.org/10.1002/ijc.29010.
Received 06.11.2024
Accepted 10.12.2024
About the Authors
Maria V. Iurova, PhD, obstetrician-gynecologist, oncologist, Researcher at the Scientific Polyclinic Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, m_yurova@oparina4.ru, https://orcid.org/0000-0002-0179-7635Alisa O. Tokareva, PhD (Physico-Mathematical Sciences), Specialist at the Laboratory of Clinical Proteomics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)531-44-44 (ext. 3113), alisa.tokareva@phystech.edu, https://orcid.org/0000-0001-5918-9045
Vitaliy V. Chagovets, PhD (Physico-Mathematical Sciences), Head of the Laboratory of Metabolomics and Bioinformatics, V.I. Kulakov NMRC for OG&P, Ministry of Health
of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)438-21-98, vvchagovets@gmail.com
Natalia L. Starodubtseva, PhD (Bio), Head of the Laboratory of Clinical Proteomics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0000-0001-6650-5915
Vladimir E. Frankevich, Dr. Sci. (Physico-Mathematical Sciences), Deputy Director of the Institute of Translational Medicine, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, v_frankevich@oparina4.ru
Corresponding author: Maria V. Iurova, m_yurova@oparina4.ru



