Comparative study of the equivalence of the biosimilar follitropin alpha preparation (solution for subcutaneous injection) and the original follitropin alpha preparation (lyophilisate for preparation of solution for subcutaneous administration) in women with different responses to ovarian stimulation in an in vitro fertilization program: results of a phase IV clinical trial
The efficacy and safety of any medication should be proven in randomized prospective clinical trials. However, the data obtained in real clinical practice are important for the formation of the evidence base and make the results of the study on the effectiveness and safety of the medications available to a wide population of patients.Perminova S.G., Nazarenko Т.А., Korneeva I.Е., Bashmakova N.V., Mityurina Е.V., Alimova О.А., Belova I.S., Ershov А.V., Khramtsova А.Yu., Dzhalilova E.R.
Objective: To determine the equivalence of the biosimilar follitropin alpha preparation (Primapur, solution for subcutaneous administration) and the original follitropin alpha preparation (Gonal-F, lyophilisate for preparation of solution for subcutaneous administration) in women with different responses to ovarian stimulation in an in vitro fertilization program.
Materials and methods: This is a retrospective, non-interventional (observational), cohort, comparative, multicenter study of the efficacy and safety of the biosimilar follitropin alpha preparation (Primapur, solution for subcutaneous administration, IVFarma, Russia), and the original follitropin alpha preparation (Gonal-F, lyophilisate for preparation of solution for subcutaneous administration, Merck-Serono, Italy). A total of 240 patients were included in the study: 120 patients received Primapur, a solution for subcutaneous administration, and 120 patients used Gonal-F, lyophilisate for preparation of solution for subcutaneous administration. The patients were stratified depending on the parameters of the ovarian reserve and the expected ovarian response.
Results: The results of the study confirmed the therapeutic equivalence of the biosimilar follitropin alpha preparation (Primapur, solution for subcutaneous administration) and the original follitropin alpha preparation (Gonal-F, lyophilisate for preparation of solution for subcutaneous administration) in women with different responses to ovarian stimulation in an in vitro fertilization program. There were no statistical differences in the parameters of the stimulated cycle: in the initial, daily and total dose of drugs, in the number of aspirated and mature oocytes, zygotes, blastocysts, blastocysts of good quality and cryopreserved embryos in the compared groups. The outcomes of in vitro fertilization programs (the rate of biochemical pregnancy, clinical pregnancy, early reproductive losses and ongoing pregnancy) were also comparable in the subgroups with hypo-response, normal response and hyper-response and did not differ statistically.
Conclusion: The biosimilar follitropin alfa preparation (Primapur, solution for subcutaneous injection) can be recommended for a wide use in clinical practice in various categories of patients in the in vitro fertilization programs, regardless of the parameters of the ovarian reserve and the type of ovarian response.
Keywords
Ovarian stimulation is the most important stage of the in vitro fertilization (IVF) program, which largely determines its effectiveness. In order to obtain a sufficient number of oocytes, and, consequently, to increase the chances of successful treatment results, it is necessary to choose the right preparations for ovarian stimulation. Nowadays, clinicians carry out ovarian stimulation for multifollicular growth by administering follicle-stimulating hormone (FSH) preparations and/or human menopausal gonadotropins [1].
The first recombinant FSH follitropin alpha was developed in Europe in 1995 (GONAL-f, Merck KGaA, Darmstadt, Germany) [2] and in the USA in 1997 (GONAL-f RFF; EMD Serono, Inc., Rockland, MA) [3]. Follitropin beta (Puregon; NV Organon, Oss, Netherlands) received marketing authorization in Europe in 1996 and in the USA (Follistim AQ) in 2004 [4]. Recombinant FSH (rFSH) preparations are produced using genetically modified Chinese hamster ovary cells cultivated in bioreactors; immunochromatographic purification which is performed using affine antibodies that specifically bind to FSH results in a highly purified preparation with a constant profile of FSH isoforms and high specific activity [5]. These drugs were comparable in number of obtained oocytes and frequency of live birth when they were used in assisted reproductive technology (ART) programs. However, rFSH follitropin alpha (GONAL-f, Merck KGaA, Darmstadt, Germany) is recognized as the reference drug [6]; it has been used in 19245492 cycles to date [7] and more than 4 million children have been born after its use [8, 9].
Due to the end of patent protection for follitropin alpha and the need to expand the market of alternative preparations for ovarian stimulation, a number of European countries have started clinical development of biosimilar follitropin alpha preparations in recent decades. A biosimilar preparation is a biologic medical product that contains a version of the active substance of a registered biological original (reference) preparation and that is similar in quality, biological activity, efficacy and safety in comparison with a reference preparation on the basis of comparative studies [10].
According to the recommendations of the European Medicines Agency (EMA), the similarity of a biosimilar preparation with an original medical preparation must be established in physical and chemical properties, qualitative characteristics, biological activity, safety and efficacy on the basis of comprehensive studies which should be carried out before a biosimilar preparation can be approved for medical use in clinical practice [6, 10–13]. Biosimilar preparations containing rFSH meet similar requirements [14, 15].
To date, the results of three clinical studies of biosimilar follitropin alpha preparations have been published: Bemfola (Gedeon Richter, Hungary), Ovaleap (Teramex, Ireland) and Primapur (IVFarma, Russia) [10]. The studies were conducted in accordance with the EMA requirements for rFSH [15].
The first Russian biosimilar follitropin alpha preparation (Primapur) was registered in the Russian Federation in 2019. All stages of its production were observed starting from the active pharmaceutical substance to the complete dosage form as a solution for subcutaneous injection in a pre-filled pen [15-17]; all necessary studies have been carried out in accordance with the Russian recommendations [16] and the EMA recommendations for rFSH [15], including comparative physicochemical and preclinical studies [18, 19], as well as a study of pharmacokinetics with the participation of healthy volunteers (the number of the protocol and the results of the study are in the international database of clinical trials ClinicalTrials.gov: NCT03857230) [20].
The results of two clinical studies on the evaluation of the therapeutic efficacy of the biosimilar follitropin alpha preparation (Primapur) have been published in the Russian literature. Barakhoeva Z.B. et al. (2018) conducted a multicenter, randomized, comparative phase III study in parallel groups, blind at the embryological stage, which was devoted to the confirmation of the therapeutic equivalence of the biosimilar follitropin alpha preparation (Primapur) to the original preparation (GONAL-f) during the ovarian stimulation in the IVF program (2018). Their study showed that the biosimilar follitropin alpha preparation is equivalent to the original one in terms of the number of aspirated and mature oocytes, zygotes; the number of days of stimulation; the amount of the preparation administered for the course of treatment; the rate of biochemical and clinical pregnancy (ClinicalTrials.gov: NCT03088137) [21]. However, only women with normal parameters of ovarian reserve (anti-Muller hormone (AMH) ≥ 1 ng/ml) were included in this clinical study.
The results of a large-scale multicenter clinical trial in real clinical practice conducted in 35 ART clinics in Russia and evaluating the results of 5484 ovarian stimulation cycles with the use of Primapur preparation were published in 2021. These results confirmed its efficacy and safety in a non-selective population of patients who underwent various ovarian stimulation protocols (ClinicalTrials.gov: NCT04854707) [22]. However, there was no comparative assessment of the equivalence of the forms of follitropin alpha preparation as a solution for subcutaneous administration that was produced in Russia and the original follitropin alpha in the form of lyophilisate for the solution for subcutaneous administration; there was no analysis of the protocols of ovarian stimulation which was performed depending on the parameters of the ovarian reserve and the expected ovarian response in the patients included in the study.
The group of patients undergoing ART treatment is known to be extremely heterogeneous and includes women with diminished, normal and high ovarian reserve. In this regard, it is interesting to compare the equivalence of the biosimilar follitropin alpha preparation (Primapur, solution for subcutaneous administration, IVFarma, Russia) and the original follitropin alpha (GONAL-f, lyophilisate for the solution for subcutaneous administration, Merck-Serono, Italy) in women with different responses to ovarian stimulation in the IVF program.
The aim of the study is to determine the equivalence of the biosimilar follitropin alpha preparation (Primapur, solution for subcutaneous administration) and the original follitropin alpha preparation (GONAL-f, lyophilisate for the solution for subcutaneous administration) in women with different responses to ovarian stimulation in IVF program.
Materials and methods
Study design. This was a retrospective, non-interventional (observational), cohort, comparative, multicenter study of the efficacy and safety of the biosimilar follitropin alpha preparation (Primapur, solution for subcutaneous administration, IVFarma, Russia), and the original follitropin alpha preparation (GONAL-f, lyophilisate for the solution for subcutaneous administration, Merck-Serono, Italy) which were used according to the indications stated in the approved instructions for medical use.
Investigational Medicinal Products. Primapur (INN: follitropin alpha, marketing authorization No. LP-005826), solution for subcutaneous injection, disposable pre-filled pens 900 IU (66 mcg); GONAL-f (INN: follitropin alpha, marketing authorization No. LS-000200, lyophilisate for the solution for subcutaneous administration, 75 IU (5.5 mcg).
Clinical Centers. The study was conducted at Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, in Moscow (Clinical Centre No.1) and Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, in Yekaterinburg (Clinical Centre No.2)
Patients’ population. A total of 240 patients were included in the study: 120 patients received Primapur, a solution for subcutaneous administration, and 120 patients used GONAL-f, lyophilisate for the solution for subcutaneous administration.
The patients were stratified depending on the parameters of the ovarian reserve and the expected ovarian response.
The main group (Primapur, solution for subcutaneous injection) consisted of 60 patients with suspected hyper–response (polycystic ovary syndrome (PCOS), multifollicular ovaries) (AMH more than 3 ng/ml) with more than 15 oocytes, 40 patients with normal response (AMH 2-3 ng/ml) with 9-14 oocytes, and 20 patients with hypo–response (AMH less than 1 ng/ml) with less than 8 oocytes.
The women of the comparison group (GONAL-f, lyophilisate for the solution for subcutaneous administration) were stratified according to a similar principle; it included 60 women with hyper–response, 40 women with normal response, and 20 women with hypo–response.
Inclusion criteria:
- Women with identified causes of infertility and indications for the ART treatment, according to the Order of the Ministry of Health of the Russian Federation dated July 31, 2020 No. 803n “On the procedure for the use of assisted reproductive technologies, contraindications and restrictions to their use”.
- Age 20-38 years.
- No more than two attempts of IVF / ICSI in the previous history.
- 18 ≤ body mass index (BMI) ≤30 kg/m2.
- Basal FSH level 12 IU/L.
- AMH level ≥ 0.5 ng/ml.
Exclusion criteria:
- Women with identified contraindications to the use of the ART treatment, according to the Order of the Ministry of Health of the Russian Federation dated July 31, 2020 No. 803n “On the procedure for the use of assisted reproductive technologies, contraindications and restrictions to their use”.
- stage III/IV endometriosis; hydrosalpinx.
- Submucous uterine fibroids, regardless of size; subserous and interstitial myomatous nodes exceeding 4.5 cm in size.
- AMH level <0.5 ng/ml.
- Less than 3 oocytes obtained in the previous IVF/ICSI program.
- Severe forms of oligoastenoteratozoospermia, azoospermia: indications for TESE/TESA/MESA, donor sperm.
Analysis of the outcomes of ovarian stimulation protocols. There was an assessment of anthropometric, demographic data, duration and causes of infertility, the number of IVF/ICSI attempts, the total number of aspirated oocytes, mature oocytes (stage MII), zygotes (2PN), the total dose of the administered drug, the number of transferred embryos, the rate of biochemical pregnancies after embryo transfer (ET) (hCG level>20 IU/L on the 12th-14th day after ET), the rate of clinical pregnancies after ET (the presence of a gestational sac and heartbeat on the 5th-6th week after ET), the rate of ongoing pregnancy (more than 12 weeks), the rate of early reproductive loss (miscarriage before 12 weeks).
Stimulation protocol. The patients of both groups underwent ovarian stimulation with a gonadotropin-releasing hormone (GnRH) antagonist. The initial dose of follitropin alpha preparations (Primapur or GONAL-f) was selected depending on the parameters of the ovarian reserve: it was 300 IU per day in patients with hypo–response, 225 IU per day in normal response, and 150 IU per day in hyper–response. Then the ovarian response was evaluated on the 5th-6th day of ovarian stimulation and the dose was changed if necessary. GnRH antagonist was administered when the diameter of the dominant follicle was 14-15 mm. The ovulation trigger (human chorionic gonadotropin (hCG)) was prescribed when the diameter of the dominant follicles was 17-18 mm in case of normal and hypo-response. In order to reduce the risk of ovarian hyperstimulation syndrome (OHSS) in women with hyper-response, hCG trigger was replaced with GnRH agonist at a dose of 0.2 mg subcutaneously. Luteal phase was supported using micronized progesterone at a dose of 600 mg per day intravaginally starting from the day after transvaginal puncture (TVP) of the ovarian follicles. Depending on the parameters of the sperm, fertilization was attempted using IVF/ICSI methods. ET was performed on the 3rd or 5th days of cultivation, no more than two embryos were transferred.
Biochemical pregnancy was diagnosed at hCG level >20 IU/L on the 12th-17th day after ET; clinical pregnancy was confirmed on the basis of the presence of the gestational sac, embryo, and heartbeat in six weeks after ET.
Statistical analysis
Statistical data analysis and plotting were performed using the Microsoft Excel Spreadsheet Software (Microsoft, USA) and the GraphPad Prism statistical software package (GraphPad Software, USA). The normality of the distribution was determined using the D’Agostino-Pearson test. The data with a normal distribution are presented as mean value (standard deviation), t-test was used for their comparison. Qualitative data are presented as an absolute value (n) and as a percentage, Fisher’s exact test was used to compare them. The differences were considered statistically significant at the level of p<0.05.
Results and discussion
Efficacy and safety of biosimilar follitropin alpha preparations have been actively discussed in the literature since their development and introduction into clinical practice in different countries [23]. This is due to the fact that it is necessary to prove the equivalence of the biosimilar and original preparations in efficacy and safety not only during randomized controlled registration clinical trials but also in various groups of patients undergoing IVF treatment with the use of follitropin alpha preparations in all dosage forms available on the market.
The present study was aimed at the comparative assessment of the clinical (therapeutic) equivalence of the biosimilar follitropin alpha preparation (solution for subcutaneous administration) and the original follitropin alpha preparation (lyophilisate for the solution for subcutaneous administration) depending on the ovarian response to stimulation in 240 patients undergoing IVF (Table 1).
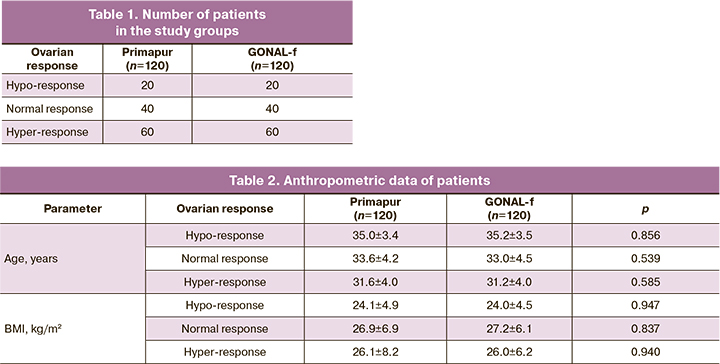
The main group (Primapur, solution for subcutaneous injection) consisted of 60 patients with suspected hyper–response (polycystic ovary syndrome (PCOS), multifollicular ovaries) (AMH more than 3 ng/ml) with more than 15 oocytes, 40 patients with normal response (AMH 2-3 ng/ml) with 9-14 oocytes, and 20 patients with hypo–response (AMH less than 1 ng/ml) with less than 8 oocytes.
The women of the comparison group (GONAL-f, lyophilisate for the solution for subcutaneous administration) were stratified according to a similar principle; it included 60 women with hyper–response, 40 women with normal response, and 20 women with hypo–response.
GnRH antagonist was used for ovarian stimulation.
The patients of the main group (Primapur, solution for subcutaneous administration) and comparison group (GONAL-f, lyophilisate for the solution for subcutaneous administration) were compared in anthropometric data (age, BMI), clinical and anamnestic data and infertility factors within a subgroup (hypo-response, normal response, hyper-response) (Table 2).
As one can see from the presented data, there were no statistically significant differences in age and BMI in patients with different ovarian response to gonadotropic stimulation between the groups of Primapur and GONAL-f preparations (p>0.05).
The analysis of clinical and anamnestic data of patients who participated in the study showed that the incidence of primary infertility in the groups of Primapur and GONAL-f preparations was comparable in women with hypo-response, 8 (40.0%) and 10 (50.0%), (p=0.376), normal response, 15 (37.5%) and 16 (40.0%), (p=0.500) and hyper-response, 18 (30.0%) and 22 (36.7%), (p=0.281), respectively (Table 3). Similarly, the incidence of secondary infertility was not statistically significantly different in both groups: 6.1±2.1 and 5.8±2.5 (p=0.683); 4.9±2.0 and 4.8±1.9, (p=0.819); 2.5±1.1 and 2.7±0.8, (p=0.257).
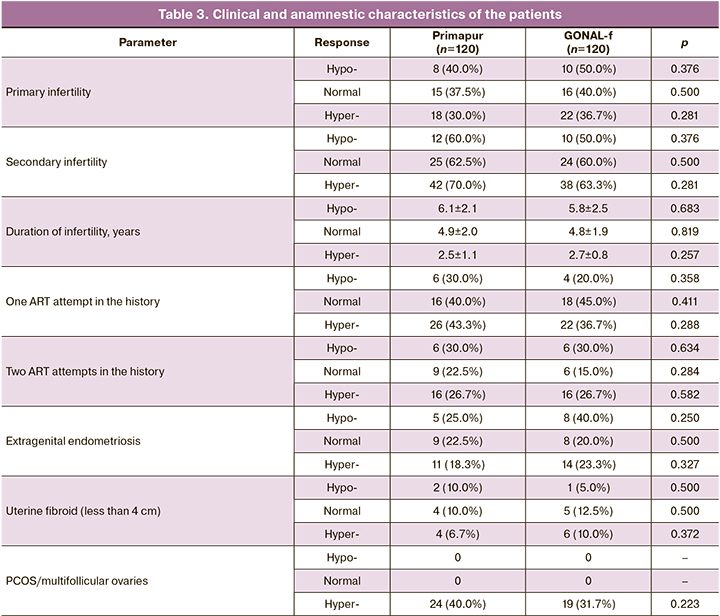
Women with hypo-response had the longest duration of infertility compared to women with normal and hyper-response, however, the differences were not statistically significant depending on the preparation used for ovarian stimulation.
The analysis of the results of previous infertility treatment showed that all patients had a history of one or two ART programs with different outcomes; their number was comparable in the groups of Primapur and GONAL-f preparations.
As for gynecological pathologies, extragenital endometriosis prevailed in women with hypo-response; there were ovarian surgeries for endometrioid cysts in the history. However, the incidence of extragenital endometriosis did not differ depending on the preparation used for the ovarian stimulation, Primapur and GONAL–f, (5 (25.0%) and 8 (40.0%), respectively, (p=0.250)). The incidence of uterine fibroids was comparable in groups of patients with different ovarian response and did not depend on the ovulation inducer. Hyper-response was due to the presence of PCOS or multifollicular ovaries; their incidence in both groups was comparable (24 (40.0%) and 19 (31.7%), (p=0.223)).
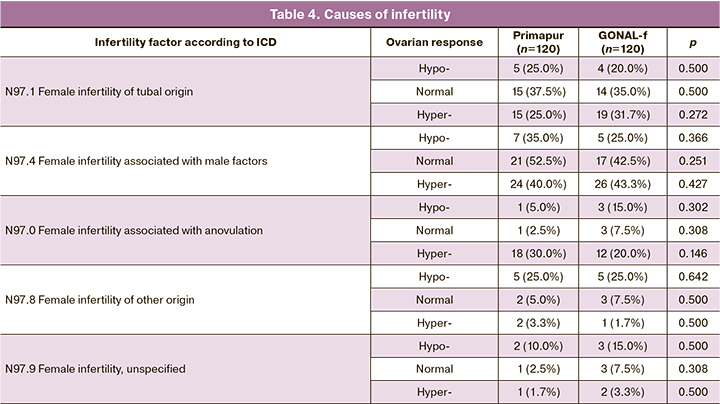
The analysis of the causes of infertility also failed to reveal significant differences within each subgroup (hypo-, normal, and hyper-response) depending on the ovulation inducer (Table 4).
Thus, there were no statistically significant differences in clinical and anamnestic characteristics between the study groups of Primapur and GONAL-f preparations.
The parameters of ovarian stimulation and the embryological stage are presented in Table 5.
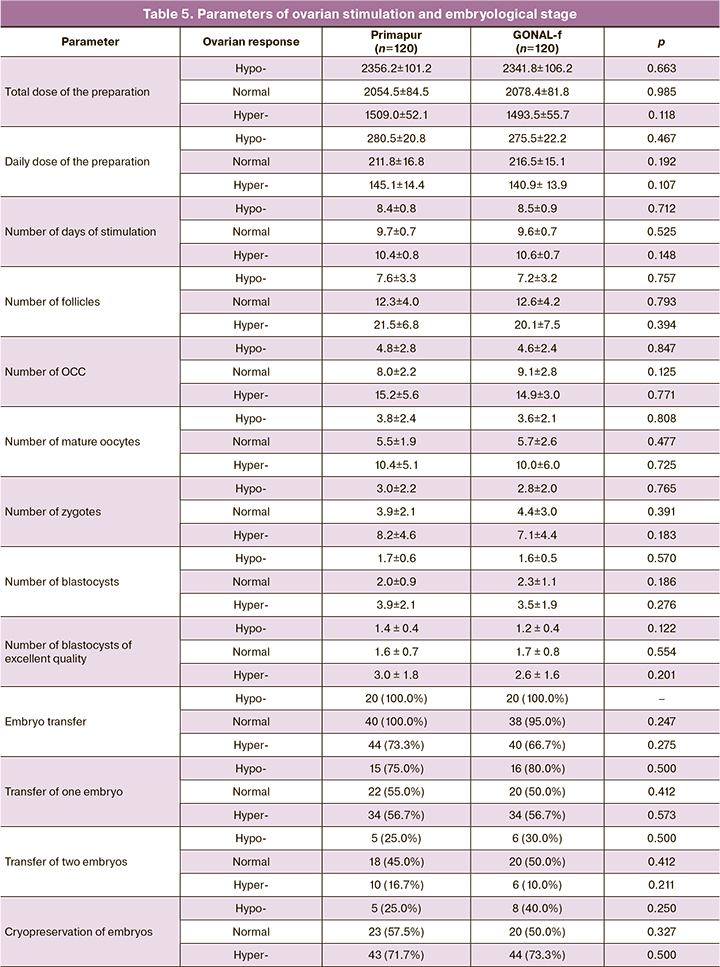
As one can see from the presented data, there were no significant differences in the daily and total doses of preparations and the duration of stimulation within each subgroup with a different ovarian response, depending on the preparation used for ovarian stimulation.
However, women with hypo-response received higher daily doses of the ovarian inducer compared to the women with normal response, both in the Primapur group and in the GONAL-f group; the number of follicles increased to 7.6±3.3 and 7.2±3.2, respectively (p=0.757).
The primary endpoint of the registration study in assessing the efficacy of biosimilar follitropin alpha preparations established by European and Russian recommendations [15, 16] was the assessment of the number of aspirated oocytes. The results of a comparative assessment of the obtained oocyte-cumulus complexes (OCC) in this study showed (Fig. 1) that a comparable number of OCC was obtained in the group of women with normal response when they used Primapur and GONAL-f preparations (8.0±2.2 and 9.1±2.8, respectively, p=0.125). Similarly, the number of OCC in women with hyper-response was comparable (15.2±5.6 and 14.9±3.0, p=0.771).
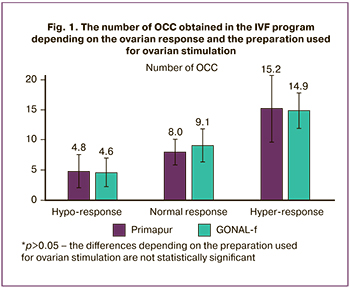
Therefore, the equivalence of Primapur and GONAL-f preparations was confirmed for women with normal and elevated parameters of the ovarian reserve (AMH 2-3 ng/ml in patients with normal response and AMH > 3 ng/ml in patients with hyper-response); these findings are consistent with the results of other studies [10, 21, 24].
Women with diminished ovarian reserve are known to be a particularly difficult category of ART patients; doctors often choose unreasonably high initial doses of gonadotropins at the start of ovarian stimulation due to fear of the lack of an adequate ovarian response. Therefore, it is important to note that in the group with hypo-response (AMH less than 1 ng/ml, less than 8 oocytes), the number of OCC obtained with the use of Primapur did not significantly differ from the same indicator with the use of GONAL-f (4.8±2.8 and 4.6±2.4, p=0.847) with comparable initial doses (300 IU per day), daily and total doses of the ovulation inducer. This fact confirms the equivalence of the Russian biosimilar follitropin alpha preparation to the original follitropin alpha preparation in women with suboptimal ovarian response, which is extremely important for clinical practice.
Another important efficiency parameter is the number of mature oocytes (MII) capable of fertilization.
The evaluation of the number of mature oocytes (MII) depending on the preparation used for ovarian stimulation (Primapur and GONAL-f) did not reveal any statistically significant differences in patients with hypo-response (3.8±2.4 and 3.6±2.1, p=0.808), normal response (5.5±1.9 and 5.7±2.6, p=0.477), and hyper-response (10.4±5.1 and 10.0±6.0, p=0.725). Similar tendencies were revealed after evaluating the number of zygotes with two pronuclei (3.0±2.2 and 2.8±2.0, p=0.765; 3.9±2.1 and 4.4±3.0, p=0.391; 8.2±4.6 and 7.1±4.4, p=0.183); the numbers of blastocysts (1.7±0.6 and 1.6±0.5, p=0.570; 2.0±0.9 and 2.3±1.1, p=0.186; 3.9±2.1 and 3.5±1.9, p=0.276) and the numbers of blastocysts of excellent quality (1.4±0.4 and 1.2±0.4, p=0.122; 1.6±0.7 and 1.7±0.8, p=0.554; 3.0±1.8 and 2.6±1.6, p=0.201).
Thus, there were no statistically significant differences in the parameters of ovarian stimulation and the embryological stage between the groups using Primapur and GONAL-f preparations.
In the Primapur and GONAL-f groups, ET was performed in all patients with hypo-response, in 40 (100%) and 38 (95%) women with normal response, (p=0.247), and in 44 (73.3%) and 40 (66.7%) women with hyper-response, (р=0.275). The reasons for the cancellation of ET were mainly the risk of OHSS, as well as the detection of hydrosalpinx or endometrial polyp during stimulation (despite the absence of pathology according to the examination data before starting the IVF program). The number of women with the transfer of one and two embryos was comparable in all groups. The frequency of embryo cryopreservation in the Primapur and GONAL-f groups was comparable and did not differ significantly in women with hypo-response (5 (25.0%) and 8 (40.0%), p=0.250); normal response (23 (57.5%) and 20 (50.0%), p=0.327) and hyper-response (43 (71.7%) and 44 (73.3%), p=0.500).
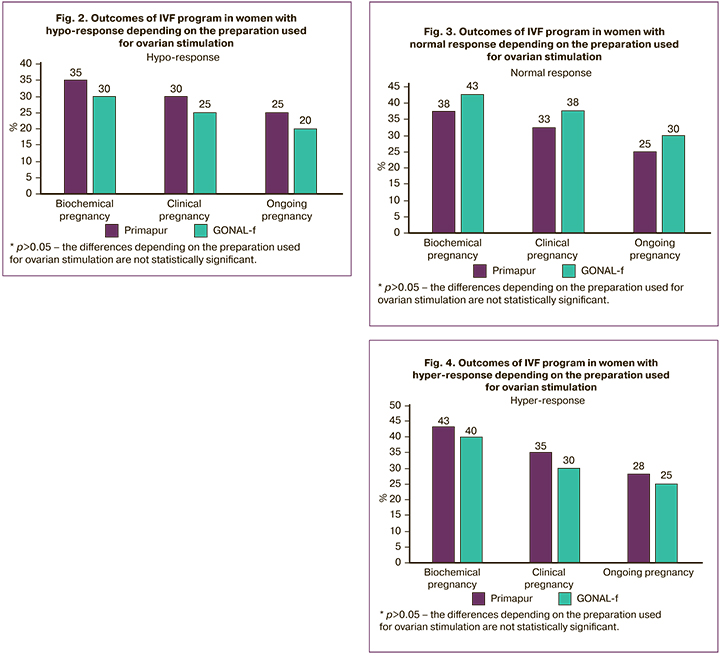
The results of the comparative evaluation of the effectiveness of IVF programs depending on the preparation used for ovarian stimulation and the character of the ovarian response are presented in Table 6 and Figures 2-4.
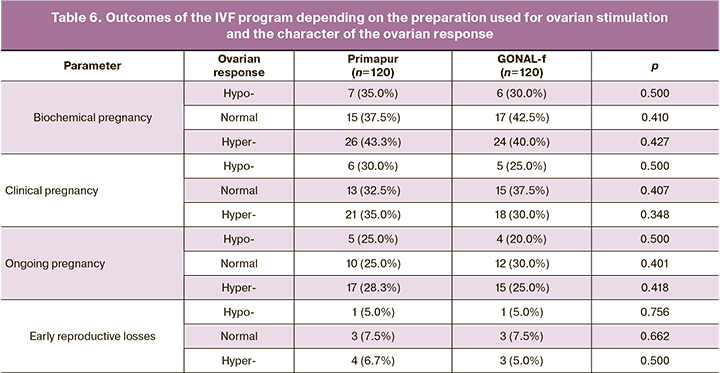
Thus, women with hypo-response in the Primapur and GONAL-f groups did not show any significant differences in the rate of biochemical pregnancy (7 (35.0%) and 6 (30.0%), p=0.500), clinical pregnancy (6 (30.0%) and 5 (25.0%), p=0.500), ongoing pregnancy (5 (25.0%) and 4 (20.0%), p=0.500) and the rate of early reproductive losses (1 (5.0%) and 1 (5.0%), p=0.756). A similar tendency was detected in groups with normal response (15 (37.5%) and 17 (42.5%), p=0.410) and hyper-response (26 (43.3%) and 24 (40.0%), p=0.427), 21 (35.0%) and 18 (30.0%, p=0.348), 17 (28.3%) and 15 (25.0%, р=0.418).
The results obtained in this study are not consistent with the meta-analysis data of Longobardi et al. (2019) [25], who analyzed the results of three randomized controlled clinical trials [24, 26, 27], where the rate of clinical pregnancy (RR 0.83, 95% CI: 0.71–0.96) and the rate of live birth (RR 0.82, 95% CI: 0.70–0.97) were significantly lower after the use of the biosimilar rFSH preparation compared to the original rFSH preparation.
On the contrary, a later meta-analysis conducted by Budani et al. (2021) revealed no differences in the rate of biochemical pregnancy and rate of live births in the group which used the original rFSH preparation compared to the group which used the biosimilar preparation [10].
Thus, the results of this clinical study showed that the outcomes of IVF programs in patients receiving the biosimilar follitropin alpha preparation (Primapur) and the original (reference) follitropin alpha preparation (GONAL-f) were comparable within the subgroups with hypo-response, normal response and hyper-response.
The administration of these two follitropin alpha preparations did not cause any adverse reactions.
Conclusion
The results of the study confirmed the therapeutic equivalence of the biosimilar follitropin alpha preparation (Primapur, solution for subcutaneous administration) and the original follitropin alpha preparation (GONAL-f, lyophilisate for the solution for subcutaneous administration) in women with different responses to ovarian stimulation in an in vitro fertilization program. There were no statistical differences in the parameters of the stimulated cycle (initial, daily and total dose of drugs), in the number of aspirated and mature oocytes, zygotes, blastocysts, blastocysts of good quality and cryopreserved embryos in the compared groups. The outcomes of in vitro fertilization programs (the rate of biochemical pregnancy, clinical pregnancy, early reproductive losses and ongoing pregnancy) were also comparable in the subgroups with hypo-response, normal response and hyper-response and did not differ statistically.
The biosimilar follitropin alfa preparation (Primapur, solution for subcutaneous injection) can be recommended for a wide use in clinical practice in various categories of patients in the in vitro fertilization programs, regardless of the parameters of the ovarian reserve and the character of ovarian response.
References
- Lunenfeld B., Bilger W., Longobardi S., Alam V., D’Hooghe T., Sunkara S.K. The development of gonadotropins for clinical use in the treatment of infertility. Front. Endocrinol. (Lausanne). 2019; 10: 429. https://dx.doi.org/10.3389/fendo.2019.00429.
- European Medicines Agency. GONAL-f (follitropin alfa): summary of product characteristics. 2010. Available at: https://www.ema.europa.eu/en/documents/product-information/gonal-f-epar-product-information_en.pdf Accessed October 2020.
- Food and Drug Administration: GONAL-F® RFF* REDI-JECT™ (follitropin alfa): prescribing information. 2013. Available https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021684s036lbl.pdf Accessed October 2020.
- Drugs.com. Follistim AQ Approval History. 2018. Available at:https://www.drugs.com/history/follistim-aq.html Accessed March 26, 2019.
- Howles C.M. Genetic engineering of human FSH (Gonal-F). Hum. Reprod. Update.1996; 2(2): 172-91. https://dx.doi.org/10.1093/humupd/2.2.172.
- European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance:non-clinical and clinical issues. 2014. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinalproducts-containing-biotechnology-derived-proteins-active_en-2.pdf Accessed October 2020.
- Velthuis E., Hubbard J., Longobardi S., D’Hooghe T. The frequency of ovarian Hyperstimulation syndrome and thromboembolism with originatorrecombinant human Follitropin Alfa (GONAL-f) for medically assisted reproduction: a systematic review. Adv. Ther. 2020; 37(12): 4831-47. https://dx.doi.org/10.1007/s12325-020-01512-w.
- Al-Inany H.G., Abou-Setta A.M., Aboulghar M.A., Mansour R.T., Serour G.I. Efficacy and safety of human menopausal gonadotrophins versus recombinant FSH:a meta-analysis. Reprod. Biomed. Online. 2008;16(1): 81-8.https://dx.doi.org/10.1016/s1472-6483(10)60559-7.
- Coomarasamy A., Afnan M., Cheema D., van der Veen F., Bossuyt P.M., vanWely M. Urinary hMG versus recombinant FSH for controlled ovarian hyperstimulation following an agonist long down-regulation protocol in IVFor ICSI treatment: a systematic review and meta-analysis. Hum. Reprod. 2008; 23(3): 10-5. https://dx.doi.org/10.1093/humrep/dem305.
- Budani M.C., Fensore S., Di Marzio M., Tiboni G.M. Efficacy and safety of follitropin alpha biosimilars compared to their reference product: a meta-analysis. Gynecol. Endocrinol. 2021; 37(5): 406-414. https://dx.doi.org/10.1080/09513590.2020.1792437.
- World Health Organization. Expert Committee on Biological Standardization. WHO Guidelines on evaluation of similar biotherapeutic products (SBPs). Geneva: WHO; 2009. Available at: http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf
- European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) EMA/CHMP/437/04 Guideline on similar biological medicinal products (Rev.1). 2014. Available at: https://www.ema.europa.eu/en/similar-biological-medicinal-products
- European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). EMEA/CHMP/ BMWP/42832/2005 Rev1. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues; 2014b. Available at:https://www.ema.europa.eu/en/documents/scientific- guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived- proteins-active_en-2.pdf
- European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) EMA/CHMP/41467/2013 Assessment report. Ovaleap. International non-proprietary name: follitropin alfa. 2013b. Available at:https://www. ema.europa.eu/en/documents/assessment-report/ovaleap-epar-public-assessment-report_en.pdf
- European Medicines Agency. Committee for Medicinal Products for Human Use. Guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant human follicle stimulating hormone(r-hFSH). EMA/597110/2012., 2013. Available at: https://www.ema.europa.eu/documents/scientific-guideline/guideline-nonclinical-clinical-development-similar-biological-medicinal-productscontaining_en.pdf
- Министерство здравоохранения Российской Федерации. Федеральное государственное бюджетное учреждение «Научный центр экспертизы средств медицинского применения». Руководство по экспертизе лекарственных средств. т.IV. М.; 2014. [Ministry of Health of the Russian Federation, Scientific Center for Expertise of Medicinal Products. Guidelines for the examination of medicines. Vol. IV. Moscow: 2014.(in Russian)].
- Подкорытов А.Б., Жиляев О.В., Ползиков М.А. Шприц-ручка для самостоятельного введения раствора фоллитропина альфа с минимальным шагом устанавливаемой дозы 5 МЕ. Акушерство, гинекология и репродукция. 2017; 11(4): 35-42. https://dx.doi.org/10.17749/2313-7347.2017.11.4.035-042. [Podkorytov A.B., Zhilyaev O.V., Polzikov M.A. A pen injector for self-administration of follitropin alpha solution with a minimal dose increment of 5 IU. Obstetrics, Gynecology and Reproduction. 2017;11(4):35-42. (in Russian)]. https://dx.doi.org/10.17749/2313-7347.2017.11.4.035-042.
- Vorob’ev I.I., Khodak Y.A., Proskurina O.V., Gosudarev A.I., Semikhin A.S., Byrikhina D.V., et al. Physicochemical properties, toxicity, and specific activity of a follitropin alpha biosimilar. Pharm. Chem. J. 2017; 50(11): 753-60.https://dx.doi.org/10.1007/s11094- 017-1525-3.
- Orlova N.A., Kovnir S.V., Khodak Y.A., Polzikov M.A., Nikitina V.A., Skryabin K.G., Vorobiev I.I. High-level expression of biologically active human follicle stimulating hormone in the Chinese hamster ovary cell line by a pair of tricistronic and monocistronic vectors. PLoS One. 2019; 14(7): e0219434. https://dx.doi.org/10.1371/journal.pone.0219434.
- Тюлькина Е.Е., Гордеев И.Г., Гребенкин Д.Ю., Казей В.А., Цикаришвили М.М., Лучинкина Е.Е., Абдулла Б.Х., Самандари С., Воробьев И.И.,Шигабутдинов А.Ф., Ползиков М.А. Сравнительное рандомизированное перекрестное исследование переносимости и фармакокинетики препаратов Примапур® и Гонал-ф® при однократном подкожном введении здоровым добровольцам. Экспериментальная и клиническая фармакология. 2017; 80(4): 13-7. https://dx.doi.org/10.30906/0869-2092-2017-80-4-13-17. [Tulkina E.E., Gordeev I.G., Grebenkin D.Yu. et al. Comparative randomized cross-over study of the tolerability and pharmacokinetics of Primapur® and Gonal-f® with single-single subcutaneous administration in healthy volunteers. Experimental and Clinical Pharmacology. 2017;80(4):13-7. (in Russian)]. https://dx.doi.org/10.30906/0869-2092-2017-80-4-13-17.
- Барахоева З.Б., Вовк Л.А., Зорина И.В., Белоусова Н.Ю., Тетерина Т.А., Яковенко С.А., Апрышко В.П., Фетисова Ю.А., Марилова Н.А., Морозова Е.Г.,Овчинникова М.М., Тищенко М.А., Щербатюк Ю.В., Колотовкина А.В., Мискун А.А., Касьянова Г.В., Сичинава Л.Г., Шалина Р.И., Ползиков М.А. Основные результаты сравнительного многоцентрового исследования III фазы биоаналогового фоллитропина альфа (Примапур®) и оригинального фоллитропина альфа (Гонал-ф®). Акушерство, гинекология и репродукция. 2018; 12(3): 5-16. https://dx.doi.org/10.17749/2313-7347.2018.12.3.005-016. [Barakhoeva Z.B., Vovk L.A., Zorina I.V., Belousova N.Yu., Teterina T.A.et al. Major results of a phase III comparative multicenter study on the follitropin alfa biosimilar (Primapur®) and the original follitropin alfa (Gonal-f®). Obstetrics, Gynecology and Reproduction. 2018;12(3):5-16. (in Russian)]. https://dx.doi.org/10.17749/2313-7347.2018.12.3.005-016.
- Камилова Д.П., Овчинникова М.М., Абляева Э.Ш., Левиашвили М.М.,Стулева Н.С., Бройтман Е.В., Ганихина М.А., Маясина Е.Н., Исхакова Л.Ф.,Боярский К.Ю., Овсянникова Е.Н., Барахоева З.Б., Никитин С.В.,Бендусов И.А., Фетисова Ю.А., Юдина М.А., Тарарашкина Е.С., Хетагурова Д.Т., Блинов Д.В., Ползиков М.А. Эффективность применения биоаналогового фоллитропина альфа в реальной клинической практике: результаты наблюдательного исследования «ФОЛЛИТРОПИН». Акушерство, гинекология и репродукция. 2021; 15(1): 5-21.https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2021.212. [Kamilova D.P., Ovchinnikova M.M., Ablyaeva E.Sh., Leviashvili M.M., Stuleva N.S. et al. An observational study «FOLLITROPIN» comparing the efficacy of follitropin alpha biosimilar: the real-world data. Obstetrics, Gynecology and Reproduction. 2021; 15(1): 5-21. (in Russian)]. https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2021.212.
- de Mora F., Howles C.M. Overlapping biosimilar and originator follitropin alfa preparations: How much closer can they get? Drug Discov. Today. 2022; 27(8): 2071-5. https://dx.doi.org/10.1016/j.drudis.2022.04.022.
- Barakhoeva Z., Vovk L., Fetisova Y., Marilova N., Ovchinnikova M., Tischenko M., Scherbatyuk Y., Kolotovkina A., Miskun A., Kasyanova G., Teterina T., Zorina I., Belousova N., Morozova E., Yakovenko S., Apryshko V., Sichinava L., Shalinа R., Polzikov M. A multicenter, randomized, phase III study comparing the efficacy and safety of follitropin alpha biosimilar and the original follitropin alpha. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 241: 6-12.https://dx.doi.org/10.1016/j.ejogrb.2019.07.032.
- Longobardi S., Mol B., Chua S.J., Wang R., Venetis C., Papsch R. et al. A systematic review and meta-analysis, comparing biosimilar versus originator recombinant follitropin alfa in women undergoing IVF/ICSI’. Hum. Reprod. 2019; 34(Suppl. 1): 106.[ Presented at 35th Annual Meeting of the European-Society-of-Human-Reproduction-and-Embryology (ESHRE). Vienna, Austria 24-26 June 2019.]
- Strowitzki T., Kuczynski W., Mueller A., Bias P. Randomized, active-controlled, comparative phase 3 efficacy and safety equivalence trial of Ovaleap® (recombinant human follicle-stimulating hormone) in infertile women using assisted reproduction technology (ART). Reprod. Biol. Endocrinol. 2016; 14: 1. https://dx.doi.org/10.1186/s12958-015-0135-8.
- Rettenbacher M., Andersen A.N., Garcia-Velasco J.A., Sator M., Barri P., Lindenberg S. et al. A multi-centre phase 3 study comparing efficacy and safety of Bemfola((R)) versus Gonal-f((R)) in women undergoing ovarian stimulation for IVF. Reprod. Biomed. Online. 2015; 30(5): 504-13.https://dx.doi.org/10.1016/j.rbmo.2015.01.005.
Received 19.09.2022
Accepted 17.10.2022
About the Authors
Svetlana G. Perminova, Dr. Med. Sci., Leading Researcher at Reproductology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, perisvet@list.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.Tatiana A. Nazarenko, Dr. Med. Sci., Professor, Director of the Institute of Reproductive Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)531-44-44, t_nazarenko@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Irina E. Korneeva, Dr. Med. Sci., Professor, Senior Researcher at the Scientific and Clinical Department of Assisted Reproductive Technology named
after F. Paulsen, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, irina.korneeva@inbox.ru,
117997, Russia, Moscow, Ac. Oparina str., 4.
Nadezda V. Bashmakova, Dr. Med. Sci., Professor, Honored Doctor of the Russian Federation, Head of the Department of ART, Chief obstetrician-gynecologist of the Ural Federal District, Chief Researcher, Urals Research Institute of Maternity and Child Care, Ministry of Health of Russia, +7(343)371-87-68, BashmakovaNV@niiomm.ru,
620028, Russia, Yekaterinburg, Repin str., 1.
Elena V. Mityurina, PhD, Senior Researcher at Reproductology Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, e_mityurina@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Olga A. Alimova, PhD, Reproductologist at the Department of Assisted Reproductive Technologies, Urals Research Institute of Maternity and Child Care, Ministry of Health
of Russia, +7(343)371-87-68, 620028, Russia, Yekaterinburg, Repina str., 1.
Irina S. Belova, graduate student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,
irina-belova00@mail.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Andrey V. Ershov, Junior Researcher at the Department of Assisted Reproductive Technologies, Urals Research Institute of Maternity and Child Care, Ministry of Health
of Russia, +7(343)232-55-12, omm.vrt@ya.ru, 620109, Yekaterinburg, Russia, Repin str., 17.
Alexandra Yu. Khramtsova, obstetrician-gynecologist, researcher, Department of ART, Urals Research Institute of Maternity and Child Care, Ministry of Health of Russia, +7(343)232-55-12, aleksaxr@mail.ru, 620028, Russia, Yekaterinburg, Repin str., 1.
El’mira R. Dzhalilova, obstetrician-gynecologist at the Department of Assisted Reproductive Technologies, Urals Research Institute of Maternity and Child Care,
Ministry of Health of Russia, +7(343)232-55-12, vip.dzhalilova2017@mail.ru, 620109, Russia, Yekaterinburg, Repin str., 17.
Authors’ contributions: Perminova S.G., Nazarenko T.A., Bashmakova N.V. – conception and design of the study; Alimova O.A., Khramtsova A.Yu., Dzhalilova E.R., Ershov A.V., Korneeva I.E., Belova I.S., Mityurina E.V. – collecting and processing the material; Perminova S.G., Nazarenko T.A., Bashmakova N.V., Alimova O.A. – writing the article; Perminova S.G., Bashmakova N.V. – editing.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The work was carried out without sponsorship.
Acknowledgements: The authors express their gratitude to Irina A. Proskurina, Ph.D., Head of the Department No. 2 for the Effectiveness and Safety of Medications, Scientific Centre for Expert Evaluation of Medicinal Products, Ministry of Health of Russia, for assistance in preparing the article.
Patient Consent for Publication. All patients participating in the study signed an informed consent.
Authors’ Data Sharing Statement. The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Perminova S.G., Nazarenko Т.А., Korneeva I.Е., Bashmakova N.V.,
Mityurina Е.V., Alimova О.А., Belova I.S., Ershov А.V., Khramtsova А.Yu., Dzhalilova E.R. Comparative study of the equivalence of the biosimilar follitropin alpha preparation (solution for subcutaneous injection) and the original follitropin alpha preparation (lyophilisate for preparation of solution for subcutaneous administration) in women with different responses to ovarian stimulation in an in vitro fertilization program: results of a phase IV clinical trial.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 10: 138-149 (in Russian)
https://dx.doi.org/10.18565/aig.2022.10.138-149



