Morphological characteristics of the endometrium in the preconception period, chorion and placenta during pregnancy resulting from in vitro fertilization in patients with chronic endometritis (secondary analysis of the results of the TULIP 2 randomized controlled trial)
Sukhanov A.A., Dikke G.B., Kukarskaya I.I., Pesotskaya А.V.
Background: Chronic endometritis (CE) is associated with unsuccessful attempts of in vitro fertilization (IVF) in 7.7–67.5% of patients.
Objective: To determine the characteristics of the morphological structure of the endometrium, chorion and placenta in patients with favourable and unfavourable pregnancy outcomes resulting from IVF with cryotransfer, after treatment of chronic endometritis in the preconception period using a complex of natural antimicrobial peptides and cytokines.
Materials and methods: A total of 600 patients with CE and infertility who underwent IVF (cryopreservation of one embryo) were selected from the electronic database. The analysis included the results of morphological examination of their endometrium, chorion and placenta after the treatment of CE in the preconception period. The patients of group I received the Superlymph drug, an antibiotic and a progestogen, patients of group II received an antibiotic and a progestogen, and then only a progestogen for up to 6 months in both groups). The morphological structure of the endometrium was assessed before and after treatment before conception, and the chorion/placenta after pregnancy.
Results: The average age of the patients was 36 (33–38) years, duration of infertility was 6.0 (4.5–7.0) and 5.5 (4.0–6.5) in groups I and II, respectively, p=0.06. The pregnancy rate as a result of cryotransfer was 57.0 versus 38.7%, respectively (RR=1.47; 95% CI: 1.24–1.75, p<0.001). Live birth rate was 45.3 and 20.7%, respectively (RR=2.19; 95% CI: 1.70–2.83, p<0.0001).
After treatment in the preconception period, the structure of the endometrium in patients in group I corresponded to the cycle phase in 85.3%, in group II – in 60.3%. After spontaneous miscarriage in the first trimester, more patients in group I than in group II had a complete gravidar transformation (RR=9.06, 95% CI: 2.35–35.0, p<0.001) with fewer signs of hematogenous infection in the chorion and its gestational immaturity. In both groups, the histological structure of the placenta after miscarriage at 120–216 weeks corresponded to the gestation period with the deposition of intercellular fibrinoid, ectasia and vascular fullness, and signs of infection. After preterm birth, the weight and thickness of the placenta were statistically significantly higher in patients in group I, however, the morphological structure differed only in a smaller number of syncytial kidneys and fibrosis of the basal plates, the remaining indicators in both groups showed that the placenta did not match the gestation period. In patients who had full-term birth, the placenta mass was comparable; as for the rest of the parameters, there was a statistically significant difference between patients of groups I and II with a decrease in the risk of defective structure in most indicators by 2 to 9 times in favour of group I. This difference determined a 2.7-fold lower risk of complications during pregnancy in patients of group I (RR=0.37, 95% CI: 0.27–0.52, p<0.001).
Conclusion: There are morphological signs of chorionic/placental inferiority in patients with CE and unsuccessful outcomes of pregnancy resulting from IVF, which determine the necessity of restoring the structure and function of the endometrium before conception. Morphological examination of the endometrium before IVF planning in patients who received a course of complex treatment, including Superlymph during preconception period, indicates a significant improvement in the structure of the endometrium compared with the control group (matching the day of the cycle, reduction of spiral artery sclerosis and periglandular fibrosis), which improves the results of IVF and pregnancy outcomes.
Authors' contributions: Sukhanov A.A. – collecting clinical material, forming an electronic database, writing fragments of an article and editing it; Dikke G.B. – analysis of the results of statistical processing of clinical material and their interpretation, search for literary sources, writing an article and editing it after reviewing; Kukarskaya I.I. – organization of research on a clinical basis, supervision during the study; Pesotskaya A.V. – analysis of the results of morphological research.
Conflicts of interest: Authors declare lack of the possible conflicts of interest.
Funding: The study was conducted without sponsorship.
Acknowledgements: The authors express their gratitude to the staff of the UNIM Laboratory of Pathomorphology, Histology and Immunohistochemistry (Novosibirsk), doctors Sergey V. Demchenko, Evgenia Yu. Kozhevnikova, Konstantin V. Kropotkin.
Ethics approval. The study was approved by the Ethical Review Board of the Perinatal Medical Center, Tyumen, Russia).
Patients’ consent to publication. The patients provided an informed consent for the publication of their data.
Research data exchange. The data confirming the conclusions of this study are available upon request from the author responsible for the correspondence after the approval of the lead researcher.
For citation: Sukhanov A.A., Dikke G.B., Kukarskaya I.I., Pesotskaya А.V. Morphological characteristics of the endometrium in the preconception period, chorion and placenta during pregnancy resulting from in vitro fertilization in patients with chronic endometritis (secondary analysis of the results of the TULIP-2 randomized controlled trial).
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (5): 118-132 (in Russian)
https://dx.doi.org/10.18565/aig.2024.122
Keywords
Chronic endometritis (CE) is a common disease with its incidence ranging from 14.1–24.4% in the population (or 0.2–46% according to other data, depending on the patient’s profile and biopsy method). CE accounts for 2.8–56.8% of infertility causes, and 7.7–67.5% of recurrent implantation failures (RIF) following assisted reproduction treatment [1–4]. It is possible for a woman with CE to become pregnant, but pregnancy often ends with spontaneous miscarriages in early gestation (40–62.4%) or preterm birth (14.3–27%) [5, 6]. The risk of adverse outcomes persists in pregnancy resulting from ART as well [6].
Recent studies indicate that the impairment of reproductive function in women with CE is due to a change in endometrial receptivity, which results from a proinflammatory state in the endometrium owing to activation of local immune defenses accompanied by the secretion of cytokines and chemokines [7]. The etiology of CE is associated with the changes in the vaginal microbiota with a predominance of opportunistic pathogens (most often staphylococci, streptococci, eubacteria) and viruses, ascending infection, inflammatory changes in the afterbirth and placental insufficiency [8].
Endometrial dysfunction plays a role in the pathogenesis of reproductive disorders in CE. This dysfunction is characterized by pathognomonic signs such as inconsistency of the structure of the endometrium with the day of the menstrual cycle, decreased expression of estrogen and progesterone receptors, pathological activation of neoangiogenesis, and cell cycle disorders [9]. Changes in the endometrium lead to a disorder of implantation, the process of trophoblast invasion, the development of the placenta with deficient blood flow, with subsequent complications of pregnancy, which, in combination with inflammatory changes in the chorion and placenta, results in its early termination.
Antibacterial therapy of CE is effective and promotes fertility restoration and an increase in the live birth rate in ART programs if CE is resolved (OR=6.82 in comparison with persistent CE) [10, 11]. However, CE is resolved after one course of antibacterial therapy only in 31.3% of patients [12] and it is necessary to repeat courses of antibacterial therapy or prescribe medications that complement antibacterial therapy in patients, whose disease was not cured.
The repeated course of antibacterial therapy makes it possible to cure another 30% of patients, and the third course can cure 19% [12]; however, an increase in the rate of pregnancies/live births is not reported in this study. The combined use of systemic and intrauterine administration of antibacterial therapy can lead to a more effective cure of the disease, namely by 40%, but it does not increase the rate of pregnancies/live births [13].
The attempts of using therapy with other means (without antibacterial therapy) on the basis of the concept of the autoimmune nature of CE turned out to be ineffective, and the theory itself was untenable, since it was proved that patients with CE did not differ in either systemic inflammatory or autoimmune profiles from patients without CE [9, 14–16].
According to several systematic reviews and meta-analyses, the most effective additional methods of treating CE that can contribute to the achievement of pregnancy/live birth after ART are intrauterine infusion of platelet-rich plasma [17], immunomodulatory agents [18], including intrauterine administration of autologous peripheral blood mononuclear cells (PBMC) obtained from patients before IVF [19, 20]. There is evidence of the effectiveness of local therapy with exogenous products of PBMC secretion, or secretome therapy, which is prescribed as a medication containing a complex of natural antimicrobial peptides and cytokines obtained from PBMC of porcine blood (Superlymph) [20–22].
Therefore, it is worth studying the morphological changes of the endometrium, chorion and placenta in order to understand the causes of miscarriage and preterm birth in CE, as well as the characteristics of their structure after complex treatment using the Superlymph medication at the preconception stage. There are no publications devoted to the study of the morphology of the chorion and placenta in unsuccessful and favorable pregnancy outcomes in patients with CE.
Understanding the relationship between the histological structure of the endometrium, chorion/placenta, the clinical course and outcomes of pregnancy in patients after treated CE will clarify the pathophysiological processes that occur in this pathology, as well as optimize the management of women.
The objective of the study is to determine the characteristics of the morphological structure of the endometrium, chorion and placenta in patients with favorable and unfavorable outcomes of pregnancy resulting from IVF with cryopreservation, after treatment of chronic endometritis in the preconception period using a complex of natural antimicrobial peptides and cytokines.
Materials and methods
Design. This the secondary analysis of the results of the study “The course and outcomes of pregnancy resulting from in vitro fertilization in patients with chronic endometritis who received complex treatment using the Superlymph drug at the preconception stage” (the TULIP 2 randomized controlled trial) [23].
Materials. The data of 600 female patients from the electronic database were used in the study. The women were diagnosed with uterine infertility, chronic inflammatory uterine disease (N71.1) which were confirmed histologically and immunohistochemically; the patients met the inclusion/exclusion criteria, they underwent treatment for CE and IVF (cryopreservation) in the Perinatal Medical Center (Tyumen, Russia) from September 2019 to June 2023 [23]. The patients were divided into groups: group I (n=300) received antibacterial therapy at the preconception stage, progestogens in the second phase of the cycle, and a complex of natural antimicrobial peptides and cytokines (Superlymph); group II (n=300) received antibacterial therapy/progestogen (control group). Progestogen was taken until cryopreservation (no more than 6 months).
Methods. Endometrial aspirate obtained before and after treatment of patients before conception on the 21st-24th day of the menstrual cycle and gestational tissue after the end of pregnancy were used for histological examination; the end of pregnancy is referred as follows: early miscarriage – up to 116 weeks (26 and 23 patients from groups I and II, respectively), late miscarriage – 120–216 weeks (8 and 12 patients, respectively), preterm birth – 220–366 weeks (14 and 12 patients, respectively) and full-term delivery – 370–416 weeks (123 and 59 patients, respectively). The morphological structure of the endometrium was assessed according to the criteria for CE diagnosis [24, 25]; the placenta was assessed according to the classification and criteria of macro- and microscopic characteristics of the placenta [26].
Statistical analysis
Statistical processing was performed using the program SPSS v.27.0. The distribution of variables was determined using the Kolmogorov–Smirnov test. In case of normal distribution, quantitative variables were expressed in the form of average values (M) and standard deviation (SD), others were shown in the form of median (Me) and interquartile range (Q1–Q3), qualitative variables were expressed in absolute numbers (n), and their fractions were presented in relative values (%). Quantitative parameters different from the normal distribution were compared using the Mann–Whitney U-test. The percentages were compared using Pearson’s chi-squared (χ2) test. Differences were considered significant at p<0.05 and first and second level errors α=5% and β=20%, respectively. The dependence of intervention and outcome was determined by calculating the relative risk (RR) with a 95% confidence interval (CI).
Results
General information about patients and treatment outcomes. The age of the patients ranged from 18 to 45 years, Me (Q1–Q3) is 36 (33–38) years. Most of the patients were aged 35 years and older, namely 87.4% (202/300) and 63.0% (189/300) in groups I and II, respectively, p=0.27. The social and clinical characteristics of the patients are presented in the article [23]. The patients in groups I and II differed only in education, but there were no statistically significant differences in other parameters. The data on general examination and somatic status of patients in both groups were comparable.
The duration of infertility averaged 6.0 (4.5–7.0) and 5.5 (4.0–6.5) years, respectively, p=0.06. Among the patients of the first group, 21 (7.0%) patients underwent one course of treatment using Superlymph, 142 (47.3%) patients had two courses, 100 (33.3%) patients received three courses and 37 (12.3%) women had four courses of treatment. The overall pregnancy rate and pregnancy outcomes are presented in Table 1.
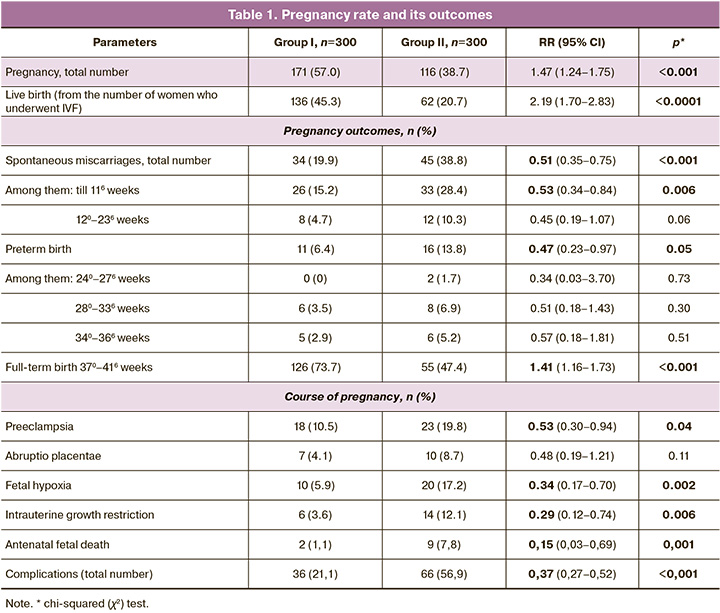
Thus, the preparation of the endometrium in patients with CE in the preconception period using the Superlymph/antibacterial therapy/progestogen complex contributed to a 2-fold reduction in the risk of spontaneous miscarriages in the early stages and earlier than 370 weeks, as well as pregnancy complications by 2–3 times in patients who became pregnant after IVF.
The results of the morphological study
Morphological structure of the endometrium in patients in the preconception period. All patients underwent histological examination of the endometrium (Table 2).
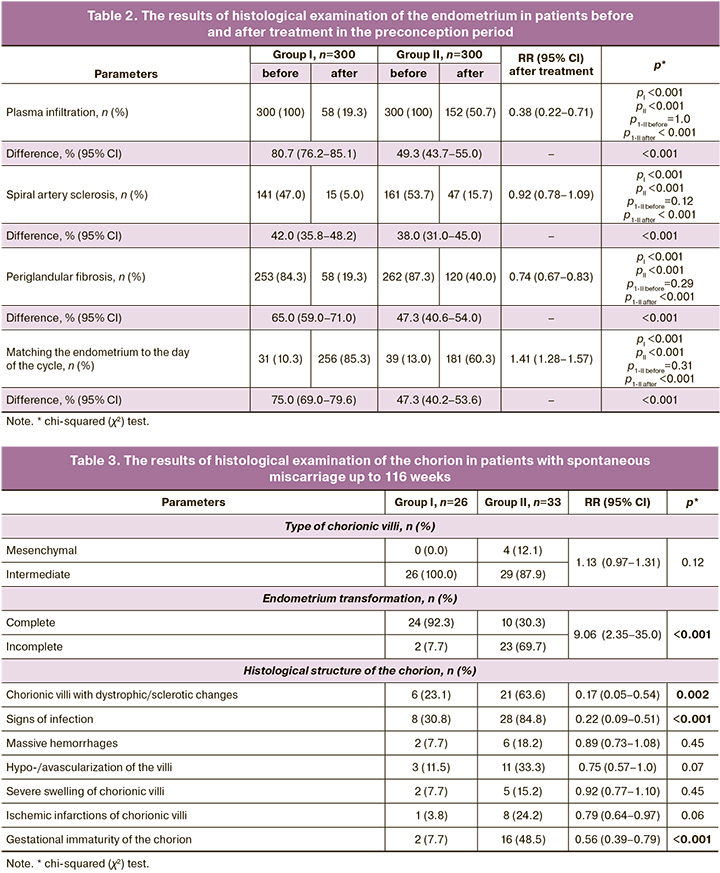
The analysis of Table 2 showed that the frequency of detection of spiral artery sclerosis in group I after treatment was statistically significantly lower compared to group II. Similar results were obtained for periglandular fibrosis, the frequency of which decreased in both groups, but was significantly lower in group I. At the same time, plasma cell infiltration was absent in 80.7% versus 49.3% of patients, respectively (RR=1.64, 95% CI: 1.44–1.86, p<0.001), indicating the advantage of complex treatment and, thus, the probability of detecting CE was 1.6 times lower in women receiving Superlymph.
Morphological structure of the chorion in patients with spontaneous miscarriage in the first trimester. Spontaneous miscarriage occurred in 26/171 (15.2%) and 33/116 (28.5%) patients in the first trimester, respectively. The results are presented in Table 3. The endometrium transformation in group I was better than in group II, the intermediate type of chorionic villi was more common, and dystrophic/sclerotic changes with signs of infection were less common.
There was no proliferation of chorial syncytium in both groups. At the same time, such changes as a sharp edema of the villi, their hypo-/avascularization with the presence of ischemic infarcts and massive hemorrhages were detected with the same frequency in both groups, which, apparently, was the cause of spontaneous miscarriage in the early stages.
The histological structure of the chorion in spontaneous miscarriage in the early stage is shown in Figure 1.
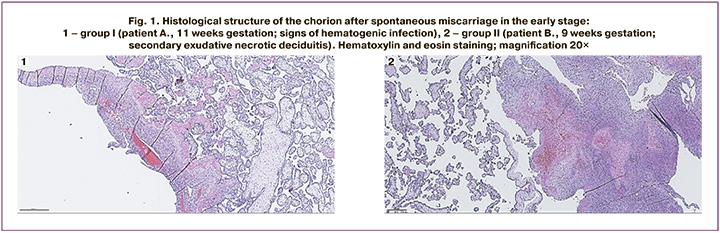
Figure 1.1 shows the chorion obtained after spontaneous miscarriage from a patient who was 11 weeks pregnant. There are immature intermediate villi with hypovascularization, dystrophic changes, interstitial fibrinoid and relative anemia of the vascular bed; anemia of the intervillous space, basal plate with focal monocytic infiltration. Conclusion: missed miscarriage with signs of hematogenous infection of unexplained etiology (focal productive villusitis).
Figure 1.2 shows the chorion obtained after spontaneous miscarriage from a patient who was 9 weeks pregnant. There are immature intermediate villi with hypovascularization, and some of the villi have signs of reduction of the vascular bed; the stroma of the villi with dystrophic changes and fibrinoid deposition in the interstitial space. In the placental bed, there is diffuse monocytic infiltration with a focal admixture of neutrophils, focal exudative necrotic changes and hemorrhages. Conclusion: missed miscarriage, basal secondary exudative necrotic deciduitis.
In both observations, the presence of dystrophic changes in chorionic villi and fibrinoid deposits in the interstitial space suggests an immune rejection reaction as an etiological factor of pregnancy termination. This termination results from persistent inflammation in the endometrium, which is confirmed by the presence of focal monocytic infiltration with focal exudative necrotic changes and hemorrhages.
Morphological structure of the chorion/placenta in patients with spontaneous miscarriage in the late stages. Spontaneous miscarriage at 120-216 weeks gestation occurred in 8/171 (4.7%) and 12/116 (10.3%) patients in groups I and II, respectively (Table 4).
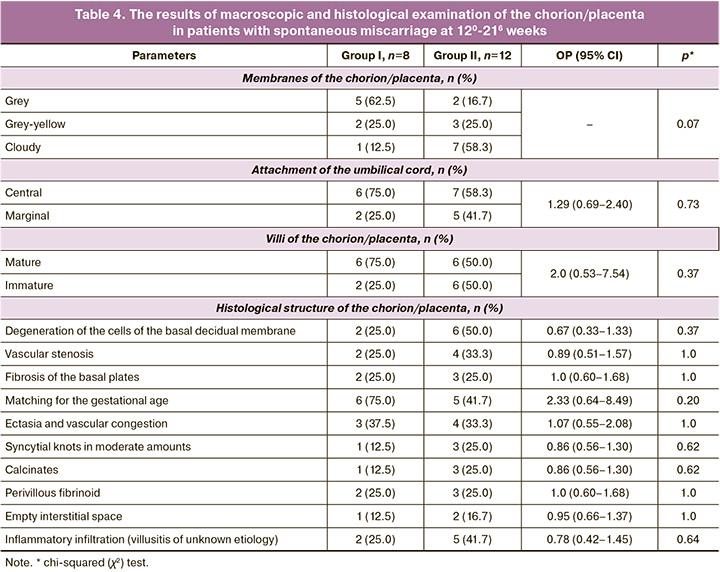
According to the analysis of the indicators presented in Table 3, it can be concluded that in both groups there were no statistically significant differences in the structure of the chorion/placenta; inflammatory infiltration, deposition of interstitial fibrinoid, and dystrophy of the basal decidual membrane cells were revealed.
The defective morphological structure of the chorion/placenta is manifested by a decrease in its vascularization during these periods; it indicates a lack of functioning due to incomplete restoration of the endometrial structure under the influence of treatment at the preconception stage.
The results of histological examination of placentas in patients after late spontaneous miscarriage are shown in Figure 2.
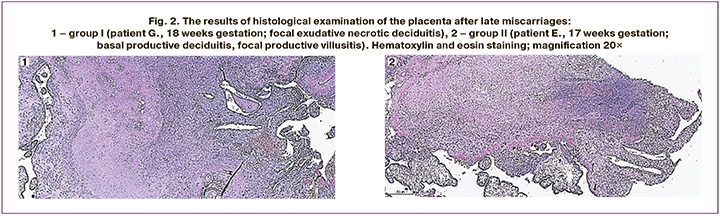
Figure 2.1 shows the placenta after spontaneous miscarriage in a patient at 18 weeks gestation. There are villi of an intermediate immature and mature structure with hypovascularization; stroma with dystrophic and sclerotic changes, sometimes with edema; single chorionic villi with monocytic infiltration in the stroma; basal plate with diffuse monocytic infiltration, focal exudative necrotic changes. Conclusion: placental tissue corresponds to the gestational age, hematogenic infection of unknown etiology (basal and parietal exudative necrotic deciduitis, focal productive villusitis).
Figure 2.2 shows the placenta after spontaneous miscarriage at 17 weeks gestation. There is the placenta of an intermediate immature structure with partial reduction of the bloodstream, moderate blood filling of the capillaries; stroma of the villi with dystrophic changes; hemorrhages and single monocytes in the stroma of the villi; large trophoblast proliferations, sometimes with dystrophic changes; basal plate with diffuse monocytic infiltration; chorial plate without pathological changes; deposits of calcium salts. Conclusion: the histological structure of the placental tissue does not correspond to the gestational age, hematogenic infection of unknown etiology (basal productive deciduitis, focal productive villusitis).
These observations indicate the development of secondary placental insufficiency due to infectious pathology of the fetoplacental system with manifestations of hematogenic infection.
Histological structure of the placenta in patients with preterm birth. Preterm birth occurred in 14/171 (8.2%) and 12/116 (10.3%) patients. Most of the women had full-term birth at 280–366 weeks (10/14 (71.4%) and 9/12 (75.0%), respectively). The morphology of the placentas is presented in Table 5.
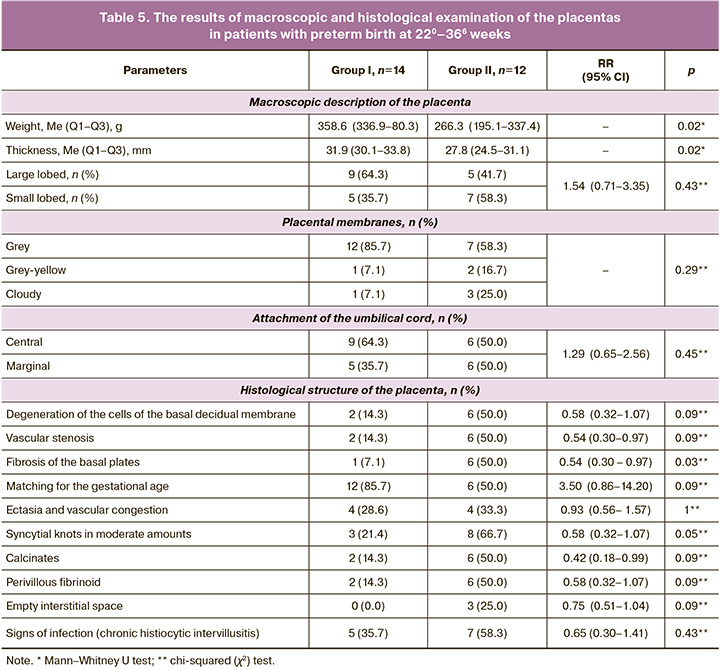
Table 5 shows that the mass and thickness of the placentas were significantly higher in patients in group I, however, the morphological structure differed only in a smaller number of syncytial knots and fibrosis of the basal plates. The rest of the indicators in both groups indicated its deficiency, as well as in patients with late spontaneous miscarriage.
The examples of the morphological structure of the placentas in patients from two groups after preterm birth are shown in Figure 3.
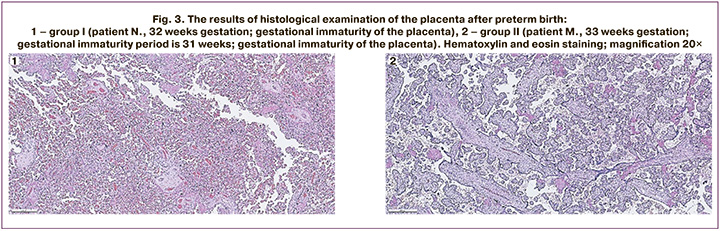
Figure 3.1 shows the results of histological examination of the placenta after delivery at 32 weeks gestation. There is the placenta of the intermediate and terminal type of villous tree development with moderate blood filling of the vascular bed of all generations villi; groups of villi with fibrinoid alteration; a large number of syncytial knots; basal plate with perivascular monocytic infiltration, vessels filled with blood and focal exudative infiltration in fibrinoid; chorial plate with ectasia of intraplacental veins; small focal deposits of calcium salts. Conclusion: the histological structure of the placental tissue does not correspond to the gestational age (gestational immaturity).
Figure 3.2 shows the results of histological examination of the placenta after delivery at 31 weeks gestation. There is the placenta of an intermediate and terminal type of structure with moderate blood filling of the vascular bed of all generations; groups of necrotic villi with fibrinoid alteration; uneven blood filling of the intervillous space; diffuse monocytic infiltration in the basal plate, a thickened fibrinoid layer with large-focal exudative necrotic changes and delaminating hemorrhages; chorial plate without pathological changes; deposits of calcium salts. Conclusion: the histological structure of the placental tissue does not correspond to the gestational age (gestational immaturity).
Morphological structure of the placenta in patients who had full-term birth. The results of macroscopic and histological examination of the placentas from women who gave birth at 370–416 weeks are presented in Table 6.
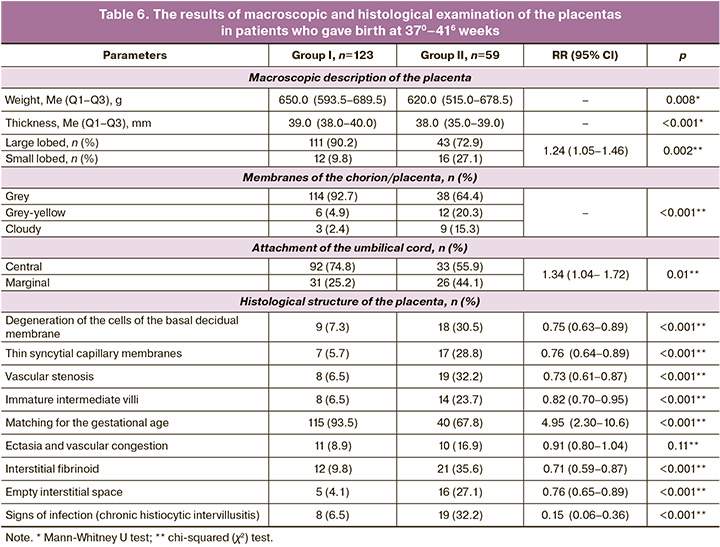
The number of patients who had full-term birth differed significantly between the groups, namely 71.9 and 50.9% of the number of pregnancies, p<0.001. The weight of the placentas was comparable, but in all other parameters there was a statistically significant difference (except ectasia and vascular stenosis) between patients of groups I and II with a decrease in the risk of defective structure in certain indicators from 2 to 9 times in favor of group I. This difference determined a 2.7-fold lower risk of complications during pregnancy in patients of group I.
The examples of histological examination of the placentas after full-term birth in patients from two groups are shown in Figure 4.
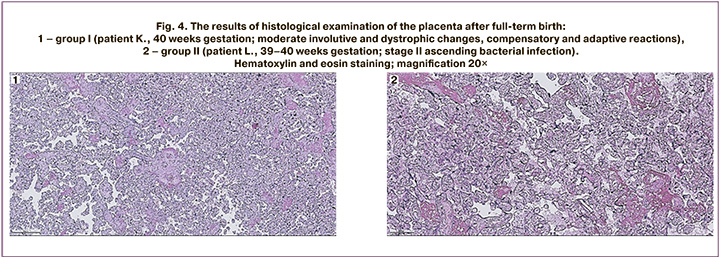
Figure 4.1 shows the results of histological examination of the placenta after full-term birth at 40 weeks gestation. There is the placenta of the terminal type of villous tree development with moderate blood filling of the vascular bed of villi of all generations; a large number of syncytial knots; groups of villi with fibrinoid alteration, sclerotic stroma changes; basal plate with large focal hemorrhages, diffuse monocytic infiltration, vessels filled with blood, foci of exudative necrotic changes; a chorial plate is unremarkable; deposits of calcium salts. There is the umbilical cord with moderate edema of the stroma, three blood vessels, vascular ectasia; blood is in the lumen of the vessels. The membranes are represented by an amnion of normal structure. Conclusion: the histological structure of the placental tissue corresponds to the gestational age, moderate involutive and dystrophic changes, compensatory and adaptive reactions that are evenly distributed.
Figure 4.2 shows the results of morphological examination of the placenta after full-term birth at 39–40 weeks gestation. There is the placenta of the terminal type of villous tree development with paresis and blood filling of the vascular bed of the villi of all generations; a large number of syncytial knots; uneven blood filling of the intervillous space; basal plate with diffuse monocytic infiltration; diffuse exudative infiltration in the subchorial fibrinoid. There are the membranes with the amniotic layer partially detached, the dark cell trophoblastic layer, decidual membrane of the spongy type with focal exudative infiltration. There is the umbilical cord with moderate swelling of the stroma, blood vessels, large-focal perivascular hemorrhages. Conclusion: the histological structure of the placental tissue corresponds to the gestational age, stage II ascending bacterial infection (exudative placental deciduitis, exudative subchorial intervillusitis), severe acute circulatory disorders in the placental tissue.
Discussion
There are two factors among the reasons for unsuccessful IVF attempts, namely the inability of embryos obtained in vitro to intrauterine implantation (embryonic factor), or a decrease in endometrial receptivity (endometrial factor); provided that embryos are obtained of good quality, endometrial factor acquires a leading role, causing up to 70% of IVF failures, which motivates scientists to search for methods of influencing the endometrium [27].
The morphological characteristics of the endometrium in CE have been thoroughly studied and its main manifestations have been identified, which are infiltration of the endometrial stroma by plasmocytes, the presence of lymphoid infiltrates, sclerosis of spiral arteries, focal hyperplasia of the basal layer [9]. Severe dystrophic and atrophic changes in the mucous membrane and glandular epithelium occur simultaneously with polymorphic cellular infiltration and fibroplastic stromal transformation of varying intensity [28]. Characteristic structural changes for CE are intensive processes of apoptosis of the epithelium of the uterine mucosa with insignificant proliferative activity, while there is an inadequate reception of progesterone by target cells [29]. There were also the formation of scar tissue and impaired hemodynamics which lead to focal hemorrhages in the uterine mucosa [30].
The most frequently discussed method of treating CE is antibacterial therapy, however, research focuses on reproductive results, and morphological changes in the endometrium under the influence of therapy have not been described [31]. There is no information in the literature on the characteristics of the chorion/placenta structure, their connection with the structure of the endometrium before conception, and the dependence of pregnancy outcomes on them in patients with CE.
This study showed that in the treatment of patients with infertility caused by CE, the morphological structure of the endometrium improves in both groups at the preconception stage. The treatment with the use of Superlymph/antibacterial therapy/progestogen and antibacterial therapy/progestogen led to the normalization of secretion/proliferation processes in accordance with the phases of the menstrual cycle and a decrease in fibrosis; however, the results were 1.5 times better in group I, while the resolution of CE was 1.6 times higher according to the CD138 criterion, which indicates the advantage of a complex treatment.
As for the repeated examination of the endometrium (morphology in combination with immune histochemical test) in women with CE, Liu W.J. et al. (2022) showed that treatment with doxycycline can improve reproductive results in cryopreservation ART programs, however, if CE persisted after the first course, then repeated use of antibiotics (1 or 2 courses of 14 days each) did not improve outcomes [32]. Morphological characteristics of the endometrium under the influence of treatment are not given in this work. It is likely that the potential effect of antibacterial therapy on endometrial structures is limited by the elimination of plasma cells without restoring functional activity of the tissues. It is known that, even if the morphological structure of the endometrium corresponds to the phase of the menstrual cycle, its functional properties necessary for implantation processes may be impaired [33]. This point is confirmed by the results obtained in this study: pregnancy occurred only in 38.7% of patients after antibacterial therapy/progestogen, whereas it occurred in 57.0% in patients who additionally received Superlymph, with a 1.5-fold increase in the chances of pregnancy.
The examination of the chorion after miscarriage in the study of Kaku S. et al. (2020) showed the presence of plasma cells, which indicated the presence of CE [33]. However, the morphological characteristics of the chorion have not been studied in this work. In the present study, there were the following statistically significant differences in the morphology of gestational tissues after spontaneous miscarriage in the first trimester: complete transformation of the endometrium (92.3 versus 30.3%, respectively, p<0.001), the presence of chorionic villi with dystrophic/sclerotic changes (23.1 versus 63.6%, p=0.002), inflammation in the chorion (30.8 versus 84.8%, p<0.001), gestational immaturity of the chorion (7.7 versus 48.5%, p<0.001). A greater number of disorders in the structure of the chorion indicates its functional deficiency, which obviously resulted in a higher frequency of miscarriages in the early stages.
There were no significant changes in the chorion/placenta in spontaneous miscarriages between 120 and 216 weeks. After preterm birth, the mass and thickness of the placenta were statistically significantly higher in patients in group I, however, the morphological structure differed only in a smaller number of syncytial knots (21.4 and 66.7%, p=0.05) and fibrosis of the basal plate (7.1 and 50.0%, p=0.03), respectively.
Normalization of the structure and function of the endometrium is known to ensure the normal process of trophoblast invasion after implantation, which is critical for the adequate functioning of the placenta in the future and affects the course of pregnancy. This is confirmed by the results of this study, namely, a 2–fold reduction in the risk of preeclampsia, hypoxia and fetal growth retardation by 3 times, as well as antenatal fetal death by 6 times. Pregnancy outcomes also indicate the advantage of using Superlymph before conception: the increase in the chances of live birth was twice as high, the risk of spontaneous miscarriage in the early stages and preterm birth was twice as low; and this effect was more significant in comparison with the outcomes that are presented in the studies of other scientists [34, 35]. A lower incidence of complications and improved outcomes may be due to the fact that the expression of progesterone receptors in the epithelium and stroma of the endometrium increases by 3 and 2.7 times, respectively; in addition, there was an improvement in angiogenesis and vascularization of the endometrium under the influence of Superlymph, which was shown in a study by Yu.E. Dobrokhotova et al. [36]. The authors also revealed the restoration of the expression of innate immunity factors, control of CE and normalization of endometrial tissue receptivity [37].
It is noted in articles that women who had preterm birth more often have vascular malperfusion [38] and impaired maturation of villi [39], proliferative sinusitis, post-inflammatory hypovascularization, abnormal differentiation of the vascular-stromal component of villi, circulatory disorders, compensatory hyperplasia of terminal villi, capillaries and their syncytial membranes [40]; these findings are consistent with the results of this study.
Preston M. et al. reported that placental abnormalities play a role in the etiology of preterm births along with inflammatory changes [41]. Women who gave birth prematurely have vascular lesions of the placenta. Despite convincing evidence that placenta abnormalities can contribute to the development of preterm birth, the underlying mechanisms of these processes have not been studied. According to one theory, when uteroplacental ischemia is so severe that it leads to decidual necrosis and bleeding, thrombin plays a role in initiating labor because it has powerful oxytocin-like activity.
Placental abnormality is well known to be the cause of intrauterine growth retardation, when spiral arteries cannot transform, and this leads to an increase in vascular resistance and a decrease in blood flow to the placenta and fetus [41].
The above changes in the placenta were detected by us in women with CE treated in the preconception period; some characteristics were also identified, which are probably due to the inflammatory response of the immune system to the persistence of opportunistic pathogens and viruses.
Vitagliano A. et al. (2018) believe that a control biopsy with histological examination of the endometrium after treatment with CE is necessary in all cases to confirm the resolution of CE, before IVF [42]. Our study also demonstrates the need to diagnose the complete restoration of the endometrium in the preconception stage; however, as it was shown in our other works, it should be done not only morphologically, but also functionally with the help of additional modern research methods [43], as well as the use of predictive tools for pregnancy and its outcomes, developed on the basis of neural network technology [44].
Conclusion
Patients with CE and unsuccessful IVF outcomes show morphological signs of chorionic/placental insufficiency (placental insufficiency), which determines the need to restore the structure and function of the endometrium before conception. Morphological examination of the endometrium before IVF planning in patients who received a course of complex treatment, including the Superlymph complex during the preconception period, indicates a significant improvement in its structure compared with the control (matching the day of the cycle, reduction of spiral artery sclerosis and periglandular fibrosis), which improves the results of IVF and pregnancy outcomes.
References
- Cicinelli E., Trojano G., Mastromauro M., Vimercati A., Marinaccio M., Mitola P.C. et al. Higher prevalence of chronic endometritis in women with endometriosis: a possible etiopathogenetic link. Fertil. Steril. 2017; 108(2): 289-95.e1. https://dx.doi.org/10.1016/j.fertnstert.2017.05.016.
- Yasuo T., Kitaya K. Challenges in clinical diagnosis and management of chronic endometritis. Diagnostics. 2022; 12: 2711. https://dx.doi.org/10.3390/ diagnostics1211271.
- Singh N., Sethi A. Endometritis – diagnosis, treatment and its impact on fertility – A scoping review. JBRA Assist. Reprod. 2022; 26(3): 538-46. https://dx.doi.org/10.5935/1518-0557.20220015.
- Kimura F., Takebayashi A., Ishida M., Nakamura A., Kitazawa J., Morimune A. et al. Review: Chronic endometritis and its effect on reproduction. J. Obstet. Gynaecol. Res. 2019; 45(5): 951–60. https://dx.doi.org/10.1111/jog.13937.
- Pirtea P., Cicinelli E., De Nola R., de Ziegler D., Ayoubi J.M. Endometrial causes of recurrent pregnancy losses: endometriosis, adenomyosis, and chronic endometritis. Fertil. Steril. 2021; 115(3): 546-60. https:/dx.doi.org/10.1016/j.fertnstert.2020.12.010.
- Zhang Q., Yang G., Tan J., Xiong Y., Xu Y., Xu Y. et al. Antibiotic cured chronic endometritis remains a risk factor for early pregnancy loss in the subsequent frozen euploid embryo transfer. Reprod. Biomed. Online. 2024; 48(2): 103611. https:// dx.doi.org/10.1016/j.rbmo.2023.103611.
- Buzzaccarini G., Vitagliano A., Andrisani A., Santarsiero C.M., Cicinelli R., Nardelli C. et al. Chronic endometritis and altered embryo implantation: a unified pathophysiological theory from a literature systematic review. J. Assist. Reprod. Genet. 2020; 37(12): 2897-911. https://dx.doi.org/10.1007/s10815-020-01955-8.
- Дадаева Д.Г., Соснина А.К., Траль Т.Г., Толибова Г.Х., Будиловская О.В., Крысанова А.А., Савичева А.М., Коган И.Ю. Воспалительные изменения в последе и их связь с микробиотой влагалища до родов. Журнал акушерства и женских болезней. 2021; 70(1): 59-68. [Dadaeva D.G., Sosnina A.K., Tral T.G., Tolibova G.Kh., Budilovskaya O.V., Krysanova A.A., Savicheva A.M., Kogan I.Yu. Placental inflammatory changes and their association with the vaginal microbiota before delivery. Journal of Obstetrics and Women's Diseases. 2021; 70(1): 59-68. (in Russian)]. https://dx.doi.org/10.17816/J0WD52962.
- Толибова Г.Х., Траль Т.Г. Хронический эндометрит – затянувшаяся дискуссия. Уральский медицинский журнал. 2023; 2: 142-51. [Tolibova G.Kh., Tral T.G. Chronic endometritis – a protracted discussion. Ural Medical Journal. 2023; (2): 142-51. (in Russian)]. https://dx.doi.org/10.52420/2071-5943-2023-22-2-142-152.
- Liu J., Liu Z.A., Liu Y., Cheng L., Yan L. Impact of antibiotic treatment for chronic endometritis on pregnancy outcomes in women with reproductive failures (RIF and RPL): A systematic review and meta-analysis. Fron.t Med. (Lausanne). 2022; 9: 980511. https:/dx.doi.org/10.3389/fmed.2022.980511.
- Li J., Li X., Ding J., Zhao J., Chen J., Guan F. et al. Analysis of pregnancy outcomes in patients with recurrent implantation failure complicated with chronic endometritis. Front. Cell Dev. Biol. 2023; 11: 1088586. https://dx.doi.org/10.3389/fcell.2023.1088586.
- Cicinelli E., Resta L., Loizzi V., Pinto V., Santarsiero C., Cicinelli R. et al. Antibiotic therapy versus no treatment for chronic endometritis: a case-control study. Fertil. Steril. 2021; 115(6): 1541-8. https://dx.doi.org/10.1016/j.fertnstert.2021.01.018.
- Pantos K., Simopoulou M., Maziotis E., Rapani A., Grigoriadis S., Tsioulou P. et al. Introducing intrauterine antibiotic infusion as a novel approach in effectively treating chronic endometritis and restoring reproductive dynamics: a randomized pilot study. Sci. Rep. 2021; 11(1): 15581. https://dx.doi.org/10.1038/s41598-021-95072-w.
- Kushnir V.A., Solouki S., Sarig-Meth T., Vega M.G., Albertini D.F., Darmon S.K. et al. Systemic inflammation and autoimmunity in women with chronic endometritis. Am. J. Reprod. Immunol. 2016; 75(6): 672-7. https://dx.doi.org/10.1111/aji.12508.
- You S., Zhu Y., Li H., He F., Liu S., Yang X. et al. Recombinant humanized collagen remodels endometrial immune microenvironment of chronic endometritis through macrophage immunomodulation. Regen. Biomater. 2023; 10: rbad033. https:// dx.doi.org/10.1093/rb/rbad033.
- Мальцева Л.И., Шарипова Р.И., Железова М.Е. Хронический эндометрит – смена привычных представлений. Практическая медицина. 2018; 6:99-105. [Maltseva L.I., Sharipova R.I., Zhelezova M.E. Chronic endometritis is a change in habitual ideas. Practical Medicine. 2018; (6): 99-105. (in Russian)]. https://dx.doi.org/10.32000/2072-1757-2018-16-6-99-105.
- Huang W., Liu B., He Y., Xie Y., Liang T., Bi Y. et al. Variation of diagnostic criteria in women with chronic endometritis and its effect on reproductive outcomes: A systematic review and meta-analysis. J. Reprod. Immunol. 2020; 140: 103146. https://dx.doi.org/10.1016/j.jri.2020.103146.
- Liu M., Yuan Y., Qiao Y., Tang Y., Sui X., Yin P., Yang D. The effectiveness of immunomodulatory therapies for patients with repeated implantation failure: a systematic review and network meta-analysis. Sci. Rep. 2022; 12(1): 18434. https:// dx.doi.org/10.1038/s41598-022-21014-9.
- Wang C., Guan D., Li R., Bing Z., Yang Y., Yang K. Comparative efficacies of different immunotherapy regimens in recurrent implantation failure: A systematic review and network meta-analysis. J. Reprod. Immunol. 2021; 148: 103429. https:/dx./doi.org/10.1016/j.jri.2021.103429.
- Тапильская Н.И., Толибова Г.Х., Савичева А.М., Копылова А.А., Глушаков Р.И., Будиловская О.В., Крысанова А.А., Горский А.Г., Гзгзян А.М., Коган И.Ю. Эффективность локальной цитокинотерапии хронического эндометрита пациенток с бесплодием. Акушерство и гинекология. 2022; 2: 91-100. [Tapilskaya N.I., Tolibova G.Kh., Savicheva A.M., Kopylova A.A., Glushakov R.I., Budilovskaya O.V., Krysanova A.A., Gorskii A.G., Gzgzyan A.M., Kogan I.Yu. The effectiveness of local cytokine therapy for chronic endometritis in patients with infertility. Obstetrics and Gynecology. 2022; (2): 91-100. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.2.91-100.
- Доброхотова Ю.Э., Ганковская Л.В., Боровкова Е.И., Зайдиева З.С., Скальная В.С. Модулирование локальной экспрессии факторов врожденного иммунитета у пациенток с хроническим эндометритом и бесплодием. Акушерство и гинекология. 2019; 5: 125-32. [Dobrokhotova Yu.E., Gankovskaya L.V., Borovkova E.I., Zaidieva Z.S., Skalnaya V.S. Modulation of the local expression of innate immune factors in patients with chronic endometritis and infertility. Obstetrics and Gynecology. 2019; (5): 125-32. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.5.125-132.
- Дикке Г.Б., Суханов А.А., Кукарская И.И., Остроменский В.В. Цитокиновый профиль пациенток с хроническим эндометритом и нарушением репродуктивной функции. Вопросы гинекологии, акушерства и перинатологии. 2021; 20(6): 82-91. [Dikke G.B., Sukhanov A.A., Kukarskaya I.I., Ostromensky V.V. Cytokine profile of patients with chronic endometritis and reproductive dysfunction. Gynecology, Obstetrics and Rerinatology. 2021; 20(6): 82-91. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2021-6-82-91.
- Суханов А.А., Дикке Г.Б., Остроменский В.В., Кукарская И.И., Шилова Н.В. Течение и исходы беременности, наступившей в результате экстракорпорального оплодотворения, у пациенток с хроническим эндометритом, получавших комплексное лечение с использованием препарата «Суперлимф» на прегравидарном этапе (рандомизированное контролируемое испытание «ТЮЛЬПАН 2»). Акушерство и гинекология. 2023; 8: 123-34. [Sukhanov A.A., Dikke G.B., Ostromensky V.V., Kukarskaya I.I., Shilova N.V. Course and outcomes of pregnancy following IVF in patients with chronic endometritis receiving complex treatment with the Superlymph medication at the preconception stage (TULIP 2 randomized controlled trial). Obstetrics and Gynecology. 2023; (8): 123-34. (inRussian)]. https://dx.doi.org/10.18565/aig.2023.190.
- Толибова Г.Х., Траль Т.Г., Клещеев М.А. Эндометриальная дисфункция: алгоритм клинико-морфологического исследования. Санкт-Петербург; 2016. 44 с. [Tolibova G.Kh., Tral T.G., Kleshcheev M.A. Endometrial dysfunction: algorithm for clinical and morphological research. Saint Petersburg; 2016. 44 p. (in Russian)].
- Khong Y., Cheung A.N.Y., Zheng W. Diagnostic Endometrial Pathology. 2nd. ed. Тaylor & Francis Group; 2019. 200 p. https://dx.doi.org/10.1201/9781315228686.
- Щеголев А.И. Современная морфологическая классификация повреждений плаценты. Акушерство и гинекология. 2016; 4: 16-23. [Shchegolev A.I. Current morphological classification of damages to the placenta. Obstetrics and Gynecology. 2016; (4): 16-23. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.4.16-23.
- Краснопольская К.В., Назаренко Т.А., Ершова И.Ю. Современные подходы к оценке рецептивности эндометрия (обзор литературы). Проблемы репродукции. 2016; 22(5): 61-9. [Krasnopol'skaia K.V., Nazarenko T.A., Ershova I.Yu. Modern approaches to endometrial receptivity assessment (a review). Russian Journal of Human Reproduction. 2016; 22(5): 61-9. (in Russian)]. https://dx.doi.org/10.17116/repro201622561-69.
- Beruchashvili M., Gogiashvili L., Datunashvili E., Topuria Z., Tsagareli Z. Morphological peculiarities of endometrium in chronic endometritis associated with bacterial vaginosis. Georgian Med. News. 2010; (181):59-63.
- Шилов А.В., Мнихович М.В., Лучинин В.В., Васин И.В., Снегур С.В., Казанцева Г.П., Соломатина Л.М. Патоморфологическая и иммуноморфологическая характеристика хронического эндометрита. Вестник новых медицинских технологий. Электронное издание. 2018; 4. [Shilov A.V., Mnikhovich M.V., Luchinin V.V., Vasin I.V., Snegur S.V., Kazantseva G.P., Solomatina L.M. Pathomorphological and immunomorphological characteristics of chronic endometritis. Bulletin of New Medical Technologies. Electronic edition. 2018; 4. (in Russian)]. https://dx.doi.org/10.24411/2075-4094-2018-16138.
- Газизова Г.Х., Ящук А.Г., Масленников А.В., Даутова Л.А., Батталова Г.Ю. Морфологические особенности эндометрия у пациенток с хроническим эндометритом атрофической формы и нарушением гемодинамики. Архив акушерства и гинекологии им. В.Ф. Снегирева. 2022; 9(4): 231-7. [Gazizova G.K., Yashchuk A.G., Maslennikov A.V., Dautova L.A., Battalova G.Y. Morphological features of the endometrium in patients with atrophic chronic endometritis and impaired hemodynamics. V.F. Snegirev Archives of Obstetrics and Gynecology. 2022; 9(4): 231-7]. https://dx.doi.org/10.17816/2313-8726-2022-9-4-231-237.
- Liu W.J., Huang J., Sun L., Huang L., Zhang Q.Y., Nong Y.Q. et al. New biopsy after antibiotic treatment: effect on outcomes of assisted reproduction in patients with infertility and chronic endometritis. Reprod. Biomed. Online. 2022; 45(6): 1167-75. https://dx.doi.org/10.1016/j.rbmo.2022.07.020.
- Толибова Г.Х., Траль Т.Г., Клещёв М.А., Кветной И.М., Айламазян Э.К. Эндометриальная дисфункция: алгоритм гистологического и иммуногистохимического исследования. Журнал акушерства и женских болезней. 2015; 64(4): 69-77. [Tolibova G.Kh., Tral T.G., Kleshchev M.A., Kvetnoy I.M., Ailamazyan E.K. Endometrial dysfunction: algorithm for histological and immunohistochemical. Journal of Obstetrics and Women's Diseases. 2015; 64(4): 69-77. (in Russian)]. https://dx.doi.org/10.17816/JOWD64469-77.
- Kaku S., Kubo T., Kimura F., Nakamura A., Kitazawa J., Morimune A. et al. Relationship of chronic endometritis with chronic deciduitis in cases of miscarriage. BMC Womens Health. 202; 20(1): 114. https://dx.doi.org/10.1186/s12905-020-00982-y.
- Yang J., Feng L. Intrauterine administration of autologous peripheral blood mononuclear cells (PBMCs) activated by HCG improves the implantation and pregnancy rates in patients with repeated implantation failure: a prospective randomized study. Am. J. Reprod. Immunol. 2016; 76(3): 212-6. https://dx.doi.org/10.1111/aji.12542.
- Амян Т.С., Перминова С.Г., Кречетова Л.В., Вторушина В.В. Эффективность внутриматочного введения аутологичных мононуклеарных клеток периферической крови перед переносом эмбриона у пациенток с повторными неудачами имплантации в программах вспомогательных репродуктивных технологий. Гинекология. 2018; 2: 28-33 [Amyan T.S., Perminova S.G., Krechetova L.V., Vtorushina V.V. Efficacy of intrauterine administration of autologous peripheral blood mononuclear cells before embryo transfer in patients with repeated implantation failures in assisted reproductive technology programs. Gynecology. 2018; (2): 28-33. (in Russian)]. https://dx.doi.org/10.26442/2079-5696_2018.2.28-33.
- Доброхотова Ю.Э., Боровкова Е.И., Скальная В.С., Ильязов Т.К., Рассохина О.В. Клинико-иммунологические параллели у пациенток с бесплодием и хроническим эндометритом до и после экзогенной цитокинотерапии. Акушерство и гинекология. 2019; 12: 154-60. [Dobrokhotova Yu.E., Borovkova E.I., Skalnaya V.S., Ilyazov T.K., Rassokhina O.V. Clinical and immunological parallels in patients with infertility and chronic endometritis before and after exogenous cytokine therapy. Obstetrics and Gynecology. 2019; (12): 154-60. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.12.154-160.
- Доброхотова Ю.Э., Ганковская Л.В., Боровкова Е.И., Нугуманова О.Р. Экзогенная цитокинотерапия в лечении пациенток с хроническим эндометритом. Акушерство и гинекология. 2021; 2: 119-26. [Dobrokhotova Yu.E., Gankovskaya L.V., Borovkova E.I., Nugumanova O.R. Exogenous cytokine therapy in the treatment of patients with chronic endometritis. Obstetrics and Gynecology. 2021; (2): 119-26. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.2.119-26.
- Yogeshkumar S., Dhananjay S., Gowdar S., Gowdar G., Kulkarni V., Byranahalli S. et al. Morphological study of the placenta in deliveries with pre-eclampsia: Results from a prospective, observational study in India and Pakistan (PURPOSe). BJOG. 2023; 130(Suppl. 3): 36-42. https://dx.doi.org/10.1111/1471-0528.17617.
- Jaiman S., Romero R., Bhatti G., Jung E., Gotsch F., Suksai M. et al. The role of the placenta in spontaneous preterm labor and delivery with intact membranes. J. Perinat. Med. 2022; 50(5): 553-66. https://dx.doi.org/10.1515/jpm-2021-0681.
- Малышкина А.И., Назарова А.О., Кулида Л.В., Козырина А.А., Жолобов Ю.Н., Назаров С.Б. Патоморфологические особенности плацент у женщин с преждевременными родами в зависимости от срока гестации. Акушерство, гинекология и репродукция. 2017; 11(4): 23-9. [Malyshkina A.I., Nazarova A.O., Kulida L.V., Kozyrina A.A., Zholobov Yu.N., Nazarov S.B. Pathomorphology of placenta in women with preterm births at different age of gestation. Obstetrics, Gynecology and Reproduction. 2017; 11(4): 23-9. (in Russian)]. https://dx.doi.org/10.17749/2313-7347.2017.11.4.023-029.
- Preston M., Hall M., Shennan A., Story L. The role of placental insufficiency in spontaneous preterm birth: A literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024; 295: 136-42. https://dx.doi.org/10.1016/j.ejogrb.2024.02.020.
- Vitagliano A., Saccardi C., Noventa M., Di Spiezio Sardo A., Saccone G., Cicinelli E. et al. Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: a systematic review and meta-analysis. Fertil. Steril. 2018; 110(1): 103-12.e1. https://dx.doi.org/10.1016/j.fertnstert.2018.03.017.
- Суханов А.А., Дикке Г.Б., Остроменский В.В., Кукарская И.И., Шилова Н.В. Предикторы успеха экстракорпорального оплодотворения с криопереносом у пациенток с хроническим эндометритом по данным лазерного конверсионного тестирования. Проблемы репродукции. 2024; 30(2): 62-73. [Sukhanov A.A., Dikke G.B., Ostromensky V.V., Kukarskaya I.I., Shilova N.V. Predictors of success of in vitro fertilization with cryotransfer in patients with chronic endometritis according to laser conversion testing. Russian Journal of Human Reproduction. 2024; 30(2): 62-73. (in Russian)]. https://dx.doi.org/10.17116/repro20243002162.
- Суханов А.А., Дикке Г.Б., Мудров В.А., Кукарская И.И. Прогнозирование успеха экстракорпорального оплодотворения у пациенток с хроническим эндометритом и нарушением репродуктивной функции с помощью нейросетевой технологии (вторичный анализ результатов рандомизированного контролируемого испытания «ТЮЛЬПАН 2»). Акушерство и гинекология. 2024; 4: 103-14. [Sukhanov A.A., Dikke G.B., Mudrov V.A., Kukarskaya I.I. Predicting the success of in vitro fertilization in patients with chronic endometritis and reproductive disorders using neural network technology (secondary analysis of the results of the TULIP-2 randomized controlled trial). Obstetrics and Gynecology. 2024; (4): 103-14. (in Russian)]. https://dx.doi.org/10.18565/aig.2024.47.
Received 17.05.2024
Accepted 24.05.2024
About the Authors
Anton A. Sukhanov, PhD, Head of the Department of Family Planning and Reproduction, Tyumen Perinatal Center, 1 Daudelnaya str., Tyumen, 625002, Russia;Associate Professor, Department of Obstetrics and Gynecology, Tyumen State Medical University, Ministry of Health of Russia, 10 Permyakov str., Tyumen,
625013, Russia, such-anton@yandex.ru, https://orcid.org/0000-0001-9092-9136
Galina B. Dikke, Dr. Med. Sci., Professor of the Department of Obstetrics and Gynecology with a Course of Reproductive Medicine, F.I. Inozemtsev Academy of Medical Education, 22 Liter M, Moskovskiy Ave., Saint Petersburg, 190013, Russia, galadikke@yandex.ru, https://orcid.org/0000-0001-9524-8962
Irina I. Kukarskaya, Dr. Med. Sci., Professor of the Department of Obstetrics, Gynecology and Reanimatology with a Course of Clinical Laboratory Diagnostics, Tyumen State Medical University, Ministry of Health of Russia, 10 Permyakov str., Tyumen, 625013, Russia; Chief Physician, Tyumen Region Perinatal Center, 1 Daudelnaya str., Tyumen, 625002, Russia; Chief Specialist in Obstetrics and Gynecology, Department of Health of the Tyumen Region, kukarskay@mail.ru, https://orcid.org/0000-0002-8275-3553
Anastasia V. Pesotskaya, Head of the Pathological Laboratory with the Department of Immunohistochemistry, Altai Regional Clinical Perinatal Center,
656045, Russia, Altai Territory, Barnaul, Fomina str., 154, pesokpesotskaya@yandex.ru, https://orcid.org/0000-0002-4625-0916
Corresponding author: Anton A. Sukhanov, such-anton@yandex.ru



