The use of androgen priming in infertile women with diminished ovarian reserve undergoing assisted reproductive technology (IVF/ICSI)
Objective: To study the effectiveness of androgen priming in infertile women with diminished ovarian reserve (DOR) undergoing assisted reproductive technology (ART) treatment. Materials and methods: A prospective cohort study included infertile women with DOR undergoing in vitro fertilization (IVF) treatment. According to the ESHRE criteria (Bologna Criteria, 2011), the study group included 305 women; among them there were 203 patients who received androgen priming during one month prior to the IVF/ICSI program. The hormonal study including the androgen fractions in the blood serum was carried out using immunochemiluminescent assay (ICMA) in all women. Results: The use of androgen priming in infertile women with DOR led to statistically significant improvements in the parameters of oogenesis and embryogenesis. The use of dehydroepiandrosterone (DHEA) revealed statistically significant differences in the number of oocytes from 2.9 (0.3) to 4.4 (0.3), including mature oocytes from 1.8 (1) to 3.3 (1.05), zygotes from 1.2 (0.6) to 2.1 (0.7), blastocysts from 0.8 (0.4) to 1.4 (0.6), and the frequency of blastula formation from 27.6% to 31.8% (p<0.001). There was an increase in the pregnancy rate from 8.6% to 11.4% (p=0.32). The use of testosterone-containing gel also revealed statistically significant differences in the number of oocytes from 3.0 (0.5) to 4.1 (0.5) and mature oocytes from 1.74 (0.5) to 3.4 (0.6), zygotes from 1.1 (0.4) to 2.2 (0.6), blastocysts from 0.6 (0.3) to 1.5 (0.7), the frequency of blastula formation from 20.0% to 36.6% and pregnancy rate as well (p<0.001); however, pregnancy rate was found to be higher (14%) in this group after receiving therapy compared to the DHEA group. Conclusion: A decrease in the level of androgens in the blood serum is associated with impaired folliculogenesis, oogenesis, embryogenesis and leads to reduced effectiveness of the IVF/ICSI program. The study demonstrated an increase in the number of mature oocytes, the percentage of fertilization and the number of blastocysts of good and excellent quality, which can be the main factors leading to the clinical effectiveness of ART programs; however, there was no statistically significant difference in the pregnancy rate between the groups of patients who received treatment.Gavisova A.A., Kamaletdinov N.S., Nazarenko T.A., Dolgushina N.V.
Keywords
Despite the active development of technologies in reproductive medicine at the beginning of the XXI century, the rate of diminished ovarian reserve (DOR) in infertile women undergoing in vitro fertilization (IVF) amounts from 9 to 24% and it continues to increase [1, 2].
In order to improve the results of IVF/ICSI programs, various techniques have been developed. The administration of high doses of gonadotropins, modified ovarian stimulation protocol, DuoStim, combinations of various neoadjuvant therapy regimens made it possible to choose personalized therapy [3]. One of the promising areas of neoadjuvant therapy has recently been the use of androgens before and/or during ovarian stimulation, since DOR is associated with a decrease in the level of androgens, including testosterone, according to some Russian and international studies [4, 5].
Numerous studies show that women after 30–35 years have a decrease in the synthesis of androgens and this is clearly seen in the model of ovarian hyperandrogenism in polycystic ovary syndrome (PCOS) [6]. This process involves organs and systems where one can observe the expression of androgen receptors that initiate various functional activities, including sexual ones, as part of the reproductive process.
According to the “two cells – two gonadotropins” theory, androgens play an important role in ensuring steroidogenesis in the ovaries [7]; they are substrates for the aromatase activity of granulosa cells, and when they are converted into estrogens, they have a direct autocrine and/or paracrine effect on the regulation of folliculogenesis through the receptors with the same name in the ovaries [8, 9]. The number and expression of androgen receptors change at various stages of folliculogenesis, their number decreases gradually during the transition of the follicle to its final stage of maturation [10]. The expression of androgen receptors positively correlates with the number and expression of markers of cell proliferation in the ovaries and negatively correlates with the rate of cellular apoptosis [11].
The existing data on the role of androgens in the maturation of antral follicles suggest their influence on aromatase activity in preovulatory follicles [12–14], and the initiation of ovulatory peak. However, the role of androgens in folliculogenesis continues to be a subject for the discussion. Androgens are known to cause follicle atresia [15, 16]; they demonstrate a synergistic effect with follicle-stimulating hormone (FSH), stimulate the synthesis of insulin-like growth factor-1 (IGF-1) and promote follicle recruitment. There are changes similar to the morphological picture of multifollicular ovaries even after short-term use of exogenous androgens in ovarian tissue: the total number of preantral and antral follicles increases by 2.5–4.5 times [17–19].
In order to improve the results of assisted reproductive technologies (ART), various doses of androgen-containing drugs were used in different studies on the role of androgen priming. At the early and intermediate stages of follicle maturation, the addition of testosterone as priming can improve the transformation from a dormant into a growing pool [20] and increase the number of preantral and antral follicles. There is evidence of a possible effect of testosterone on the increased expression of FSH receptors in granulosa cells, and therefore potential increase in the sensitivity of the ovaries to FSH. This study evaluated the effect of androgenic priming, namely testosterone-containing gel and tablet form of dehydroepiandrosterone (DHEA) in women with DOR and infertility undergoing ART treatment (IVF/ICSI) [21].
The aim of this study was to determine and evaluate the therapeutic effect of androgenic priming, DHEA and testosterone, on the outcomes of ART programs (IVF/ICSI) in women with infertility and DOR.
Materials and methods
The study was conducted on the basis of the 1st Gynecological Department, Institute of Reproduction, National Medical Research Center for Obstetrics, Gynecology and Perinatology in Moscow according to the Order of the Ministry of Health of the Russian Federation No. 803n. The study initially included 380 women with infertility and DOR, but 75 of them were excluded before random distribution because they did not meet the following inclusion criteria: age under 43 years, a history of DOR (AMH≤1.2 ng/ml and CAF≤5 on the 3rd day of the menstrual cycle), the absence of embryos of good and excellent quality according to the findings of embryological stage in previous IVF/ICSI programs; they decided to achieve conception with donor oocytes or stopped treatment before ovarian stimulation (Figure). The exclusion criteria were surgical interventions on the ovaries, endometrioid ovarian cysts, oncological diseases, endocrine and metabolic disorders in the history.
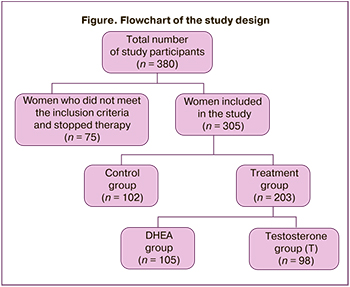
Figure shows a flowchart of women included in the study: 305 patients out of 380 women with DOR met the inclusion criteria. All women were randomly divided into two groups: treatment group and control group. The treatment group consisted of 203 women who were recommended androgen priming: group T (n=98) included patients who were administered 1% testosterone-containing gel (Androgel) and group DHEA (n=105) included patients who received DHEA. The control group included 102 women who did not receive therapy.
The patients in group T applied 12.5 mg of transdermal 1% testosterone (Androgel 50 mg, Besin Healthcare, Belgium) to clean, dry, healthy skin on both shoulders once a day in the evening for 3 months. The patients in the DHEA group took a tablet of DHEA 25 mg 3 times a day for 3 months. If contraception was necessary, all women used the barrier method for 3–6 months before undergoing the IVF treatment.
The patients of all groups underwent ovarian stimulation in a protocol with gonadotropin-releasing hormone (GnRH) antagonists. Ultrasound examination in the IVF/ICSI program was performed using GE Voluson P6 ultrasound machine (2018, Korea) with 5–7 MHz transvaginal probe. Embryo cultivation was carried out in G1-PLUS/G2-PLUS sequential media (Vitrolife, Västra Frölunda, Sweden), embryos were classified according to the standard morphological assessment (ALPHA Consensus, 2011) depending on the number and size of cells, fragmentation and quality of the embryo. The assessment of the blastocyst quality was carried out according to the Gardner and Schoolcarft system. Blastocysts assessed as ≥3BB were classified as embryos of excellent quality. Embryo transfer was performed on the 5th day of cultivation, at the blastocyst stage. In 10 days after embryo transfer, pregnancy was diagnosed on the basis of serum concentration of beta subunits of human chorionic gonadotropin (β-hCG). The pregnancy test was evaluated as positive when the level of β-hCG was more than 20 IU/L.
Laboratory tests were carried out on the basis of the clinical and diagnostic laboratory of the National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia. On the 2nd–3rd day of the menstrual cycle, venous blood was taken from the ulnar vein on in the morning on an empty stomach using S-Monovette blood collection system (Sarstedt, Germany). The serum obtained by centrifugation at 3000 rpm for at least 10 minutes with the Eppendorf 5804 apparatus (Germany) was used for analysis no later than one hour after blood collection. When additional studies were necessary, aliquots of blood serum samples were taken and stored at -70°C. The androgen profile was determined using immunochemiluminescent assay (ICLA) in blood serum with an automatic immunochemical analyzer Cobas e411 (Roche Diagnostics GmbH, Germany) and commercial kits from this manufacturer.
Statistical analysis
Statistical data processing was performed using Excel spreadsheets and Statistica M10 software package (StatSoft Inc., USA). Quantitative indicators were checked for normal distribution using the Kolmogorov–Smirnov test; the results are presented in the form of mean values and standard deviation M(SD). Qualitative indicators are presented in the form of absolute and relative values. Initial characteristics and outcomes in groups were compared using ANOVA for continuous variables (in comparison of three groups) and Student’s t-test (for two groups), the chi-square test (χ2), as well as the Fisher’s exact test for proportions. The changes in indicators during treatment were evaluated using the Student’s t-test. The value of p<0.05 was considered statistically significant; in case of applying the Bonferroni correction for multiple comparison of indicators between groups, the level of p<0.017 was considered significant.
The protocol of this study No.2 was approved the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, dated February 07, 2019.
Results
This was a randomized controlled study of 305 women with infertility and DOR who presented to the Center to achieve pregnancy and childbirth.
The patients of both groups were initially compared for average age, body mass index (BMI), ovarian reserve parameters, hormones evaluated on the 2nd-3rd day of the menstrual cycle, pelvic ultrasound examination and the number of antral follicles (CAF) (p>0.05) (Table 1). The average age was 37.6 (4.9) years. All women had a regular menstrual cycle. The average BMI was 23.5 (4.2) kg/m2, the average duration of infertility was 5.7 (2.0) years.
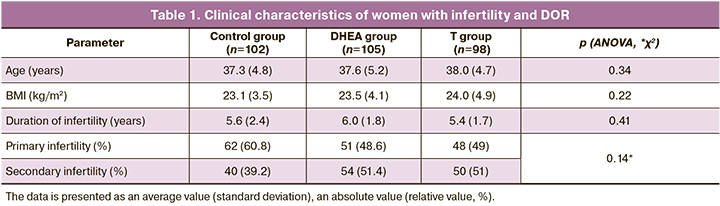
The parameters of the hormonal profile in the treatment group were compared and assessed before and after three months of androgen priming.
The average values of hormones were compared and analyzed according to the ICLA in the blood serum in the early follicular phase; their analysis showed lower concentrations of total testosterone at baseline, whereas its level increased from 0.29 (0.05) to 0.39 (0.05) nmol/L (p<0.001) after one month of androgenic DHEA priming (Table 2). The concentration of androstenedione significantly differed before and after DHEA priming; it was 1.2 (0.1) and 5.2 (0.15) nmol/L before and after treatment, respectively (p<0.001). AMH fluctuation was observed from 0.84 (0.2) ng/ml to an average of 1.1 (0.25) ng/ml in the DHEA group, but it was not statistically significant (p=0.34).
After one-month administration of the testosterone-containing gel, there was a statistically significant increase in the concentration of total testosterone from 0.4 (0.2) to 0.44 (0.05) ng/ml (p<0.001). The analysis of androstenedione concentration after androgen priming showed that the patients in the testosterone group also had statistically significant changes from 1.3 (0.6) to 5.6 (0.6) nmol/L (p<0.001). There were no statistically significant differences in AMH in this group (Table 3).
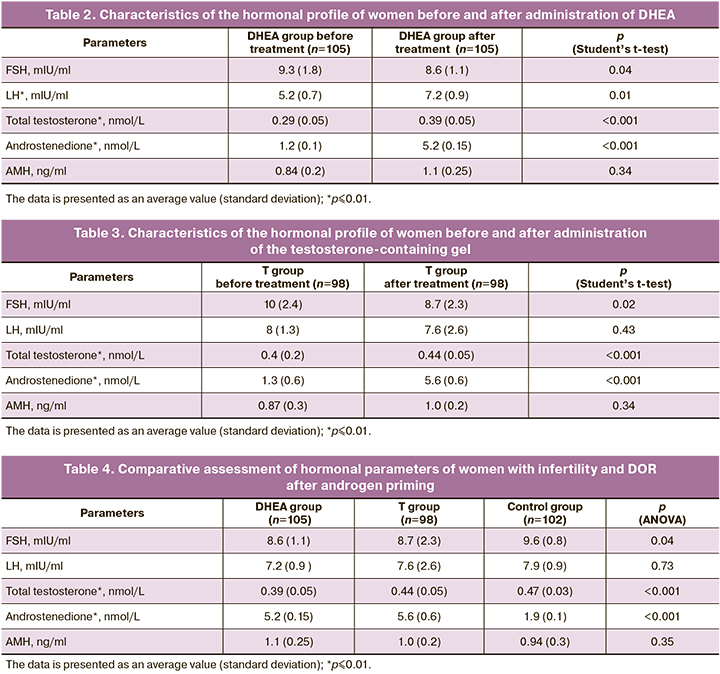
The comparative analysis of the hormonal profile of the treatment group with the control group showed statistically significant differences in the androstenedione index (p<0.001) (Table. 4), which means that androstenedione, as a precursor of androgens, can be a predictor for the diagnosis of androgen deficiency.
The comparative analysis of the indicators of oogenesis and embryogenesis was carried out before and after androgen priming to assess the effect of the androgen profile on the embryological stage.
The analysis of the embryological stage after administration of DHEA revealed statistically significant differences in the number of oocytes from 2.9 (0.3) to 4.4 (0.3), including mature ones from 1.8 (1) to 3.3 (1.05), zygotes from 1.2 (0.6) to 2.1 (0.7), blastocysts from 0.8 (0.4) to 1.4 (0.6), blastulation rate from 27.6% to 31.8% (p<0.001) (Table 5). There was an increase in the pregnancy rate from 8.6% to 11.4% (p=0.32).
The analysis of the embryological stage after administration of testosterone-containing gel also revealed statistically significant differences in the number of oocytes from 3.0 (0.5) to 4.1 (0.5) and mature oocytes from 1.74 (0.5) to 3.4 (0.6), zygotes from 1.1 (0.4) to 2.2 (0.6), blastocysts from 0.6 (0.3) to 1.5 (0.7), blastulation rate from 20.0% to 36.6% and pregnancy rate (p<0.001); however, a higher pregnancy rate was detected in this group, namely 14% after therapy compared to the DHEA group (Table 6).
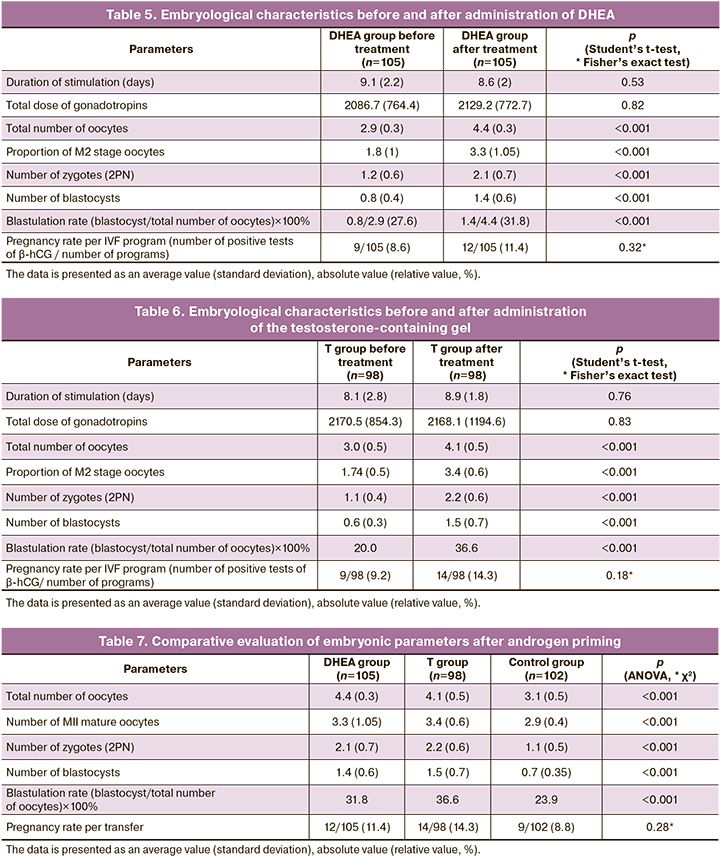
The comparison of the parameters of the embryological stage in the treatment group after 3 months of androgen priming with the control group revealed a statistically significant change in the number of oocytes, including mature ones, an improvement in early embryogenesis and blastulation, which may be evident of the positive effect of androgen therapy on early ART outcomes (Table 7).
The use of androgenic priming had no effect on the duration of the stimulation and the total dose of gonadotropins in all the study groups. The use of androgen priming did not cause any side effects.
Discussion
We carried out the research on the effect of androgen priming on the results of IVF/ICSI programs in reproductive-aged women with infertility and DOR. The choice of rational therapy in this cohort of patients is debatable [22, 23].
According to our data, administration of androgen priming during IVF/ICSI programs results in a higher number of mature oocytes, higher percentage of fertility and number of blastocysts and a statistically significantly higher percentage of clinical pregnancy; these findings are consistent with the results of numerous studies on the effect of androgen priming on ART outcomes. For instance, Davison S.L. et al. revealed a direct positive correlation between the concentration of testosterone and the number of aspirated oocytes [24].
The Cochrane review (2015) showed that androgenic priming with both DHEA and testosterone improves the effectiveness of IVF/ICSI treatment in women with DOR and infertility and increases the rate of pregnancy maintenance after taking DHEA up to 15–26% (instead of 12% when using placebo) and after taking testosterone up to 10–32% (instead of 8% when using placebo).
According to the meta-analysis of 2022, the use of DHEA did not influence the effectiveness of the IVF program, but androgenic priming with testosterone increased the number of oocytes, as well as the rate of clinical pregnancy and live birth [26].
Our study did not reveal any side effects from the use of the medications administered at these doses, although hair loss, hirsutism, tendency to acne and an increase in the activity of the sebaceous glands were described in the literature after taking DHEA at the dose of 75 mg [4, 27]. Singh A.B. et al. noted that the application of transdermal gel resulted in episodes of acne, increased activity of the sebaceous glands, hirsutism [28]; however, most studies do not report significant side effects [29, 30].
The duration of the treatment course with androgens may be crucial for the induction of folliculogenesis and their growth. But the data on the optimal duration of androgen use has not been presented yet due to the heterogeneity of groups of women with diminished ovarian reserve. The most common duration of androgenic priming is from 4 to 12 weeks at a dose of 50–100 mg [31]; in our study priming in both groups was used for 3 months. According to Yeung T.W.Y.et al., priming with DHEA increased serum concentrations of testosterone (T) and dehydroepiandrosterone sulfate (DHEA-S) after 3–4 weeks, but it did not affect the follicular concentrations of these hormones, and it increased intrafollicular concentrations of DHEA-S after 16 weeks [32]. The duration of follicle development from 70 to 120 days explains the need for more prolonged therapy in women with DOR and infertility [33]. A further promising direction for the study of androgenic priming in ART programs is its stratification according to the duration of use and selection of the most effective duration of the course.
Conclusion
Thus, according to the results of the study, we concluded that the personification of the treatment of women with infertility and DOR using androgenic priming, both DHEA and testosterone-containing gel, leads to an improvement in the indicators of the embryological stage and increase in the effectiveness of IVF/ICSI programs compared to the group of women who did not receive therapy. These findings confirm the advantages of androgens as “conductors” of folliculogenesis and show their clinical effectiveness when they are administered in due time to women with infertility and DOR.
References
- Jeve Y., Bhandari H. Effective treatment protocol for poor ovarian response: a systematic review and meta-analysis. J. Hum. Reprod. Sci. 2016; 9(2):70-81. 9(2):70-81. https://dx.doi.org/10.4103/0974-1208.183515.
- Ferraretti A.P., La Marca A., Fauser B.C.J.M., Tarlatzis B., Nargund G., Gianaroli L. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum. Reprod. 2011; 26(7): 1616-24. https://dx.doi.org/10.1093/humrep/der092.
- MacLaughlin D.T., Donahoe P.K. Sex determination and differentiation. N. Engl. J. Med. 2004; 350(4): 367-78. https://dx.doi.org/10.1056/NEJMra022784.
- Neves A.R., Montoya-Botero P., Polyzos N.P. The role of androgen supplementation in women with diminished ovarian reserve: time to randomize, not meta-analyze. Front. Endocrinol. (Lausanne). 2021; 12: 653857.https://dx.doi.org/10.3389/fendo.2021.653857.
- Richardson A., Jayaprakasan K. The use of androgen priming in women with reduced ovarian reserve undergoing assisted reproductive technology. Semin. Reprod. Med. 2021; 39(5-06): 207-19.https://dx.doi.org/10.1055/s-0041-1735646.
- Astapova O., Minor B.M.N., Hammes S.R. Physiological and pathological androgen actions in the ovary. Endocrinology. 2019; 160(5): 1166-74.https://dx.doi.org/10.1210/en.2019-00101.
- Ryan K.J., Petro Z., Kaiser J. Steroid formation by isolated and recombined ovarian granulosa and tehcal cells. J. Clin. Endocrinol. Metab. 1968; 28(3):355-8. https://dx.doi.org/10.1210/jcem-28-3-355.
- Horie K., Takakura K., Fujiwara H. Immunohistochemical localization of androgen receptor in the human ovary throughout the menstrual cycle in relation to oestrogen and progesterone receptor expression. Hum. Reprod. 1992; 7(2): 184-90. https://dx.doi.org/ 10.1093/oxfordjournals.humrep.a137614.
- Suzuki T., Sasano H., Kimura N., Tamura M., Fukaya T., Yajima A., Nagura H. Immunohistochemical distribution of progesterone, androgen and oestrogen receptors in the human ovary during the menstrual cycle: relationship to expression of steroidogenic enzymes. Hum. Reprod. 1994; 9(9): 1589-95. https://dx.doi.org/10.1093/oxfordjournals.humrep.a138757.
- Hillier S.G., Tetsuka M., Fraser H.M. Location and developmental regulation of androgen receptor in primate ovary. Hum. Reprod. 1997; 12(1): 107-11.https://dx.doi.org/10.1093/humrep/12.1.107.
- Weil S.J., Vendola K., Zhou J., Adesanya O.O., Wang J., Okafor J., Bondy C.A. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J. Clin. Endocrinol. Metab. 1998; 83(7): 2479-85. https://dx.doi.org/10.1210/jcem.83.7.4917.
- Harlow C.R., Shaw H.J., Hillier S.G., Hodges J.K. Factors influencing follicle-stimulating hormone-responsive steroidogenesis in marmoset granulosa cells: effects of androgens and the stage of follicular maturity. Endocrinology. 1988; 122(6): 2780-7. https://dx.doi.org/10.1210/endo-122-6-2780.
- Shaw H.J., Hillier S.G., Hodges J.K. Developmental changes in luteinizing hormone/human chorionic gonadotropin steroidogenic responsiveness in marmoset granulosa cells: effects of follicle-stimulating hormone and androgens. Endocrinology. 1989; 124(4): 1669-77. https://dx.doi.org/10.1210/endo-124-4-1669.
- Hillier S.G., Ross G.T. Effects of exogenous testosterone on ovarian weight, follicular morphology and intraovarian progesterone concentration in estrogen-primed hypophysectomized immature female rats. Biol. Reprod. 1979; 20(2): 261-8.
- Billig H., Furuta I., Hsueh J.W. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology. 1993; 133(5): 2204-12.https://dx.doi.org/10.1210/endo.133.5.8404672.
- Vendola K.A., Zhou J., Adesanya O.O., Weil S.J., Bondy C.A. Androgens stimulate early stages of follicular growth in the primate ovary. J. Clin. Invest. 1998; 101(12): 2622-9. https://dx.doi.org/10.1172/JCI2081.
- Spinder T., Spijkstra J., Van Den Tweel J. The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J. Clin. Endocrinol. Metab. 1989; 69(1): 151-7. https://dx.doi.org/10.1210/jcem-69-1-151.
- Jonard S., Robert Y., Cortet-Rudelli C., Pigny P., Decanter C., Dewailly D. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum. Reprod. 2003; 18(3): 598-603. https://dx.doi.org/10.1093/humrep/deg115.
- Pigny P., Merlen E., Robert Y., Cortet-Rudelli C., Decanter C., Jonard S., Dewailly D. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J. Clin. Endocrinol. Metab. 2003; 88(12): 5957-62.https://dx.doi.org/10.1210/jc.2003-030727.
- Gervásio C.G., Bernuci M.P., Silva-de-Sá M.F., de Rosa-e-Silva A.C.J.S. The role of androgen hormones in early follicular development. ISRN Obstet. Gynecol. 2014; 2014 : 818010. https://dx.doi.org/10.1155/2014/818010.
- Casson P.R., Lindsay M.S., Pisarska M.D., Carson S.A., Buster J.E. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum. Reprod. 2000; 15(10): 2129-32.https://dx.doi.org/10.1093/humrep/15.10.2129.
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum. Reprod. 2011; 26(6):1270-83. https://dx.doi.org/ 10.1093/humrep/der037.
- Slater C.C., Souter I., Zhang C., Guan C., Stanczyk F.Z., Mishell D.R. Pharmacokinetics of testosterone after percutaneous gel or buccal administration. Fertil. Steril. 2001; 76(1): 32-7. https://dx.doi.org/10.1016/s0015-0282(01)01827-1.
- Davison S.L., Bell R., Donath S., Montalto J.G., Davis S.R. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab. 2005; 90(7): 3847-53. https://dx.doi.org/10.1210/jc.2005-0212.
- Nagels H.E., Rishworth J.R., Siristatidis C.S., Kroon B. Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction. Cochrane Database Syst. Rev. 2015; 11: CD009749.
- Neves A.R., Montoya-Botero P., Polyzos N.P. Androgens and diminished ovarian reserve:the long road from basic science to clinical implementation. A comprehensive and systematic review with meta-analysis. Am. J. Obstet. Gynecol. 2022; 227(3): 401-413.e18. https://dx.doi.org/10.1016/j.ajog.2022.03.051.
- Yeung T.W.Y., Li R.H.W., Lee V.C.Y., Ho P.C., Ng E.H.Y. A randomized double-blinded placebo-controlled trial on the effect of dehydroepiandrosterone for 16 weeks on ovarian response markers in women with primary ovarian insufficiency. J,. Clin. Endocrinol. Metab. 2013; 98(1): 380-8. https://dx.doi.org/10.1210/jc.2012-3071.
- Singh A.B., Lee M.L., Sinha-Hikim I., Kushnir M., Meikle W., Rockwood A. et al. Pharmacokinetics of a testosterone gel in healthy postmenopausal women. J. Clin. Endocrinol. Metab. 2006; 91(1): 136-44. https://dx.doi.org/10.1210/jc.2005-1640.
- Fabregues F., Penarrubia J., Creus M., Manau D., Casals G., Carmona F., Balasch J. Transdermal testosterone may improve ovarian response to gonadotrophins in low-responder IVF patients: a randomized, clinical trial. Hum. Reprod. 2009; 24(2): 349-59. https://dx.doi.org/10.1093/humrep/den428.
- Kim C.H., Howles C.M., Lee H.A. The effect of transdermal testosterone gel pretreatment on controlled ovarian stimulation and IVF outcome in low responders. Fertil. Steril. 2011; 95(2): 679-83. https://dx.doi.org/10.1016/j.fertnstert.2010.07.1077.
- Kim C.H., Ahn J.W., Moon J.W., Kim S.H., Chae H.D., Kang B.M. Ovarian features after 2 weeks, 3 weeks and 4 weeks transdermal testosterone gel treatment and their associated effect on IVF outcomes in poor responders. Dev. Reprod. 2014; 18(3): 145-52. https://dx.doi.org/10.12717/DR.2014.18.3.145.
- Amirikia H., Savoy-Moore R.T., Sundareson A.S., Moghissi K. The effects of long-term androgen treatment on the ovary. Fertil. Steril. 1986; 45(2): 202-8.https://dx.doi.org/10.1016/s0015-0282(16)49155-7.
- Burger H.G. Androgen production in women. Fertil. Steril. 2002; 77(Suppl. 4): S3-5. 1 https://dx.doi.org/10.1016/s0015-0282(02)02985-0.
Received 02.09.2022
Accepted 13.09.2022
About the Authors
Alla A. Gavisova, Ph.D., Senior Researcher at the 1st Gynecological Department. Academician V.I. Kulakov National Medical Research Center for Obstetrics,Gynecology and Perinatology, Ministry of Health of Russia, +7(916)829-05-90, gavialla@yandex.ru, 117997, Russia, Moscow, Akademika Oparina str., 4.
Nail S. Kamaletdinov, Embryologist at the 1st Gynecological Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, sunsh86@mail.ru, 117997, Russia, Moscow, Akademika Oparina str., 4.
Tatiana A. Nazarenko, Dr. Med. Sci., Professor, Director of the Institute of Reproductive Medicine, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)531-44-44, t_nazarenko@oparina4.ru, https://orcid.org/0000-0002-5823-1667,
117997, Russia, Moscow, Akademika Oparina str., 4.
Natalia V. Dolgushina, Dr. Med. Sci., Professor, Deputy Director – Head of the Department of Organization of Scientific Activities, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, n_dolgushina@oparina4.ru, 117997, Russia, Moscow, Akademika Oparina str., 4.
Authors’ contributions: Gavisova A.A., Dolgushina N.V. – developing the design of the study; Gavisova A.A. – collecting and processing the material, review on the topic of the article, writing the work, final approval of the version for publication; Kamaletdinov N.S. – embryological stage; Nazarenko T.A., Dolgushina N.V. – editing the text.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The study was carried out without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, dated February 07, 2019.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Gavisova A.A., Kamaletdinov N.S., Nazarenko T.A., Dolgushina N.V.
The use of androgen priming in infertile women with diminished ovarian reserve undergoing assisted reproductive technology (IVF/ICSI).
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 9: 94-101 (in Russian)
https://dx.doi.org/10.18565/aig.2022.9.94-101



