Amino acid profile of blood plasma and follicular fluid in women with infertility and diminished ovarian reserve
Shevtsova M.A., Gavisova A.A., Krasnova N.A., Aksenenko A.A., Novoselova A.V., Khazzhar F., Chagovets V.V., Frankevich V.E., Nazarenko T.A.
Objective: This study aimed to investigate the amino acid profiles of blood plasma and follicular fluid in women with infertility and diminished ovarian reserve.
Materials and methods: The study included 115 women aged 18–42 years with infertility, referred to as V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia for infertility treatment using ART (IVF/ICSI) and meeting the inclusion criteria. The groups were stratified according to ovarian reserve as follows: group 1 – 50 patients with diminished ovarian reserve (AMH <1.2 ng/ml, AFC<5); group 2 – 65 patients with normal ovarian reserve (AMH ≥1.2 ng/ml, AFC≥5) and matched for age. Analysis of amino acid levels in the blood plasma and follicular fluid on the day of oocyte aspiration was conducted using liquid chromatography with mass spectrometric detection.
Results: Analysis of the amino acid profile revealed a statistically significant decrease in sarcosine and tryptophan in women with diminished ovarian reserve compared to women with normal ovarian reserve. Additionally, there was a statistically significant decrease in the concentrations of lysine, asparagine, methionine, phenylalanine, and tryptophan in the follicular fluid of women with diminished ovarian reserve. Correlation analysis showed a strong correlation between amino acid levels in the plasma and follicular fluid, as well as between amino acid levels in the plasma and follicular fluid. It also revealed a moderate positive correlation between antral follicle counts and indicators of oogenesis and early embryogenesis, and the levels of amino acids, specifically lysine and sarcosine in blood plasma and asparagine, tryptophan, and aminobutyric acid in follicular fluid.
Conclusion: This study found a significant reduction in amino acid levels in infertile women with diminished ovarian reserve, which may serve as a marker for assessing ovarian reserve and oocyte development potential to enhance outcomes in ART programs.
Authors' contributions: Gavisova A.A., Shevtsova M.A. – data collection and analysis, review of the relevant literature, drafting of the manuscript, final approval for submission, design of the study; Novoselova A.V. – LC-MS analysis of amino acids in blood plasma and follicular fluid, processing of experimental data; Khazhzhar F. – blood plasma samples preparation and analysis; Chagovets V.V., Frankevich V.E. – statistical analysis, review of the manuscript, final approval for submission; Krasnova N.A., Aksenenko A.A. – collection of material, review of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Shevtsova M.A., Gavisova A.A., Krasnova N.A., Aksenenko A.A., Novoselova A.V., Khazzhar F., Chagovets V.V., Frankevich V.E., Nazarenko T.A. Amino acid profile of blood plasma and follicular fluid in women with infertility and diminished ovarian reserve.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (2): 79-88 (in Russian)
https://dx.doi.org/10.18565/aig.2023.270
Keywords
Diminished ovarian reserve (DOR) is one of the most common and challenging causes of infertility, resulting in lower pregnancy and live birth rates in assisted reproductive technology (ART) programs than in women with normal ovarian reserve (NOR) [1]. Numerous etiological and pathogenetic mechanisms contribute to the reduction of ovarian reserve, including hormones, genes, and receptors, which consist of protein molecules with structural elements made up of amino acids, and are involved at every stage of folliculogenesis.
Follicular fluid acts as a microenvironment for the growth and development of follicles and oocytes, facilitating the exchange of substances and energy between oocytes and the extracellular environment, which potentially reflects the level of metabolism and development potential of oocytes [2]. The metabolites contained in follicular fluid provide crucial information about the growth and differentiation of follicles [3], including hormones, growth factors, cytokines, proteins, steroids, amino acids, and polysaccharides [4]. Therefore, studying the changes in follicular fluid metabolites can identify factors influencing oocyte development, serving as potential predictors of competence in embryo development and pregnancy outcomes in patients with DOR [5].
The technology enabling qualitative and quantitative assessment of low-molecular-weight compounds in biological materials is defined as metabolomic analysis. This method allows the assessment of cellular metabolism at the molecular level using liquid chromatography with mass spectrometry (LC-MS) [6, 7]. Different stages of follicle development are characterized by distinct metabolomic characteristics, which vary depending on the woman's age [8]. In recent years, researchers have focused on studying the amino acid composition of follicular fluid as biomolecules involved in the pathogenesis of reproductive system diseases, including DOR, polycystic ovary syndrome, and endometriosis [9, 10], to identify potential diagnostic and prognostic biomarkers, as disorders in amino acid metabolism may lead to changes in folliculogenesis and oogenesis [11, 12].
Amino acids are organic substances containing an amino group (-NH2) and an acid group (-COOH) (Fig. 1). Owing to differences in their side chains, amino acids possess unique biochemical properties and functions [13].
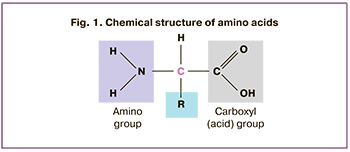
Of the more than 300 amino acids found in nature, only 20 serve as building blocks of proteins. Non-protein amino acids also play essential roles in cellular metabolism. Researchers have been interested in the role of amino acids in the implementation of reproductive functions at all stages. Research has demonstrated that both deficiency and excess of individual amino acids can suppress or increase gene expression, thereby regulating protein synthesis and numerous hormones at all stages of metabolism [15]. For instance, tyrosine and phenylalanine serve as substrates for the synthesis of adrenaline, norepinephrine, dopamine, and thyroid hormones [16]. High concentrations of amino acids, including arginine, glutamine, and leucine, stimulate hormone secretion when administered intravenously at pharmacological doses 10–20 times higher than their nutritional concentrations [17, 18].
In women with DOR, studies of the amino acid profile in follicular fluid revealed significant changes associated with the number of oocytes and quality of embryos in ART programs, indicating a significant role of amino acids. A metabolomic profile can be used to assess the ovarian reserve and predict embryonic development. Amino acids, especially leucine, glutamine, arginine, and proline, play important roles in embryogenesis and development of the placenta and fetus during pregnancy [1, 19].
This study aimed to investigate the amino acid profiles of blood plasma and follicular fluid in women with infertility and diminished ovarian reserve compared to those in women with normal ovarian reserve.
Materials and methods
This observational, cross-sectional case-control study included 115 infertile women undergoing ART (in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) programs). The patients were divided into groups according to the level of ovarian reserve. Group 1 included 50 patients with DOR (anti-Müllerian hormone (AMH) <1.2 ng/ml, antral follicle count (AFC) <5 ng/mL), and group 2 included 65 patients with NOR (AMH ≥1.2 ng/ml, AFC ≥5 ng/mL). The mean age of the women was 37.2±5.3 years.
Before entering the ART program, all patients underwent obligatory examination according to clinical guidelines, “Female Infertility” (2021) [20], and special studies, including assessment of the amino acid profile and levels of steroid hormones using high-performance LC-MS. The study was reviewed and approved by the Research Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
Inclusion criteria were age 25–42 years, absence of pregnancy for one year or more of regular sexual activity without contraception, and signed informed voluntary consent to participate in the study. Non-inclusion criteria were refusal to participate in the study, contraindications to ART, history of ovarian surgery, HIV infection and other immunodeficiency conditions, systemic connective tissue diseases, rheumatic diseases, oncological diseases of any etiology, the presence of chromosomal and genetic abnormalities, donor oocyte or embryo programs, and surrogacy programs.
Ultrasound examination was performed to monitor folliculogenesis. IVF was performed using a standard protocol with gonadotropin-releasing hormone antagonists. Urinary and recombinant gonadotropins were used to induce folliculogenesis. The daily dose of gonadotropins was determined individually, depending on the state of the ovarian reserve, but was not less than 225 IU/day; Human chorionic gonadotropin was used as an ovulation trigger. During the transvaginal puncture of the ovaries, blood and follicular fluid were collected from the first follicle.
Amino acid profile analysis was performed using LC-MS on a system consisting of an Agilent 6460 triple quadrupole mass spectrometer detector (Agilent) equipped with an electrospray ionization source and an Agilent 1260 II liquid chromatograph (Agilent). Samples were prepared as follows: 480 μL of chloroform-methanol mixture (2:1, v/v) was added to a 100 μL sample at 4°C; the sample was subjected to ultrasonication for 10 min; 150 μL of water was added and stirred for 5 min. The resulting solution was centrifuged at 13,000 g for 5 min at room temperature. Two hundred microliters of the upper water-methanol layer was taken, dried in a nitrogen stream for 30 min at 60°C, 200 μl of 3N hydrochloric acid was added to butanol, stirred for 3 min, centrifuged at 13000 g for 15 s at room temperature, and kept at 60°C for 15 min to carry out the derivatization reaction. It was then centrifuged at 13000 g for 15 s at room temperature, dried in a nitrogen stream for 30 min at 60°C, redissolved in 200 μl of acetonitrile/water solution (1/1 v/v), stirred for 5 min, centrifuged at 13,000×g for 15 s at room temperature for 10 min, and 120 μl of the resulting sample was transferred into a vial with an insert for further analysis.
Statistical analysis
Statistical analysis was performed using scripts written in R [R Core Team (2018). R: Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/] in RStudio [RStudio Team (2016). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/]. The normality of the distribution was tested using the Kolmogorov–Smirnov test and graphical data analysis.
The amino acid content of a sample was assessed semi-quantitatively by the relative level of the analyte, which was calculated by dividing the chromatographic peak area of the corresponding analyte by the total peak area of the analytes in a given sample.
Before statistical analysis, amino acid levels were transformed to have a mean of 0 and a standard deviation of 1 to graphically represent the analyzed data, as amino acid levels are distributed over a wide range [21]. Conversion formula:
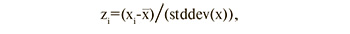
where zi is the standardized value of the parameter, xi is the initial value of the parameter, is mean value of the parameter, and stddev(x) is the standard deviation of the population.
Quantitative variables showing a normal distribution are expressed as M (SD), and a t-test was used to assess differences between groups. Data with non-normal distribution were reported as the median with the interquartile range Me (Q1; Q3), comparison of amino acid levels was carried out using the nonparametric Wilcoxon–Mann–Whitney test. To assess the correlation, nonparametric Spearman correlation analysis was used. The significance threshold was set at p<0.05.
Results
The study included infertile women of reproductive age who sought infertility treatment through the IVF/ICSI program at V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. The mean age of the women was 37.2±5.3 years. All the patients had regular menstrual cycles. The mean body mass index was 23.9±4.8 kg/m2. Patients with DOR had a significantly shorter menstrual cycle, and their mothers were at an earlier age at menopause (Table 1).
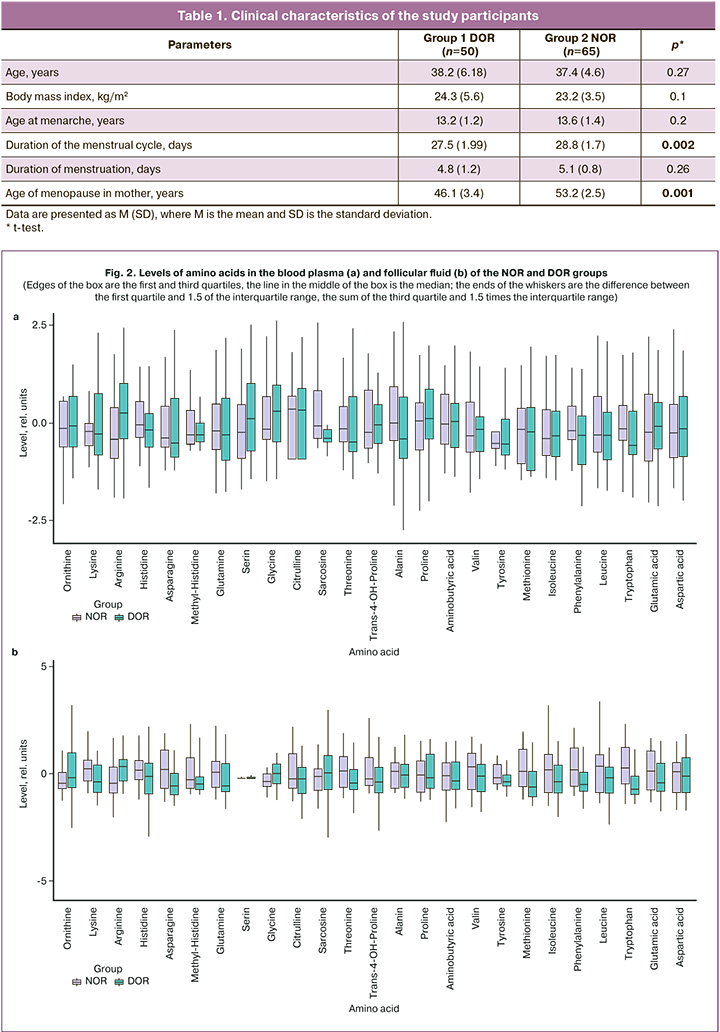
The concentrations of blood plasma and follicular fluid amino acids obtained by LC-MS on the day of oocyte aspiration were compared. In the blood plasma of women with DOR, there was a statistically significant decrease in sarcosine, -0.41 (-0.51; -0.22), and tryptophan, -0.59 (-0.82; 0.3) compared with women with NOR (sarcosine – -0.1 (-0.41; 0.7); tryptophan, -0.19 (-0.48; 0.42)) (p<0.05) (Table 2).
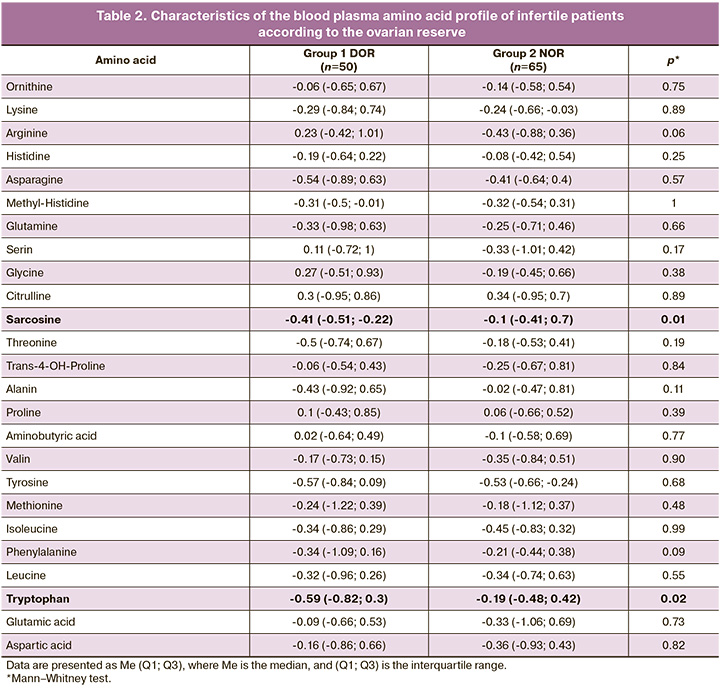
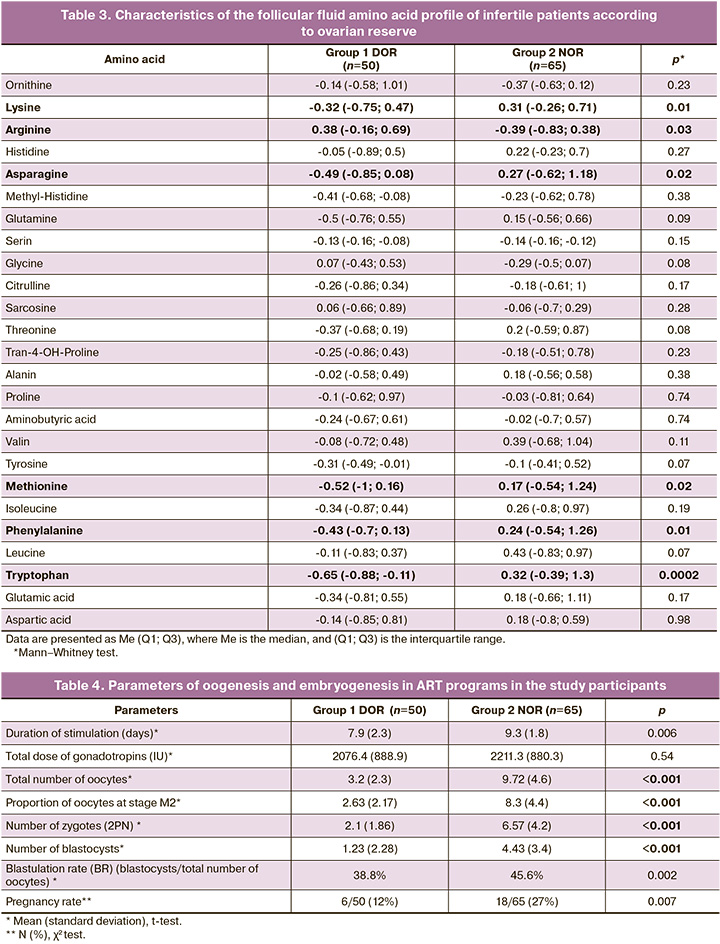
In the follicular fluid of women with DOR, there was a statistically significant decrease in lysine concentration was detected (NOR – 0.31 (-0.26; 0.71), DOR – -0.32 (-0.75; 0.47), p=0, 01), asparagine (NOR – 0.27 (-0.62; 1.18), DOR – -0.49 (-0.85; 0.08), p=0.02), methionine (NOR – 0 .17 (-0.54; 1.24), DOR – -0.52 (-1; 0.16) p=0.02), phenylalanine (NOR – 0.24 (-0.54; 1.26 ), DOR – -0.43 (-0.7; 0.13), p=0.01) and tryptophan (NOR – 0.32 (-0.39; 1.3), DOR – -0.43 (-0.7; 0.13), p=0.0002) (Table 3) and increased levels of arginine (NOR - -0.39 (-0.83; 0.38), DOR - 0.38 ( -0.16; 0.69), p=0.03).
Figure 2 shows a graphical representation of the changes in blood plasma and follicular fluid amino acid profiles.
(Edges of the box are the first and third quartiles, the line in the middle of the box is the median; the ends of the whiskers are the difference between the first quartile and 1.5 of the interquartile range, the sum of the third quartile and 1.5 times the interquartile range)
A comparative analysis of oogenesis and embryogenesis parameters revealed a statistically significant decrease in the number of oocytes, including mature oocytes, indicators of early embryogenesis and blastulation, and pregnancy rate in the DOR group (Table 4).
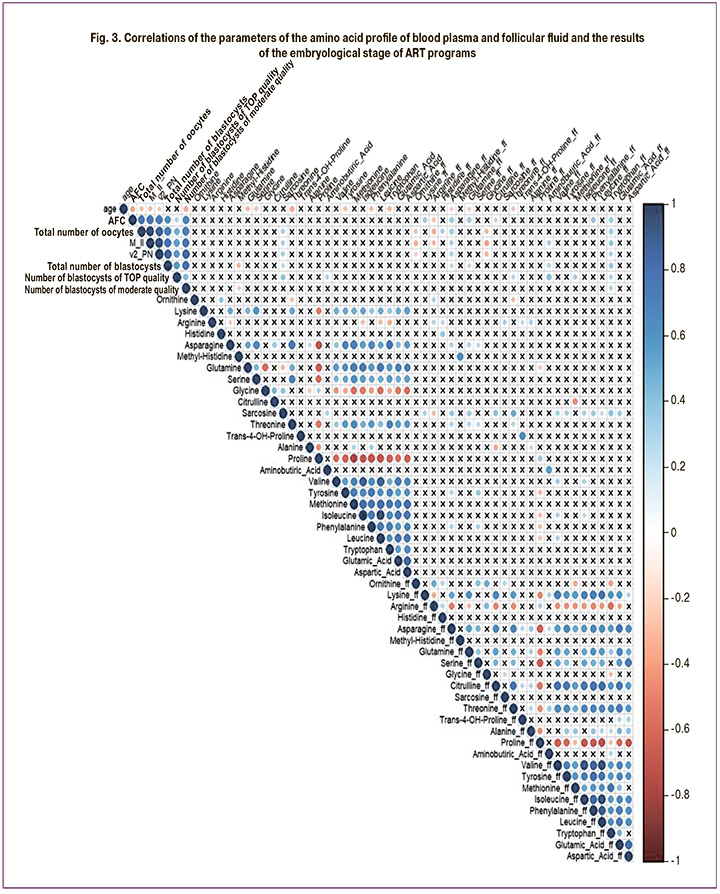
Correlation analysis of amino acid levels in the blood plasma and follicular fluid on the day of oocyte retrieval with parameters of oogenesis and early embryogenesis in each group was also performed (Fig. 2, 3).
In both the DOR and NOR groups, strong correlations were observed between the levels of amino acids in blood plasma and follicular fluid (Fig. 3). There was a moderate positive correlation between AFC and indicators of oogenesis and early embryogenesis and the levels of the blood plasma amino acids lysine and sarcosine, asparagine, tryptophan, and aminobutyric acid in the follicular fluid.
Discussion
In this study, the amino acid composition of blood plasma and follicular fluid was analyzed using LC-MS and compared between infertile patients with DOR and those with NOR.
In a study by Li J. et al., an analysis of the metabolomic profile of follicular fluid in women with DOR revealed a decrease in the level of tryptophan and its breakdown products (5-hydroxy-L-tryptophan, 5-hydroxyindoleacetic acid, the end product of serotonin metabolism, indole, indoleacetic aldehyde, and indoleacetic acid). The study also noted positive correlations between their levels and the number of oocytes, embryos, and their quality [22], which was also observed in this study. A decrease in the content of tryptophan and its breakdown products, especially in the ovarian tissue, can affect the quantity and quality of oocytes and embryos. Tryptophan, an essential amino acid, is involved in various metabolic pathways related to protein synthesis, serotonin (5-HT), and kynurenines. Tryptophan and its metabolites play crucial roles in physiological processes, such as maintaining cell growth and regulating immune function. Serotonin, the main metabolite of tryptophan, plays a significant role in regulating placental function and fetal development [23]. Serotonin may influence oocyte development by regulating progesterone secretion in granulosa cells. An experimental study by Dube et al. showed that a decrease in the level of serotonin, which is the main source of melatonin in the blood, leads to disruption of early embryonic development [24].
This study found higher concentrations of lysine, asparagine, phenylalanine, and methionine in the follicular fluid of women with NOR.
These findings are consistent with the results of Li et al., who showed decreased levels of glycine, phenylalanine, and DL-2-aminooctanoic acid and increased levels of aspartic acid, proline, L-glutamine, and pyroglutamate in the follicular fluid of patients with DOR. These changes in the amino acid profile of the follicular fluid of patients with DOR correlated with changes in oocytes and embryogenesis, indicating the role of amino acids in oocyte maturation and early embryonic development by reducing oxidative stress and enhancing mitochondrial function [22].
In a study by D'Aniello et al., D-aspartic acid concentrations in follicular fluid were found to directly correlate with oocyte morphology, maturation, percentage of mature oocytes, and fertilization rate [25].
This study showed that a decrease in amino acid concentration is associated with DOR, suggesting its potential use as a biochemical marker of ovarian reserve. The results obtained indicate the involvement of amino acids in folliculogenesis and formation of the ovarian reserve.
The majority of studies on the involvement of amino acids in the process of folliculogenesis confirm their stimulating role in the early stages of follicle growth, their support in the dynamics of follicle development, and their participation in the initiation of late-stage follicle development. These findings provide insights into the role of amino acids and their use in improving the outcomes of ART programs in infertile women with DOR.
Conclusion
The study results demonstrated a significant decrease in the levels of blood plasma and follicular fluid amino acids in infertile women with DOR and their relationship with oogenesis and embryogenesis parameters, suggesting the potential use of determining the amino acid profile as a marker for assessing ovarian reserves and the developmental potential of oocytes and embryos in ART programs.
References
- Hu S., Xu B., Jin L. Perinatal outcome in young patients with diminished ovarian reserve undergoing assisted reproductive technology. Fertil. Steril. 2020; 114(1): 118-24.e1. https//dx.doi.org/10.1016/j.fertnstert.2020.02.112.
- Dumesic D.A., Meldrum D.R., Katz-Jaffe M.G., Krisher R.L., Schoolcraft W.B. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015; 103(2): 303-16. https//dx.doi.org/10.1016/j.fertnstert.2014.11.015.
- Edwards R.G. Follicular fluid. J. Reprod. Fertil. 1974; 37(1): 189-219. https//dx.doi.org/10.1530/jrf.0.0370189.
- Klein N.A., Battaglia D.E., Miller P.B., Branigan E.F., Giudice L.C., Soules M.R. Ovarian follicular development and the follicular fluid hormones and growth factors in normal women of advanced reproductive age. J. Clin. Endocrinol. Metab. 1996; 81(5): 1946-51. https//dx.doi.org/10.1210/jcem.81.5.8626862.
- Revelli A., Delle Piane L., Casano S., Molinari E., Massobrio M., Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009; 7: 40. https//dx.doi.org/10.1186/1477-7827-7-40.
- Драпкина Ю.С., Тимофеева А.В., Чаговец В.В., Кононихин А.С., Франкевич В.Е., Калинина Е.А. Применение омиксных технологий в решении проблем репродуктивной медицины. Акушерство и гинекология. 2018; 9: 24-32. [Drapkina Yu.S., Timofeeva A.V., Chagovets V.V., Kononikhin A.S., Frankevich V.E., Kalinina E.A. Use of omics technologies to solve the problems of reproductive medicine. Obstetrics and Gynecology. 2018; (9): 24-32. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.9.24-32.
- Wilson I.D., Theodoridis G., Virgiliou C. A perspective on the standards describing mass spectrometry-based metabolic phenotyping (metabolomics/metabonomics) studies in publications. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021; 1164: 122515. https//dx.doi.org/10.1016/j.jchromb.2020.122515.
- Yang J., Feng T., Li S., Zhang X., Qian Y. Human follicular fluid shows diverse metabolic profiles at different follicle developmental stages. Reprod. Biol. Endocrinol. 2020; 18(1): 74. https//dx.doi.org/10.1186/s12958-020-00631-x.
- Liu L., Yin T.L., Chen Y., Li Y., Yin L., Ding J., Yang J., Feng H.L. Follicular dynamics of glycerophospholipid and sphingolipid metabolisms in polycystic ovary syndrome patients. J. Steroid Biochem. Mol. Biol. 2019; 185: 142-9. https//dx.doi.org/10.1016/j.jsbmb.2018.08.008.
- Karaer A., Tuncay G., Mumcu A., Dogan B. Metabolomics analysis of follicular fluid in women with ovarian endometriosis undergoing in vitro fertilization. Syst. Biol. Reprod. Med. 2019; 65(1): 39-47. https//dx.doi.org/10.1080/19396368.2018.1478469.
- Ярыгина С.А., Смольникова В.Ю., Калинина Е.А., Эльдаров Ч.М., Гамисония А.М., Макарова Н.П., Бобров М.Ю. Анализ метаболитов в различных средах культивирования эмбрионов человека. Акушерство и гинекология. 2020; 11: 114-23. [Yarygina S.A., Smolnikova V.Yu., Kalinina E.A., Eldarov Ch.M., Gamisonia A.M., Makarova N.P., Bobrov M.Yu. Analysis of human embryo culture medium metabolites. Obstetrics and Gynecology. 2020; (11): 114-23. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.114-123.
- Sun Z., Chang H.M., Wang A., Song J., Zhang X., Guo J. et al. Identification of potential metabolic biomarkers of polycystic ovary syndrome in follicular fluid by SWATH mass spectrometry. Reprod. Biol. Endocrinol. 2019; 17(1): 45. https//dx.doi.org/10.1186/s12958-019-0490-y.
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009; 37(1): 1-17. https//dx.doi.org/10.1007/s00726-009-0269-0.
- https://upload.wikimedia.org/wikipedia/commons/c/ce/AminoAcidball.svg
- Palii S.S., Kays C.E., Deval C., Bruhat A., Fafournoux P., Kilberg M.S. Specificity of amino acid regulated gene expression: analysis of genes subjected to either complete or single amino acid deprivation. Amino Acids. 2009; 37(1): 79-88. https//dx.doi.org/10.1007/s00726-008-0199-2.
- Brosnan J.T. Amino acids, then and now--a reflection on Sir Hans Krebs' contribution to nitrogen metabolism. IUBMB Life. 2001; 52(6): 265-70. https//dx.doi.org/10.1080/152165401317291101.
- Newsholme P., Brennan L., Rubi B., Maechler P. New insights into amino acid metabolism, beta-cell function and diabetes. Clin. Sci. (Lond). 2005; 108(3): 185-94. https//dx.doi.org/10.1042/CS20040290.
- Wu G., Bazer F.W., Davis T.A., Kim S.W., Li P., Marc Rhoads J. et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009; 37(1): 153-68. https//dx.doi.org/10.1007/s00726-008-0210-y.
- Wu G., Bazer F.W., Datta S., Johnson G.A., Li P., Satterfield M.C., Spencer T.E. Proline metabolism in the conceptus: implications for fetal growth and development. Amino Acids. 2008; 35(4): 691-702. https//dx.doi.org/10.1007/s00726-008-0052-7.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации «Женское бесплодие». 2021. [Ministry of Health of the Russian Federation. Clinical guidelines "Female infertility". 2021. (in Russian)].
- Misra B.B. Data normalization strategies in metabolomics: Current challenges, approaches, and tools. Eur. J. Mass Spectrom. (Chichester). 2020; 26(3):165-74. https//dx.doi.org/10.1177/1469066720918446.
- Li J., Zhang Z., Wei Y., Zhu P., Yin T., Wan Q. Metabonomic analysis of follicular fluid in patients with diminished ovarian reserve. Front. Endocrinol. 2023; 14: 1132621. https//dx.doi.org/10.3389/fendo.2023.1132621.
- Baković P., Kesić M., Perić M., Bečeheli I., Horvatiček M., George M. et al. Differential serotonin uptake mechanisms at the human maternal-fetal interface. Int. J. Mol. Sci. 2021; 22(15): 7807. https//dx.doi.org/10.3390/ijms22157807.
- Dubé F., Amireault P. Local serotonergic signaling in mammalian follicles, oocytes and early embryos. Life Sci. 2007; 81(25-26): 1627-37. https//dx.doi.org/10.1016/j.lfs.2007.09.034.
- D'Aniello G., Grieco N., Di Filippo M.A., Cappiello F., Topo E., D'Aniello E., Ronsini S. Reproductive implication of D-aspartic acid in human pre-ovulatory follicular fluid. Hum. Reprod. 2007; 22(12): 3178-83. https//dx.doi.org/10.1093/humrep/dem328.
Received 20.11.2023
Accepted 22.01.2024
About the Authors
Marina A. Shevtsova, post-graduate student, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(911)039-13-20, marina_981995@mail.ruAlla A. Gavisova, Dr. Med. Sci., Head of the 1st Gynecology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, a_gavisova@oparina4.ru
Natalya A. Krasnova, PhD, Teaching Assistant at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University); Obstetrician-Gynecologist, Reproductologist, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Academician Oparin str., 4, dr.krasnova@rambler.ru, https://orcid.org/0000-0001-8636-2560
Artem A. Aksenenko, PhD, Gynecologist at the 1st Gynecology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Akademika Oparina str., 4, +7(926)354-98-60, a_axenenko@oparina4.ru
Anastasia V. Novoselova, Researcher at the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Akademika Oparina str., 4, +7(495)438-21-98, a_novoselova@oparina4.ru
Khazzhar Fadi, PhD, Junior Researcher at the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Akademika Oparina str., 4, +7(495)438-21-98.
Vitaliy V. Chagovets, PhD (Physico-mathematical Sciences), Head of the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Akademika Oparina str., 4, +7(495)438-21-98, vvchagovets@gmail.com
Vladimir E. Frankevich, Dr. Sci. (Physico-mathematical Sciences), Deputy Director of the Institute of Translational Medicine, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Akademika Oparina str., 4, v_frankevich@oparina4.ru
Corresponding author: Marina A. Shevtsova, marina_981995@mail.ru



