Implications of mitochondrial DNA copy number in cumulus cells in late reproductive-age women
Objective. To estimate mitochondrial DNA (mtDNA) copy number in cumulus cells (CC) in patients of late reproductive age in in-vitro fertilization programs and to analyze the relationship of the indicators under study with the parameters of oogenesis, embryogenesis, embryo ploidy, and treatment outcomes.Korolkova A.I., Mishieva N.G., Martazanova B.A., Burmenskaya O.V., Veyukova M.A., Ekimov A.N., Trofimov D.Yu., Abubakirov A.N.
Subjects and methods. A prospective study was conducted to determine mtDNA copy number in 454 CC from 67 patients aged 35-45 years. The relative level of mtDNA was determined by real time polymerase chain reaction.
Results. A cohort of patients with a mean age of 37.8 years showed a statistically significant relationship between the mean level of mtDNA in CC and age (p = 0.008), as well as the level of anti-Müllerian hormone (p = 0.003). There was no correlation between mtDNA copy number in CC and oocyte maturity, fertilization rate, morphological quality, and blastocyst ploidy. There was a tendency to an increase in mtDNA levels in CC in the embryo implantation group versus the non-embryo implantation group (p = 0.08).
Conclusion. The determination of mtDNA copy number in CC can become a reliable biomarker of reproductive system aging, takin into consideration that the age given in the passport does not always reflect the true fertility potential of women. Also, the estimation of the level of mtDNA in CC may become a possible predictor of embryo implantation potential. However, further randomized trials are needed.
Keywords
An age-related decline in ovarian reserve (OR) is characterized by a decrease in ultrasound measured antral follicle count, an increase in the serum level of follicle-stimulating hormone (FSH), and a decrease in the serum level of Anti-Müllerian hormone (AMH). Low OR is often associated with changes during ovarian aging, such as an increase in the number of aneuploid embryos, a decrease in fertility, and high rates of pregnancy loss at different gestational ages [1–4]. A gradual age-related fertility decline is characterized by a decrease in oocyte quantity and quality [5]. Key indicators of the age-related follicular pool depletion are its initial size and the rate of follicular atresia, in which mitochondria play an important role. For example, some experimental studies using animal models suggested that the initial size of the follicular pool, determined during the intrauterine life of the female fetus, depends, apart from other factors, on mitochondrial biogenesis [5–7]. The distribution of mitochondrial DNA molecules (mtDNA) during the division of oogonia occurs randomly, which can lead to the production of oocytes with rearranged mtDNA. These changes affect oocytes with mutant mtDNA, which is supposed to trigger the process of follicular atresia, eliminating primary oocytes carrying mtDNA mutations. This hypothesis also explains the possible pathogenesis of premature ovarian failure [8].
Another debatable issue is the main reason for the age-related decline in the quality of oocytes in women of late reproductive age. The current literature offers several hypotheses, mostly suggesting a key role of oxidative stress, impaired microcirculation, hypoxic processes in the follicular fluid [9, 10]. However, in recent years, studies addressing this issue have been increasingly focused on the relationship of age-related oocyte quality decline with mitochondrial dysfunction [11]. Mitochondria are maternally inherited organelles that are essential for the production of most of cellular adenosine triphosphate and are the only organelles that have their DNA. It is assumed that the initial number of mitochondria and mtDNA amount can determine the energy potential of early embryos up to the three days of development since the replication of mtDNA does not occur at this stage of embryogenesis. Full mtDNA replication begins at the blastocyst stage (5-day embryo) when a higher level of energy is needed for successful division [12-14].Studies using animal models (mouse, pig oocytes) reported that cells containing higher mtDNA amounts were more successfully fertilized than oocytes with a reduced mtDNA copy number [15, 16].
However, due to ethical considerations, it is impossible to determine the oocyte mtDNA content in humans, and therefore studies have been focused on cumulus cells (CC) that are considered to be one of the best non-invasive approaches for evaluating the metabolic processes underlying oocyte quality and the developmental potential of the embryo. In particular, oocyte–cumulus complex (OCC) interactions coordinate carbohydrate, lipid, and protein metabolism to ensure the appropriate energy balance required for the meiosis and fertilization of the oocyte, and support during early embryogenesis. Thus, cumulus cell mitochondria are central agents of the energetic metabolic pathways, are directly involved in the establishment of oocyte competence during oogenesis [17, 18]. For example, M.Ogino et al. [11] identified a significant relationship between mtDNA content of CCs and the morphological quality of blastocysts. The median mtDNA content of CCs for good and poor-quality embryos was 140 and 57 relative units (r.u.), respectively (p <0.0001). Desquiret-Dumas V. et al. [18] also confirmed a statistically significant relationship between mtDNA copy number in CCs and the morphological quality of embryos. In that study, increased mtDNA copy number was associated with good quality embryos compared with fair or poor quality embryos, median 738 (250–1228), and 342 (159–818) r.u., respectively (p = 0.006). According to Taugourdeau A. et al. [19], significantly higher mtDNA copy numbers in CCs were associated with implanted embryos than with non-implanted embryos with mean 215 and 59 r.u., respectively (p<104).
Therefore, mtDNA copy number in CCs can become a reliable marker for predicting oocyte and embryo quality, as well as their implantation potential.
This study aimed to investigate mtDNA copy number in CCs in late reproductive-age women undergoing in vitro fertilization (IVF) with preimplantation genetic testing for aneuploidy (PGT-A) and to analyze the relationship between the parameters under study and oogenesis, embryogenesis, and treatment outcomes.
Materials and methods
The study was conducted at the 1st Department of Gynecology and the Laboratory of Molecular Genetics of V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. The study included 454 OCC obtained from 67 patients aged 35-45 who underwent infertility treatment by IVF/intracytoplasmic sperm injection (ICSI) followed by preimplantation genetic testing for aneuploidy (PGT-A) of the obtained embryos. The study included patients meeting the following criteria: age 35-45 years; body mass index 18–29 kg/m2; basal blood FSH level ≤15 IU/ml; the absence of stage III-IV external genital endometriosis, large uterine fibroids, polycystic ovary syndrome; normal karyotype of spouses. The study did not include smoking patients, as well as patients with severe teratozoospermia (more than 96% of sperm cells with abnormal sperm morphology, according to WHO criteria).
The patients underwent an examination per the Order No. 107н of the Ministry of Healthcare of Russia, dated 30 August 2012 “On the Procedure for the Use of Assisted Reproductive Technologies, Contraindications and Restrictions to their Application”. The ovarian stimulation protocol with gonadotropin-releasing hormone antagonists included recombinant FSH or human menopausal gonadotropin, and corifollitropin alfa. Dosages were selected individually based on the parameters of the ovarian reserve. The ovulation trigger was administered when 3 or more follicles reached ≥18 mm in diameter. Either human chorionic gonadotropin or gonadotropin-releasing hormone agonist was used as a trigger. The transvaginal US-guided ovarian puncture was performed 36 hours after the injection of the ovulation trigger.
CCs were separated from OCC using fine needles, placed in an Eppendorf tube and frozen at -80°C for subsequent mtDNA copy number assay. After the ICSI, the embryos were cultured to the blastocyst stage. On day 5-6 of development, all embryos underwent a trophectoderm (TE) biopsy to conduct PGT-A by comparative genomic hybridization. A morphological assessment of blastocyst-stage embryos was carried by the Gardner blastocyst grading system (blastocyst maturity, intracellular mass (ICM) quality, and TE quality) [20]. Based on morphological characteristics, the embryos were divided into three groups: excellent quality - blastocysts with an A-grade ICM and AB-grade TE; good quality - blastocysts with B-grade ICM and BC-grade TE; poor quality - early blastocysts, blastocysts with C-grade ICM, and C-grade TE that were excluded from PGT-A.
A relative quantitative assessment of the mtDNA copy number was performed using real-time polymerase chain reaction (PCR). Specially designed oligonucleotides and TaqMan probes were used in the reaction to amplify and quantify a specific mtDNA fragment (MT-ND2 gene - mitochondrially encoded NADH dehydrogenase 2 and MT-ND4 gene - mitochondrially encoded NADH dehydrogenase 4). Normalization was carried out on genomic DNA (LTC4S gene - leukotriene C4 synthase). The use of oligonucleotides (primers) for the MT-ND2 gene made it possible to evaluate the content of the total pool of mtDNA (mtDNAtotal), and the primers for the MT-ND4 gene allowed the content of the pool of a full-sized mtDNA lacking large deletions (mtDNAdel-) including the MT-ND4 gene, for example, del mtDNA4977, mtDNA7436, mtDNA7345, mtDNA8041.
TaqMan samples for mitochondrial and genomic DNA fragments were labeled with different fluorophores (FAM and HEX), which allowed the reaction to be performed in one tube (multiplex PCR); the reaction was performed in duplicate for each sample. Paraffin was used to provide a “hot start.” Reagents and detecting amplifiers DTprime (LLC DNA-Technology, Russia) were used by the manufacturer’s instructions and recommendations. Amplification mode: at 80° C for 1 min, incubation at 95 ° C for 1 min, and then 50 cycles: at 94°C for 15s and 64°C for 20 s with measuring fluorescence level at each cycle.
The quantity of mtDNA relative to genomic was determined by comparing threshold cycles (2∆Cp) and presented in relative units (r.u.) according to the formula 1: mtDNA/gDNA = 2 Cp gDNA-Cp mtDNA , where Cp gDNA is the value of the threshold cycle of genomic DNA amplification, Cp mtDNA is the value of the threshold cycle of mtDNA amplification.
All CCs included in the study were divided into two groups, categorized by the AMH levels: group A (n = 251) included oocytes obtained from patients with an AMH level> 1; group B (n = 203) consisted of oocytes obtained from patients with AMH level <1.
After fertilization of 454 oocytes by ICSI, 130 embryos were cultured up to 5-6 days of development. The PGT-A results showed that of 130 obtained embryos, 56 and 74 were aneuploid and euploid, respectively. The transferred euploid embryos (n = 51) were divided into 2 groups: group 1 - implanted embryos (n = 21), group 2- non-implanted embryos (n = 30).
Quantitative variables showing normal distribution were expressed as means and standard deviation M (SD); otherwise, the median and interquartile range Me (Q1; Q3) were reported. Differences in mean values between groups were assessed using the Student t-test. Variables not showing normal distribution were compared by Mann-Whitney test. The Pearson parametric test was used for the correlation analysis for variables showing normal distribution; otherwise Spearman test was used. The results were considered statistically significant at p <0.05. Statistical analysis was performed using Microsoft Excel spreadsheets and the SPSS Statistics 17.0 statistical package.
Results and discussion
A total of 454 OCCs were obtained from 67 late reproductive-age women. The mean age of patients in this study was 37.8 (2.1) years; the mean serum level of AMH was 2.66 (1.09) ng/ml. The baseline characteristics of patients included in the study are summarized in Table 1.
The results obtained using primers for the MT-ND2 and MT-ND4 genes were completely identical, which indicates the absence of large mtDNA deletions, including the MT-ND4 gene in the CCs of patients included in the study.

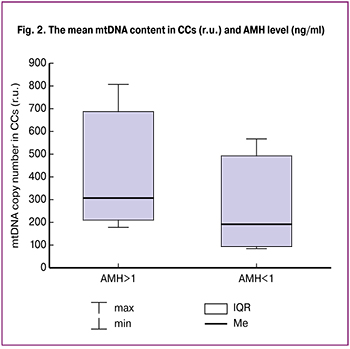 There was a negative correlation between the mean mtDNA levels in CCs and the patients’ age (r = -0.142, p = 0.008) (Fig. 1). Accordingly, in parallel with an increase in the patients’ age, a decrease in mtDNA copy number in CCs was observed. It should be noted that in our study, the age of 39 years was the threshold at which the mtDNA copy number in CCs was 1.5 times lower than in patients aged 35–38 years. In addition, the relative mtDNA copy number in CCs correlated with serum AMH (r = 0.139, p = 0.003). When the patients were categorized into groups AMH levels, no statistically significant differences were found in the prevalence of extragenital and gynecological diseases, body mass index, age, total dose of gonadotropins, and duration of treatment (Table 2). The AMH level in groups A and B was 3.2 (1.1) and 0.7 (0.3) ng/ml, respectively (p = 0.02) (Table 2). The mtDNA copy number in CCs obtained from patients with an AMH levels > 1 ng/ml was statistically significantly higher in group B (369 (203; 696) r.u.) than in group A (210 (98; 597) r.u.), p = 0.001) (Fig. 2). The number of mature oocytes was lower in group B (4.2 (0.8)) than in group A (6.5 (0.5)), respectively (p = 0.01).
There was a negative correlation between the mean mtDNA levels in CCs and the patients’ age (r = -0.142, p = 0.008) (Fig. 1). Accordingly, in parallel with an increase in the patients’ age, a decrease in mtDNA copy number in CCs was observed. It should be noted that in our study, the age of 39 years was the threshold at which the mtDNA copy number in CCs was 1.5 times lower than in patients aged 35–38 years. In addition, the relative mtDNA copy number in CCs correlated with serum AMH (r = 0.139, p = 0.003). When the patients were categorized into groups AMH levels, no statistically significant differences were found in the prevalence of extragenital and gynecological diseases, body mass index, age, total dose of gonadotropins, and duration of treatment (Table 2). The AMH level in groups A and B was 3.2 (1.1) and 0.7 (0.3) ng/ml, respectively (p = 0.02) (Table 2). The mtDNA copy number in CCs obtained from patients with an AMH levels > 1 ng/ml was statistically significantly higher in group B (369 (203; 696) r.u.) than in group A (210 (98; 597) r.u.), p = 0.001) (Fig. 2). The number of mature oocytes was lower in group B (4.2 (0.8)) than in group A (6.5 (0.5)), respectively (p = 0.01).
In this study, no association was found between the mtDNA content in CCs and the oocyte maturity or fertilization rate. Also, in our study, there was no statistically significant relationship between the mtDNA content in CCs and the morphological assessment of embryos at day 5-6 of development (p = 0.7). Besides, there were no differences in the mtDNA content of CCs and embryo ploidy: 356 r.u. and 325 r.u. for euploid and aneuploid blastocysts, respectively (p> 0.05. However, there was a tendency to a higher mtDNA content in implanted embryos compared with non-implanted ones - 390 (40) r.u. and 299 (32) r.u., respectively (p = 0.08).

These findings suggest that low mtDNA copy number in CCs probably reflects an age-related decrease in the oocyte quantity and quality. This is consistent with data by Boucret L. et al. [21] who investigated the CC mtDNA copy number in patients with normal and reduced ovarian reserve. At the same time, the group of “low ovarian reserve” consisted of patients of both late reproductive age and patients with premature ovarian failure. Women with a normal ovarian reserve had significantly higher mtDNA content in CCs than women with diminished ovarian reserve (p = 0.02).
In our study, similarly to the study by Taugourdeau A. et al. [19], there was no statistically significant association between mtDNA copy number in CCs and the morphological quality of blastocysts. However, Ogio M. et al. [11] reported a statistically significant difference in mtDNA content in CCs between good (³3ВВ) - and poor-quality (< 3BB) embryos (p <0.0001). In our study and the study by Taugourdeau A. et al. [19], embryos at days 5-6 days were divided into three groups, including excellent, good, and poor quality embryos, which could affect the lack of correlation between the studied parameters. Also, in our study, we did not observe a statistically significant relationship between mtDNA copy number in CCs and the ploidy of the obtained embryos, which is consistent with the results by other authors [18, 19, 21]. The lack of correlation between mtDNA content in CCs and the ploidy of excellent and good quality blastocyst may be explained by the fact that the oocytes have a sufficient number of mitochondria and mtDNA to provide the necessary energy level for the subsequent development of embryos to the blastocyst stage. However, mutations in the maternal genes associated with meiosis or mitosis, inheritance of aberrant maternal RNA, and accumulated mtDNA mutations can lead to impaired chromosome segregation and cell division, resulting in aneuploidy. Accordingly, the reduced mtDNA copy number and the mitochondrial potential of OCC are not always the most important factors in the development of aneuploidy.
Interestingly, in our previous study aimed at investigating mtDNA content in blastocyst TE, we observed a significantly higher mtDNA content in TE of aneuploid than euploid embryos (p = 0.003) [22]. This fact confirms the assumption about an abnormal increase in mtDNA in TE cells during embryogenesis to compensate for a defective mitochondrial pool at the end of oogenesis [7].
Besides, the findings of our study suggest a tendency toward a statistically significant relationship between the mtDNA content and the embryo implantation potential. According to Taugourdeau A. et al. [19], the mtDNA copy number in CCs in implanted embryos was significantly higher than in non-implanted ones (p <10-4). Thus, the authors concluded that the mtDNA content in CCs might be a determining factor in the successful development and implantation of embryos. The absence of a strong correlation between the mtDNA content in CCs and the implantation potential of euploid blastocysts in our study may be attributed to a lower number of transferred embryos (n = 51) than in the study by Taugourdeau A. et al. [19] (n = 84).
A considerable amount of domestic and international literature has been published about the relationship between mtDNA content in CCs and BMI, and smoking [18, 23]. High BMI is associated with mitochondrial dysfunction and a decrease in the mtDNA copy number. However, no correlation was found with embryo quality deterioration. In smoking patients, on the contrary, the mtDNA content in CCs has shown a compensatory increase. Accordingly, the determination of the mtDNA content in CCs in smoking patients and/or patients with high BMI is impractical, given that the results depend on the individual patient characteristics. Based on this fact, smoking patients and patients with high BMI were not included in this study. Nevertheless, despite abating the effects of patient-specific factors, we were unable to determine the threshold of mtDNA copy number that predicts the number or quality of embryos and their ability to implant.
Conclusion
The findings of this study suggest that the mtDNA content in CCs is associated with a decrease in both the quantity and quality of the oocytes. The determination of the mtDNA copy number in CCs could be a new reliable marker for the aging of the female reproductive system, given that the chronological age does not always reflect true female fertility potential. Also, testing for mtDNA content in CCs can be a possible predictor of the embryo implantation potential.
References
- Haadsma M.L., Groen H., Mooij T.M., Burger C.W., Broekmans F.J., Lambalk C.B., Leeuwen F.E., Hoek A. Miscarriage risk for IVF pregnancies in poor responders to ovarian hyperstimulation. Reprod Biomed Online 2010; 20: 191–200. https://doi.org/(...)6/j.rbmo.2009.11.005
- van der Stroom E.M., Konig T.E., van Dulmen-den Broeder E., Elzinga W.S., van Montfrans J.M., Haadsma M.L., Lambalk C.B. Early menopause in mothers of children with Down syndrome? Fertil Steril. 2011; 96: 985–990. doi: 10.1016/j.fertnstert.2011.07.1149.
- Lekamge D.N., Barry M., Kolo M., Lane M., Gilchrist R.B., Tremellen K.P. Anti-Mullerian hormone as a predictor of IVF outcome. Reprod Biomed Online. 2007; 14: 602–610. DOI: 10.1016/s1472-6483(10)61053-x
- Levi A.J., Raynault M.F., Bergh P.A., Drews M.R., Miller B.T., Scott R.T. Jr. Reproductive outcome in patients with diminished ovarian reserve. Fertil Steril 2001; 76: 666–669. DOI: 10.1016/s0015-0282(01)02017-9
- Aiken C.E., Tarry-Adkins J.L., Ozanne S.E. Suboptimal nutrition in utero causes DNA damage and accelerated aging of the female reproductive tract. FASEB J 2013; 27:3959–65. doi:10.1096/fj.13-234484.
- May-Panloup P., Boucret L., Chao de la Barca J.M., Desquiret-Dumas V., Ferré-L’Hotellier V., Morinière C., Descamps P., Procaccio V., Reynier P. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016; 22(6):725-743. doi:10.1093/humupd/dmw028
- Aiken C.E., Tarry-Adkins J.L., Penfold N.C., Dearden L., Ozanne S.E. Decreased ovarian reserve, dysregulation of mitochondrial biogenesis, and increased lipid peroxidation in female mouse offspring exposed to an obesogenic maternal diet. FASEB J. 2016 Apr; 30(4): 1548–1556. doi: 10.1096/fj.15-280800.
- Ene A.C., Park S., Edelmann W., Taketo T. Caspase 9 is constitutively activated in mouse oocytes and plays a key role in oocyte elimination during meiotic prophase progression. Dev Biol 2013; 377: 213–223. doi: 10.1016/j.ydbio.2013.01.027
- Klein N.A., Harper A.J., Houmard B.S., Sluss P.M., Soules M.R. Is the short follicular phase in older women secondary to advanced or accelerated dominant follicle development? J Clin Endocrinol Metab. 2013; 87: 5746–5750 DOI:10.1210/jc.2002-020622.
- Wu Y., Zhang N., Li Y.H., Zhao L., Yang M., Jin Y., Xu Y.N., Guo H. Citrinin exposure affects oocyte maturation and embryo development by inducing oxidative stress-mediated apoptosis. Oncotarget. 201728. doi:10.18632/oncotarget.15776. https://doi.org/10.18632/oncotarget.15776
- Ogino M., Tsubamoto H., Sakata K., et al. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J Assist Reprod Genet. 2016; 33(3): 367-71. https://doi.org/10.1007/s10815-015-0621-0
- Turner N., Robker R. Developmental programming of obesity and insulin resistance: does mitochondrial dysfunction in oocytes play a role? MolHumReprod. 2015; 21(1): 23-30. doi:10.1093/molehr/gau042
- Скулачев В., Богачев А., Каспаринский Ф. Мембранная биоэнергетика М.: Изд-во Московского ун-та, 2010. 367 c. eLIBRARY ID: 19502192
- Grindler N.M., Moley K.H. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod. 2013; 19(8): 486-94. http://dx.doi.org/10.1093/molehr/gat026
- Cagnone G., Tsai T-S., Makanji Y., Matthews P.M., Gould J.A., Bonkowski M.S., et al. Restoration of normal embryogenesis by mitochondrial supplementation in pig oocytes exhibiting mitochondrial DNA deficiency. Scientific Reports. 2016; 6: 1-15. 23229. https://doi.org/10.1038/srep23229
- Simsek-Duran F., Li F., Ford W., Swanson R.J., Jones H.W. Jr., Castora F.J..Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS One. 2013; 8: e64955. doi: 10.1371/journal.pone.0064955. Print 2013.
- Dumesic D.A., Guedikian A.A., Madrigal V.K., Phan J.D., Hill D.L., Alvarez J.P., Chazenbalk G.D. Cumulus cell mitochondrial resistance to stress in vitro predicts oocyte development during assisted reproduction. J Clin Endocrinol Metab 2016; 101: 2235-45. doi: 10.1210/jc.2016-1464
- Desquiret-Dumas V., Clément A., Seegers V., Boucret L., Ferré-L’Hotellier V., Bouet P.E., Descamps P., Procaccio V., Reynier P., May-Panloup P. The mitochondrial DNA content of cumulus granulosa cells is linked to embryo quality. Hum Reprod. 2017; 32(3): 607-614. doi: 10.1093/humrep/dew341
- Taugourdeau A., Desquiret-Dumas V., Hamel J. F., Chupin S., Boucret L., Ferré-L’Hotellier V., Bouet P.E., Descamps P., Procaccio V., Reynier P., May-Panloup P. The mitochondrial DNA content of cumulus cells may help predict embryo implantation. J Assist Reprod Genet. 2019; 36 (2): 223-228.https://doi.org/10.1007/s10815-018-1348-5
- Gardner D.K., Schoolcraft W.B. In Vitro Culture of Human Blastocyst. In: Jansen R. and Mortimer D., Eds., Towards Reproductive Certainty: Infertility and Genetics Beyond, Parthenon Press, Carnforth, 1999; 377-388.
- Boucret L., Chao de la Barca J.M., Moriniere C., Desquiret V., Ferre-L’Hotellier V., Descamps P., Marcaillou C., Reynier P., Procaccio V., May-Panloup P. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum Reprod. 2015; 30: 1653-64. doi: 10.1093/humrep/dev114.
- Королькова А.И., Мишиева Н.Г., Мартазанова Б.А., Бурменская О.В., Екимов А.Н., Трофимов Д.Ю., Веюкова М.А., Кириллова А.О., Абубакиров А.Н. Повышение эффективности программ ЭКО на основании определения копийности митохондриальной ДНК в трофэктодерме эмбрионов. Акушерство и гинекология. 2019; 3: 98-104. [Korolkova A.I., Mishieva N.G., Martazanova B.A., Bourmenskaya O.V., Ekimov A.N., Trofimov D.Yu., Veyukova M.A., Kirillova A.O., Abubakirov A.N. Increasing the effectiveness of IVF programs by determining mitochondrial DNA copy number in embryonic trophectoderm. Akusherstvo i Ginekologiya/Obstetrics and Gynecology.2019; (3): 98-104. (In Russian)]. https://dx.doi.org/10.18565/aig.2019.3.98-104
- Горшинова В.К., Цвиркун Д.В., Десяткова Н.В., Высоких М.Ю., Смольникова В.Ю. Дисфункция митохондрий как один из механизмов нарушения репродуктивной функции при ожирении. Акушерство и гинекология. 2014; 7: [Gorshinova V.K., Tsvirkun D.V., Desyatkova N.V., Vysokikh M.Yu., Smolnikova V.Yu. Mitochondrial dysfunction as one of the mechanisms of impaired reproductive function in obesity. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2014; 7: 9-13. (In Russian)].
Received 28.05.2019
Accepted 21.06.2019
About the Authors
Anna I. Korolkova, PhD student of the1-st gynecology department, Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of Ministryof Healthcare of Russian Federation, Mosсow. Address: 117997 Moscow, ak. Oparinastreet, 4. Тel: +7(915)322-08-79, E- mail: zaikinaai@icloud.com
Nona G. Mishieva, MD, Senior researcher of 1-st gynecology department, Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of Ministry
of Healthcare of Russian Federation, Mosсow. Address: 117997 Moscow, ak. Oparina street, 4. Тel: +7(910)424-41-97, E- mail: nondoc555@mail.ru.
Beiia A. Martazanova, Ph.D. of the1-st gynecology department, Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of Ministry of Healthcare of Russian Federation, Mosсow. Address: 117997 Moscow, ak. Oparina street, 4. Тel: +7(910)424-41-97, E-mail: bellamart88@mail.ru
Olga V. Bourmenskaya, MD, Researcher of Molecular-genetic Laboratory, Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of Ministry
of Healthcare of Russian Federation, Mosсow. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +7(495)4382292. E-mail: o_bourmenskaya@oparina4.ru
Maria A. Veyukova, PhD, embryologist of the 1-st gynecology department, Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of Ministry
of Healthcare of Russian Federation, Moscow. Adress: 117997 Mosсow, ak. Oparina street, 4. Тel:+7 (495) 438-26-22. Е-mail: veymary@gmail.com
Dmitry Yu. Trofimov, MD, Head of the Laboratory of Molecular Genetic Techniques, Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology
of Ministry of Healthcare of Russian Federation, Mosсow. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: d_trofimov@oparina4.ru
Aidar N. Abubakirov, PhD, Head of the 1-st gynecology department, Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of Ministry
of Healthcare of Russian Federation, Moscow. Adress: 117997 Mosсow, ak. Oparina street, 4. Тel:+7 (495)438-26-22. Е-mail: nondoc555@yahoo.com.
Alexey N. Ekimov, MD, Geneticist, Laboratory of Molecular Genetic Techniques, Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology
of Ministry of Healthcare of Russian Federation, Mosсow. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: a_ekimov@oparina4.ru
For citation: Korolkova A.I., Mishieva N.G., Martazanova B.A., Burmenskaya O.V., Veyukova M.A., Ekimov A.N., Trofimov D.Yu., Abubakirov A.N. Implications of mitochondrial DNA copy number in cumulus cells in late reproductive-age women.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 10:108-14. (In Russian).
https://dx.doi.org/10.18565/aig.2019.10.108-114



