Characteristics of embryonic mosaicism in infertility treatment with assisted reproductive technologies
Relevance. Although preimplantation genetic testing for aneuploidy (PGT-A) has become a routine practice in ART, it is essential to assess its results accurately. Embryonic mosaicism is one of the reasons for errors in the interpretation of PGT-A results.Makarova N.P., Ekimov A.N., Kulakova E.V., Drapkina Yu.S., Sysoeva A.P., Krasnova N.A., Kalinina E.A.
Materials and methods. Trophectoderm biopsy samples from 17 embryos were analyzed using next-generation sequencing (NGS). All embryos were aneuploid. They were divided into three parts: cells of the inner cell mass (ICM), trophectoderm No.1, and trophectoderm No.2.
Results. Additional chromosomal abnormalities were found in cell samples from different parts of the embryo. The No.1 embryo had a deletion and duplication in cells of ICM in one of the chromosomes, and monosomy was found in the sample of trophectoderm No. 2. There was a duplication in cells of ICM in embryo No. 2.
Conclusion. The findings suggest that a biopsy of a part of the trophectoderm may not be representative of all trophectoderm and ICM cells. Therefore, first-trimester screening between 11 and 13 weeks gestation should be recommended for women with infertility planning to undergo ART.
Keywords
In human embryos, chromosomal mosaicism is defined as the coexistence of two or more genetically different cell lines within one embryo. Mosaicism is a widespread occurrence in humans that is widely discussed in assisted reproductive technologies (ART) [1]. Mosaicism can occur due to mitotic crossing over, somatic mutations in the zygote or the early cleavage stages, segregation errors during cell division, and gene therapy [2]. Traditionally, it was believed that only genetic abnormalities in oocytes or sperm are responsible for abnormal embryos. However, it has now been proven that mosaicism occurs in the first few divisions after fertilization [3]. During the preimplantation period, mosaicism is relatively common, but the level of mosaicism fluctuates at different stages of embryonic development. It is believed that mosaicism reaches maximum level on days 2–3 of growth and decreases by 5–6 days after fertilization. The ability of embryos to independently compensate for mosaicism as they develop reflects the possible physiological nature of this phenomenon and the need for a more thorough interpretation of the results of preimplantation genetic testing (PGT) [4].
There are several hypotheses regarding the declining incidence of mosaicism from the cleavage to blastocyst stages of preimplantation development. The embryonic mortality model indicates that embryos with high mosaicism do not survive to implant. The clonal depletion model describes apoptosis or reduced propagation of aneuploid cells within a mosaic embryo. And the third model describes the self-correction of aneuploidies, which allow cells with monosomy or trisomy to divide into cells with a euploid number of chromosomes [5, 6].
The rate of mosaicism detected by trophectoderm biopsy ranges from 4 to 32%. Newer molecular cytogenetic techniques such as next-generation sequencing (NGS) provide a detection rate of mosaicism ranging from 20 to 80% [7]. Nevertheless, depending on the severity of mosaicism and its location, the result of the embryo trophectoderm biopsy and the subsequent interpretation of the PGT-A (preimplantation genetic testing for aneuploidy) does not always correspond to the actual indicators. Mosaicism can affect the entire embryo and its two distinct parts, the trophectoderm, which will become the placenta, and the inner cell mass (ICM), which will become the fetus. When the mosaicism is confined to the ICM, the trophectoderm biopsy will always be fully euploid. If trophectoderm biopsy is performed in a mosaic embryo with mosaic cells in the trophectoderm, but the biopsy sample includes euploid cells, the result of PGT-A will be interpreted in favor of a euploid embryo. The table shows types of blastocyst mosaicism and options of trophectoderm biopsy. The presented data confirm that the percentage of mosaicism in a biopsy sample may not always be a direct indicator of the percentage of mosaicism in the entire embryo [8].
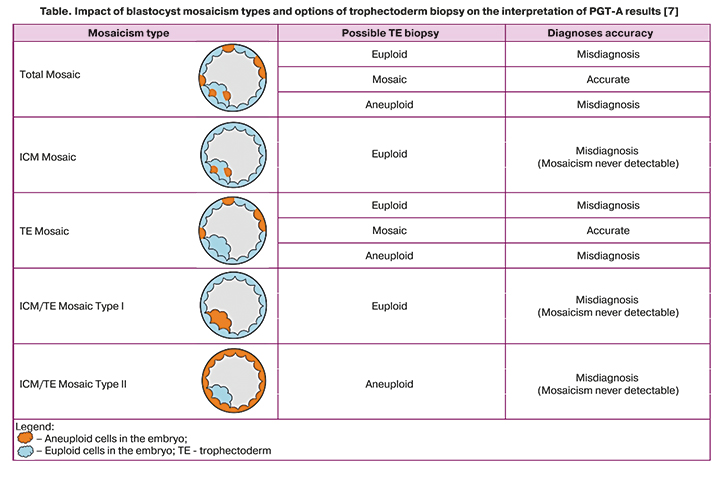
According to the 2019 Preimplantation Genetic Diagnosis International Society (PGDIS) Position Statement, if the percentage of aneuploid DNA-load in a biopsy specimen is 20–80%, an embryo is considered “mosaic.” Below 20%, a biopsy is considered “normal,” while above 80%, it is deemed “aneuploid.”
Mosaic embryos differ in the extent (low, moderate, or high) and the type of mosaicism (segmental, single chromosome, double chromosome, or complex mosaicism) [9]. The results of several large studies have shown that embryos classified as a mosaic in PGT-A might lead to ongoing pregnancies and healthy births in ART [10]. Spinella F. et al. reported that the pregnancy outcome after transferring a mosaic embryo depends on the extent of mosaicism and the type of aneuploidy [11].
There are suggestions that in somatic cells, mosaicism has a beneficial effect on the body. For example, mosaicism occurs very often in neurons, making it possible to diversify their functional status [12–14]. However, most often, mosaicism is associated with adverse outcomes, for example, increased rates of abortions, birth defects, and developmental disorders. The more significant is the extent of embryo mosaicism, the lower the likelihood of implantation and the higher the risk of miscarriage. The literature reports a statistically significant decrease in the incidence of clinical pregnancy, implantation, and live birth during the transfer of embryos with a high level of mosaicism, compared with mosaic embryos with a lower percentage of aneuploidy [11]. It should be noted that the incidence of mosaicism, in contrast to the incidence of aneuploidies, does not depend on maternal age [15].
First introduced in the late 1980s, preimplantation genetic screening (PGS) has provided unprecedented insight into the frequency and characteristics of aneuploidy in human embryos [16]. Based on the trophectoderm biopsy, PGT-A analyzes the euploid/aneuploid status of the blastocyst to select euploid embryos for transfer, increasing live birth rates after IVF [17]. Initially, the PGT-A was performed on single blastomeres of cleavage-stage embryos on day three after fertilization. Still, the results of randomized controlled trials showed no improvement in live birth rates compared with the control group (without PGT) [18]. According to modern protocols, PGT-A is recommended to be performed on samples of 5–10 trophectoderm cells collected from a blastocyst on day 5 of culture. PGT-A is recommended on day five after fertilization due to the higher development potential in such embryos, the lower risk of embryo trauma, and the possibility of biopsy of a more significant number of cells without harming further development, as well as due to the lower level of mosaicism at the blastocysts stage [19].
PGT-A made it possible to optimize the choice of embryos for transfer in ART programs, which, in turn, led to higher pregnancy rates, a decrease in reproductive losses, and the risk of having children with genetic disorders [20]. According to the approved clinical guidelines for the diagnosis and treatment of infertility, published in 2019, PGT-A is recommended in the following situations [21]: women of older reproductive age, a history of 2 or more spontaneous abortions, repeat failed "fresh" or thawed embryo transfers, and severe spermatogenesis disorders in men.
Mosaic embryo transfer in ART
Greco and colleagues reported clinical outcomes after transferring mosaic embryos for the first time in 2015 [10]. Further, multiple studies have shown that compared to euploid embryos, mosaic embryo transfer has been associated with decreased implantation rates and increased risk of miscarriage [22]. PGDIS suggested that embryos showing mosaic euploid/monosomy are preferable to euploid/trisomy, given that monosomic embryos (excepting 45, X) are not viable. When transferring an embryo with trisomy on one chromosome, it is necessary to consider the extent of mosaicism and the specificity of the chromosome. Mosaic embryos with trisomy for chromosomes 1, 3, 4, 5, 6, 8, 10, 11, 12, 17, 19, 20, 22, X, Y could be considered for transfer. Certain chromosomes (particularly 2, 7, 16) may increase the risk of intrauterine growth restriction
In the ART, it is not recommended to transfer embryos with trisomies 13, 18, and 21, which occasionally lead to births resulting in Patau, Edwards, and Down’s syndromes, respectively [23]. It is worth noting that mosaic embryos may be the only embryos available for transfer. The study by Munne et al. reported that pregnancy rates differ after transferring euploid and mosaic embryos (63 and 37%, respectively). Embryos with a low proportion of abnormal cells result in viable normal ongoing pregnancies, while high-level mosaics result in fewer viable pregnancies [24, 25]. Nevertheless, the opposite situations have also been described, when, during the transfer of a euploid embryo based on the results of PGT-A, the pregnancy was terminated in the early stages or ended in the birth of a child with chromosomal abnormalities [26]. Thus, it should be emphasized that PGT-A results should be treated in a highly differentiated manner since the cell sample obtained by trophectoderm biopsy may not always represent the chromosomal state of the entire embryo.
Materials and methods
The present study was conducted at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility and the Laboratory of Molecular Genetics. The study used the NGS to analyze the trophectoderm of 17 embryos not subject to transfer into the uterine cavity. Fertilization of oocytes was carried out by the ICSI, followed by transferring the fertilized cells to the CSCM culture medium (Irvine Sc., USA) for further culture. The onset of the stage of two pronuclei (zygote formation) was assessed 14–16 h after fertilization. All steps of culture were carried out in multi-gas incubators СООК (Ireland) in 25μl droplets under oil (Irvine Sc., USA). The CSCM medium (Irvine Sc., USA) was not changed during five days of culture. On day five after fertilization, trophectoderm biopsy was performed, followed by cryopreservation of biopsied embryos. The obtained trophectoderm cells were transferred into Eppendorf tubes containing lysis buffer for molecular genetic diagnostics of the samples under study. The PGT-A procedure consisted of several stages. At the first stage, whole genome amplification and preparation of the library for application to the chip were carried out. To create a library, special molecular barcode labels unique for each sample were attached to the DNA fragments. Then ion semiconductor sequencing was performed, followed by bioinformatic analysis of the results and preparation of a conclusion based on the data obtained according to the standard PGT-A [27].
Results
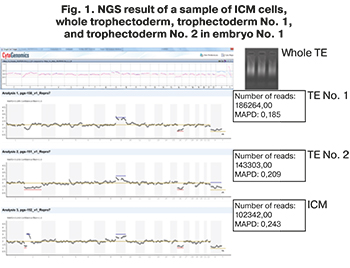 NGS findings showed that all embryos were aneuploid and not recommended for transfer. The couple having aneuploid embryos signed informed consent to use embryos for research. Aneuploid embryos were separated using a laser and a micromanipulator into 3 parts: ICM, trophectoderm sample No. 1, trophectoderm sample No. 2. The obtained cells of three parts of aneuploid embryos were sent for repeated NGS analysis. In 15 embryos, the initially detected chromosome set coincided with the samples of cells obtained after dividing the embryo into three parts. However, in two embryos, additional chromosomal abnormalities were found in a sample of cells from ICM and trophectoderm No. 1 and No. 2, shown in Figures 1 and 2. In embryo No. 1, during NGS, additional chromosomal abnormalities were found that were not detected in the sample of the whole trophectoderm cells (Fig. 1). Cells from ICM in embryo No.1 contained a deletion and duplication in one of the chromosomes, and monosomy was found in trophectoderm sample No. 2.
NGS findings showed that all embryos were aneuploid and not recommended for transfer. The couple having aneuploid embryos signed informed consent to use embryos for research. Aneuploid embryos were separated using a laser and a micromanipulator into 3 parts: ICM, trophectoderm sample No. 1, trophectoderm sample No. 2. The obtained cells of three parts of aneuploid embryos were sent for repeated NGS analysis. In 15 embryos, the initially detected chromosome set coincided with the samples of cells obtained after dividing the embryo into three parts. However, in two embryos, additional chromosomal abnormalities were found in a sample of cells from ICM and trophectoderm No. 1 and No. 2, shown in Figures 1 and 2. In embryo No. 1, during NGS, additional chromosomal abnormalities were found that were not detected in the sample of the whole trophectoderm cells (Fig. 1). Cells from ICM in embryo No.1 contained a deletion and duplication in one of the chromosomes, and monosomy was found in trophectoderm sample No. 2.
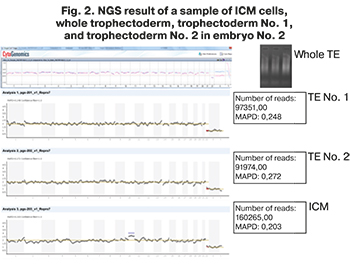 In a sample of ICM cells obtained from embryo No. 2, additional chromosomal rearrangements were also found that were not described in the NGS results of the whole trophectoderm biopsy. A duplication was found on one of the chromosomes, shown in Figure 2.
In a sample of ICM cells obtained from embryo No. 2, additional chromosomal rearrangements were also found that were not described in the NGS results of the whole trophectoderm biopsy. A duplication was found on one of the chromosomes, shown in Figure 2.
The study findings suggest that a biopsy of a part of the trophectoderm may not be representative of all trophectoderm and ICM cells. Therefore, despite current recommendations to optimize the selection of competent mosaic preimplantation embryos for further transfer, there are still some unresolved issues. One of them is an accurate diagnosis of embryonic mosaicism. Limitations in diagnostics are associated with obtaining biological material for genetic analysis, which often does not allow reliable assessment of mosaicism in the analyzed embryo.
Discussion
One of the main reasons for the misinterpretation of PGT-A results is the frequent occurrence of mosaicism, which may lead to difficulties in correctly diagnosing the actual chromosomal status of the embryo. Chromosomal mosaicism can occur in embryos during the preimplantation period. However, these errors most often are found during the first three days of culture, and as the embryo develops further, it can self-correct these abnormalities [2].
The presence of mosaicism in embryonic cells can be detected using fluorescent in situ hybridization, polymerase chain reaction, comparative genomic hybridization using microarrays (a-CGH), and the NGS [28]. NGS is the most specific and sensitive technique for diagnosing embryonic mosaicism, since the detection rate of these disorders using NGS is the highest compared to a-CGH and amounts to 10 and 3%, respectively [29]. Considering that the literature describes the cases of the birth of children with a balanced karyotype after the transfer of mosaic embryos in the ART, the presence of less than 50% of aneuploid cells in the trophectoderm biopsy in an embryo in the early stages of preimplantation development has a favorable prognosis for further pregnancy [1, 10]. However, there are opposite situations when a more detailed analysis of the embryo may reveal additional chromosomal abnormalities that affect the potential of implantation and further embryo development. To reduce the risk of false-negative results, PGT-A is recommended on day five after fertilization, when the detection rate of mosaicism is minimal due to the natural elimination of embryos with chromosomal aberrations. Although no method has been developed to solve the problem of mosaicism, additional, promising markers are being actively studied to improve the accuracy of diagnosis of chromosomal abnormalities in the embryo using PGT-A.
According to research evidence, the karyotype of blastocyst fluid is similar to that of trophectoderm cells. The fraction of extracellular DNA identified in the blastocyst fluid allows for testing for monogenic mutations and, possibly, will solve several issues in diagnosing chromosomal mosaicism in the future [30, 31]. Palini et al. (2013) demonstrated that extracellular DNA is secreted into blastocyst fluid from cells undergoing apoptosis. Nevertheless, it is not known whether the DNA of all cells (with both euploid and aneuploid chromosomes) enters blastocyst fluid and whether the secretion of extracellular DNA depends on the location of cells (ICM or trophectoderm) [32]. Thus, the question of using blastocyst as a new source of material for embryo chromosomal analysis remains promising but requires further research.
In 2021, Shitara et al. published the results of a study on the use of cell-free DNA in spent culture medium as a biomarker of the embryo chromosomal set. The study included 20 cryopreserved blastocysts donated by 12 patients. On day six after fertilization, a trophectoderm biopsy of the embryo was performed. On day 6, cell-free DNA in the spent culture medium was subjected to chromosome analysis using NGS. After a biopsy, the embryos were cultured for up to 10 days, followed by NGS on five samples of 8-day-old embryos, five samples of 9-day-old embryos, and 10 samples of 10-day-old embryos. All samples were divided into euploid (group I), aneuploid (group II), and mosaic embryos (group III) depending on the results of trophectoderm biopsy, analysis of cell-free DNA in spent culture medium, and whole genome amplification of embryos cultured for more than 6 days. It was found that the sensitivity of non-invasive diagnosis of aneuploidy using cell-free DNA in spent culture medium is 100%, the specificity is 87.5%, which exceeded these indicators when testing the trophectoderm (sensitivity – 87.5%, specificity – 77.8%) [ 33]. Thus, the determination of cell-free DNA in spent culture medium for diagnosing the embryo chromosomal status is a highly accurate, non-invasive, and promising technology that requires further study.
Considering that PGT-A is widely used as a valuable automated technology that combines all the essential qualities of the most objective diagnostic methods, the accurate interpretation of its results is critical. It should be emphasized that after euploid blastocyst transfer and the onset of ongoing pregnancy, first-trimester screening is recommended between 11 and 13 weeks of gestation. Screening of the first trimester includes ultrasound measurement of the nuchal thickness of the developing fetus and the study of biochemical blood markers (CG, PAPP-A), followed by an individual analysis of the risk of having a child with chromosomal abnormalities [34]. In a patient with a high risk of fetal aneuploidy based on the first-trimester screening, a consultation with a geneticist and prenatal diagnostics, including chorionic villus sampling or amniocentesis, depending on the gestational age, is recommended. To exclude fetal aneuploidy, the couple may additionally be offered non-invasive prenatal screening.
This study showed that the chromosomal set of cells obtained from different structures of the embryo might differ. Embryos with chromosomal mosaicism, on the one hand, can lead to the birth of healthy children with a balanced karyotype. On the other hand, depending on the extent and type of mosaicism, they can cause pregnancy loss or the birth of children with developmental defects, growth restriction, or mental retardation [35].
Conclusion
PGT-A does not guarantee the transfer of euploid blastocysts, even despite the undeniable advantages of this technology in preventing chromosomal pathology. This study’s findings suggest that a biopsy of a part of the trophectoderm may not be representative of all trophectoderm and ICM cells.
Currently, additional markers and diagnostic modalities for embryonic mosaicism are being actively investigated. Nevertheless, when a pregnancy occurs after transferring a euploid embryo based on PGT-A, the mandatory first-trimester screening allows detection of abnormal pregnancy and timely surgical correction of the identified defects.
References
- McCoy R.C. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 2017; 33(7): 448-63. https://dx.doi.org/10.1016/j.tig.2017.04.001.
- Taylor T.H., Gitlin S.A., Patrick J.L., Crain J.L., Wilson J.M., Griffin D.K. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum. Reprod. Update. 2014; 20(4): 571-81. https://dx.doi.org/10.1093/humupd/dmu016.
- Capalbo A., Bono S., Spizzichino L., Biricik A., Baldi M., Colamaria S. et al. Sequential comprehensive chromosome analysis on polar bodies, blastomeres and trophoblast: insights into female meiotic errors and chromosomal segregation in the preimplantation window of embryo development. Hum. Reprod. 2013; 28(2): 509-18. https://dx.doi.org/10.1093/humrep/des394.
- Munné S., Blazek J., Large M., Martinez-Ortiz P.A., Nisson H., Liu E. et al. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil. Steril. 2017; 108(1): 62-71. https://dx.doi.org/10.1016/j.fertnstert.2017.05.002.
- Popovic M., Dhaenens L., Boel A., Menten B., Heindryckx B. Chromosomal mosaicism in human blastocysts: the ultimate diagnostic dilemma. Hum. Reprod. Update. 2020; 26(3): 313-34. https://dx.doi.org/10.1093/humupd/dmz050. Erratum in: Hum. Reprod. Update. 2020; 26(3): 450-1.
- Малышева О.В., Бичевая Н.К., Гзгзян А.М., Глотов О.С., Кинунен А.А., Лобенская А.Ю., Мекина И.Д., Полякова И.В., Пуппо И.Л., Сайфитдинова А.Ф., Щербак С.Г., Коган И.Ю. Технологические платформы преимплантационного генетического тестирования на анеуплоидии: сравнительная эффективность диагностики хромосомной патологии. Акушерство и гинекология. 2020; 4: 65-71. [Маlysheva О.V., Bichevaya N.K., Gzgzyan А.М., Glotov О.S., Kinunen А.А., Lobenskaya А.Yu., Меkina I.D., Polyakova I.V., Puppo I.L., Saifitdinova А.F., Shcherbak S.G., Kоgan I.Yu. Technological platforms for preimplantation genetic testing for aneuplody: comparative effectiveness of diagnosing chromosomal abnormalities. Obsteterics and Gynecology. 2020; 4: 65-71. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.4.65-71.
- Vera-Rodriguez M., Rubio C. Assessing the true incidence of mosaicism in preimplantation embryos. Fertil. Steril. 2017; 107(5): 1107-12. https://dx.doi.org/10.1016/j.fertnstert.2017.03.019.
- Chuang T.H., Chang Y.P., Lee M.J., Wang H.L., Lai H.H., Chen S.U. The incidence of mosaicism for individual chromosome in human blastocysts is correlated with chromosome length. Front. Genet. 2021; 11: 565348. https://dx.doi.org/10.3389/fgene.2020.565348.
- Preimplantation Genetic Diagnosis International Society. PGDIS position statement on chromosome mosaicism and preimplantation aneuploidy testing at the blastocyst stage. Available at: https://www. pgdis-position-statement-chromosome-mosaicism-testing date: 2016. Accessed April 5, 2017.
- Greco E., Minasi M.G., Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N. Engl. J. Med. 2015; 373(21): 2089-90. https://dx.doi.org/10.1056/NEJMc1500421.
- Spinella F., Fiorentino F., Biricik A., Bono S., Ruberti A., Cotroneo E. et al. Extent of chromosomal mosaicism influences the clinical outcome of in vitro fertilization treatments. Fertil. Steril. 2018; 109(1): 77-83. https://dx.doi.org/10.1016/j.fertnstert.2017.09.025.
- Besser A.G., Mounts E.L. Counselling considerations for chromosomal mosaicism detected by preimplantation genetic screening. Reprod. Biomed. Online. 2017; 34(4): 369-74. https://dx.doi.org/10.1016/j.rbmo.2017.01.003.
- Карандашева К.О., Пащенко М.С., Демина Н.А., Акимова И.А., Макиенко О.Н., Петухова М.С., Бессонова Л.А., Анисимова И.В., Танас А.С., Залетаев Д.В., Стрельников В.В., Кузнецова Е.Б. Соматический мозаицизм при нейрофиброматозе первого типа. Медицинская генетика. 2019; 18(5): 28-36. [Karandasheva K.O., Paschenko M.S., Demina N.A., Akimova I.A., Makienko O.N., Petukhova M.S., Bessonova L.A., Anisimova I.V., Tanas A.S., Zaletaev D.V., Strelnikov V.V., Kuznetsova E.B. Somatic mosaicism in first type neurofibromatosis. Medical genetics. 2019; 18(5): 28-36. (in Russian)].
- D'Gama A.M., Walsh C.A. Somatic mosaicism and neurodevelopmental disease. Nat. Neurosci. 2018; 21(11): 1504-14. https://dx.doi.org/10.1038/s41593-018-0257-3.
- Sekhon L., Feuerstein J., Nazem T.G., Briton-Jones C., Lee J.A., Grunfeld L. et al. The incidence of mosaicism is not associated with advanced maternal age or diminished ovarian reserve. Fertil. Steril. 2017; 108(3): e217. https://dx.doi.org/10.1016/j.fertnstert.2017.07.643.
- Александрова Н.В., Шубина Е.С., Екимов А.Н., Кодылева Т.А., Мукосей И.С., Макарова Н.П., Кулакова Е.В., Левков Л.А., Барков И.Ю., Трофимов Д.Ю., Сухих Г.Т. Сравнение результатов преимплантационного генетического скрининга, проведенного методами CGH и NGS. Молекулярная биология. 2017; 51(2): 308-13. [Alexandrova N.V., Shubina E.S., Ekimov A.N., Kodyleva T.A., Mukosey I.S., Makarova N.P., Kulakova E.V., Levkov L.A., Barkov I.Yu., Trofimov D.Yu., Sukhikh G.T. Comparison of CGH and NGS preimplantation genetic screening results. Molecular biology. 2017; 51(2): 308-13. (in Russian)].
- Mastenbroek S., Twisk M., van der Veen F., Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum. Reprod. Update. 2011; 17(4): 454-66. https://dx.doi.org/10.1093/humupd/dmr003. Erratum in: Hum. Reprod. Update. 2013; 19(2): 206.
- Adler A., Lee H.L., McCulloh D.H., Ampeloquio E., Clarke-Williams M., Wertz B.H., Grifo J. Blastocyst culture selects for euploid embryos: comparison of blastomere and trophectoderm biopsies. Reprod. Biomed. Online. 2014; 28(4): 485-91. https://dx.doi.org/10.1016/j.rbmo.2013.11.018.
- Краснопольская К.В., Сесина Н.И., Воскобоева Е.Ю. Использование различных методов биопсии эмбриона для преимплантационного генетического тестирования (обзор литературы). Проблемы репродукции. 2019; 25(6): 44-50. [Krasnopolskaya K.V., Sesina N.I., Voskoboeva E. Yu. Using various methods of embryo biopsy for preimplantation genetic testing (literature review). Reproduction problems. 2019; 25(6): 44-50. (in Russian)].
- Коротченко О.Е., Сыркашева А.Г., Долгушина Н.В., Кулакова Е.В., Докшукина А.А., Екимов А.Н. Эффективность преимплантационного генетического скрининга у пациенток с привычным невынашиванием беременности и бесплодием. Акушерство и гинекология. 2018; 3: 64-9. [Korotchenko O.E., Syrkasheva A.G., Dolgushina N.V., Kulakova E.V., Dokshukina A.A., Ekimov A.N. The effectiveness of preimplantation genetic screening in patients with recurrent miscarriage and infertility. Obstetrics and gynecology. 2018; 3: 64-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.3.64-69.
- Клинические рекомендации (протокол лечения). Женское бесплодие (современные подходы к диагностике и лечению), разработанные в соответствии со статьей 76 Федерального закона от 21 ноября 2011 г. № 323-ФЗ «Об основах охраны здоровья граждан в Российской Федерации» – письмо МЗРФ от 05.03.2019 г. №15-4/ и /2-1913. [Clinical guidelines (treatment protocol) "Female infertility (modern approaches to diagnostics and treatment)", developed in accordance with Article 76 of the Federal Law of November 21, 2011 No. 323-FL "Protecting the health of citizens in the Russian Federation" – Letter of the Ministry of Healthcare of the Russian Federation dated 05.03.2019 No. 15-4 / and / 2-1913. (in Russian)].
- Abhari S., Kawwass J.F. Pregnancy and Neonatal Outcomes after Transfer of Mosaic Embryos: A Review. J Clin Med. 2021 Mar 27; 10(7): 1369. https://dx.doi.org/10.3390/jcm10071369.
- Gleicher N., Albertini D.F., Barad D.H., Homer H., Modi D., Murtinger M. et al. The 2019 PGDIS position statement on transfer of mosaic embryos within a context of new information on PGT-A. Reprod. Biol. Endocrinol. 2020; 18(1): 57. https://dx.doi.org/10.1186/s12958-020-00616-w.
- Viotti M., Victor A.R., Barnes F.L., Zouves C.G., Besser A.G., Grifo J.A. et al. Using outcome data from one thousand mosaic embryo transfers to formulate an embryo ranking system for clinical use. Fertil. Steril. 2021; 115(5): 1212-24. https://dx.doi.org/10.1016/j.fertnstert.2020.11.041.
- Munné S., Spinella F., Grifo J., Zhang J., Beltran M.P., Fragouli E., Fiorentino F. Clinical outcomes after the transfer of blastocysts characterized as mosaic by high resolution Next Generation Sequencing- further insights. Eur. J. Med. Genet. 2020; 63(2): 103741. https://dx.doi.org/10.1016/j.ejmg.2019.103741.
- Maxwell S.M., Colls P., Hodes-Wertz B., McCulloh D.H., McCaffrey C., Wells D. et al. Why do euploid embryos miscarry? A case-control study comparing the rate of aneuploidy within presumed euploid embryos that resulted in miscarriage or live birth using next-generation sequencing. Fertil. Steril. 2016; 106(6):1414-9. https://dx.doi.org/10.1016/j.fertnstert.2016.08.017.
- Gui B., Zhang Y., Liang B., Kwok Y.K.Y., Lui W.T., Yeung Q.S.Y. et al. Semiconductor sequencing for preimplantation genetic testing for aneuploidy. J. Vis. Exp. 2019 Aug 25; 150. https://dx.doi.org/10.3791/59273.
- Малышева О.В., Бичевая Н.К., Гзгзян А.М., Глотов О.С., Кинунен А.А., Лобенская А.Ю., Мекина И.Д., Полякова И.В., Пуппо И.Л., Сайфитдинова А.Ф., Щербак С.Г., Коган И.Ю. Технологические платформы преимплантационного генетического тестирования на анеуплоидии: сравнительная эффективность диагностики хромосомной патологии. Акушерство и гинекология. 2020; 4: 65-71. [Malysheva O.V., Bichevaya N.K., Gzgzyan A.M., Glotov O.S., Kinunen A.A., Lobenskaya A.Yu., Mekina I.D., Polyakova I.V., Puppo I.L., Sayfitdinova A.F., Shcherbak S.G., Kogan I.Yu. Technological platforms for preimplantation genetic testing for aneuploidy: comparative efficiency of diagnosis of chromosomal pathology. Obstetrics and gynecology. 2020; 4: 65-71. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.4.65-71.
- Sachdev N.M., McCulloh D.H., Kramer Y., Keefe D., Grifo J.A. The reproducibility of trophectoderm biopsies in euploid, aneuploid, and mosaic embryos using independently verified next-generation sequencing (NGS): a pilot study. J. Assist. Reprod. Genet. 2020; 37(3): 559-71. https://dx.doi.org/10.1007/s10815-020-01720-x.
- Скрябин Н.А., Лебедев И.Н., Артюхова В.Г., Жигалина Д.И., Степанов И.А., Кривощекова Г.В., Светлаков А.В. Молекулярное кариотипирование внеклеточной ДНК из жидкости бластоцеля как основа неинвазивного преимплантационного генетического скрининга анеуплоидий. Генетика. 2015; 51(11): 1301-7. [Skryabin N.A., Lebedev I.N., Artyukhova V.G., Zhigalina D.I. et al. Molecular karyotyping of extracellular DNA from blastocelial fluid for non-invasive preimplantation genetic screening for aneuploidy. Genetics. 2015; 51(11): 1301-7. (in Russian)].
- Qasemi M., Mahdian R., Amidi F. Cell-free DNA discoveries in human reproductive medicine: providing a new tool for biomarker and genetic assays in ART. J. Assist. Reprod. Genet. 2021; 38(2): 277-88. https://dx.doi.org/10.1007/s10815-020-02038-4.
- Palini S., Galluzzi L., De Stefani S.., Bianchi M, Wells D., Magnani M., Bulletti C. Genomic DNA in human blastocoele fluid. Reprod. Biomed. Online. 2013; 26(6): 603-10. https://dx.doi.org/10.1016/j.rbmo.2013.02.012.
- Shitara A., Takahashi K., Goto M., Takahashi H., Iwasawa T., Onodera Y. et al. Cell-free DNA in spent culture medium effectively reflects the chromosomal status of embryos following culturing beyond implantation compared to trophectoderm biopsy. PLoS One. 2021: 16(2): e0246438. https://dx.doi.org/10.1371/journal.pone.0246438.
- Шмаков Р.Г., Баев О.Р., Кан Н.Е., Пекарев О.Г., Полушкина Е.С., Клименченко Н.И. Ведение физиологической беременности: клинические рекомендации. Акушерство и гинекология. 2016; (12, Протоколы.): 20-39. [Shmakov R.G., Baev O.R., Kan N.E., Pekarev O.G., Polushkina E.S., Klimenchenko N.I. Physiological pregnancy management: clinical guidelines. Obstetrics and gynecology. 2016; (12. Protocols.): 20-39. (in Russian)].
- Victor A.R., Tyndall J.C., Brake A.J., Lepkowsky L.T., Murphy A.E., Griffin D.K., McCoy R.C., Barnes F.L., Zouves C.G., Viotti M. One hundred mosaic embryos transferred prospectively in a single clinic: exploring when and why they result in healthy pregnancies. Fertil Steril. 2019; 111(2): 280-93. https://dx.doi.org/10.1016/j.fertnstert.2018.10.019.
Received 18.03.2021
Accepted 29.03.2021
About the Authors
Natalia P. Makarova, Dr. Biol. Sci., Senior Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: np_makarova@oparina4.ru. ORCID: 0000-0003-8922-2878. 117997, Russia, Moscow, Academician Oparin str., 4.Alexey N. Ekimov, Clinical Geneticist at the Molecular Genetics Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: a_ekimov@oparina4.ru.
ORCID: 0000-0001-5029-0462. 117997, Russia, Moscow, Academician Oparin str., 4.
Elena V. Kulakova, Ph.D., Senior Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. E-mail: e_kulakova@oparina4.ru. ORCID: 0000-0002-4433-4163. 117997, Russia, Moscow, Academician Oparin str., 4.
Yulia S. Drapkina, Ph.D., Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility. Professor B.V. Leonov, V.I. Kulakov NMRC
for OG&P, Ministry of Health of Russia. E-mail: yu_drapkina@oparina4.ru. ORCID: 0000-0002-0545-1607. 117997, Russia, Moscow, Academician Oparin str., 4.
Anastasia P. Sysoeva, Embryologist at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. E-mail: sysoeva.a.p@gmail.com. ORCID: 0000-0002-6502-4498. 117997, Russia, Moscow, Academician Oparin str., 4.
Natalya A. Krasnova, Ph.D., Teaching Assistant at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University); Obstetrician-Gynecologist, Reproductologist at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: dr.krasnova@rambler.ru. ORCID: 0000-0001-8636-2560. 117997, Russia, Moscow, Academician Oparin str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: e_kalinina@oparina4.ru. ORCID: 0000-0002-8922-2878. 117997, Russia, Moscow, Academician Oparin str., 4.
For citation: Makarova N.P., Ekimov A.N., Kulakova E.V., Drapkina Yu.S., Sysoeva A.P., Krasnova N.A., Kalinina E.A. Characteristics of embryonic mosaicism in infertility treatment with assisted reproductive technologies.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 7: 144-151 (in Russian)
https://dx.doi.org/10.18565/aig.2021.7.144-151



