Clinical and molecular aspects of autologous embryo‑cumulus cells co‑culture in IVF programs
Objective: To assess the effectiveness of autologous embryo-cumulus cells co-culture in assisted reproductive technology (ART) programs for infertility treatment, as well as to analyze metabolic profile of culture media after culturing cumulus cells.Asfarova G.R., Smolnikova V.Yu., Makarova N.P., Bobrov M.Yu., Eldarov Ch.M., Zingerenko B.V., Kalinina E.A.
Materials and methods: 127 married couples who underwent infertility treatment using ART were tested. Embryological indicators and treatment effectiveness were assessed. The prospective study of metabolome in cumulus cells and culture media after cumulus cells co-culture was performed by high performance liquid chromatography and mass spectrometry using 90 samples of culture media after culturing cumulus cells, 90 samples of cumulus cells, as well as 10 control samples of culture media without cumulus cells.
Results: The prospective study of the influence of embryo-cumulus cells co-culture on the morphology of blastocysts and possibility of their implantation versus standard-based method using conventional culture media was performed. The study showed that autologous embryo-cumulus cells co-culture reduces the chances of implantation in women of late reproductive age by 1.2 times, and is not advisable for young patients. At the same time, co-culture significantly increases the number of high-quality blastocysts suitable for cryopreservation in the general cohort. This results in a greater likelihood of having a child with the use of a single ovarian stimulation protocol. Assessment of metabolism of cumulus cells showed that in culture media, co-cultivation increases the concentration of some amino acids (leucine, valine, serine) and amino-acid containing dipeptides. The obtained data suggest that cumulus cells are involved in lipid metabolism, initiating fatty acid oxidation, which is essential for normal embryonic development.
Conclusion: The results obtained in this study suggest that it is reasonable to refuse embryo-cumulus cells co-culture in women aged over 36 years, who had multiple IVF failures in anamnesis. Autologous embryo-cumulus cells co-culture significantly increases the number of high-quality embryos in young women.
Authors’ contributions: Asfarova G.R. – data collection and analysis, article writing; Smolnikova V.Yu. – patient selection, manuscript editing; Makarova N.P. – clinical study design, results analysis, article editing; Bobrov M.Yu. – design of the molecular part of the study, analysis of the results obtained; Eldarov Ch.M. – high-performance liquid chromatography and mass spectrometry of culture media; Zingerenko B.V. – embryo cultivation, assessment of embryological parameters of the study; Kalinina E.A. – editing and approval of the publication.
Conflicts of interest: The authors declare that they have no conflict of interests.
Funding: Financial support for the study was provided by the State Assignment of the Ministry of Health of the Russian Federation No. №121040600410-7 “Solution of the problem of infertility in modern conditions by developing clinical and diagnostic pathway for infertile married couples and using innovations in the programs of assisted reproduction technology”
Ethical Approval: The study was approved by the Bioethics Committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of the Russian Federation.
Patient Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Asfarova G.R., Smolnikova V.Yu., Makarova N.P.,
Bobrov M.Yu., Eldarov Ch.M., Zingerenko B.V., Kalinina E.A. Clinical and molecular aspects
of autologous embryo-cumulus cells co-culture in IVF programs.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2023; (4): 97-110 (in Russian)
https://dx.doi.org/10.18565/aig.2022.306
Keywords
The issues of infertility are relevant and significant for married couples of reproductive age worldwide, and for some of them, assisted reproductive technology (ART) for infertility treatment is the only way to give birth to a child. The quality of obtained genetic material plays a decisive role to achieve success in using ART programs. The gold standard for ovarian stimulation in ART programs is obtaining optimal number of high-quality oocytes and minimal side effects of fertility drugs used for infertility treatment [1].
The success of in vitro fertilization (IVF) programs is largely determined by the quality of obtained oocyte and embryo selection with high implantation potential [2]. At the same time, morphologically normal embryo transfer does not always result in getting pregnant, and in some cases pregnancy can be terminated at early terms. For this reason, solution of the problem of recurrent failures in IVF programs remains highly relevant. Impairment of embryo implantation is a leading reason for unsuccessful fertility treatment in IVF programs [3]. Apart from embryo quality, the major reasons of impaired implantation can be reduced endometrial receptivity or embryo-endometrial asynchrony. The sequential complex process of implantation is controlled by various molecular factors: signaling cytokines, growth factors, adhesion molecules [4–7].
Cumulus cells is a special group of cells that surround oocytes and are essential for nutrition of oocytes during growth and development. Cumulus cells coordinate oocyte maturation within the ovarian follicle, stimulate nuclear and cytoplasmic oocyte maturation, provide accumulation of energy substrates for resumption of meiosis and maturation of female gametes, that is necessary for pronucleus formation after fertilization, and determine further developmental ability [8]. The major function of cumulus cells is to ensure transport of signaling molecules and metabolites between oocyte and ovarian tissue. On the other hand, the oocyte in the maturing follicle secretes locally acting growth factors, controls the function and differentiation of cumulus cells [9].
Cumulus cells play an important role in bilateral signaling in the oocyte. The importance of these signaling pathways for the production of viable gametes is hard to overstate [9]. Taking into account bidirectional signaling between the oocyte and cumulus cells, it can be suggested that metabolic processes of cumulus oocyte complex (COC) are interrelated and significantly influence oocyte maturation and fertilization, as well as further embryonic development.
The meta-analysis by Kattan N. et al. found that autologous embryo-cumulus cells co-culture increases the effectiveness of ART programs in patients with recurrent failed IVF attempts in medical history [10]. Also, some researches demonstrated that embryo-cumulus cells co-culture improves the qualitative characteristics of blastocysts and increases the frequency of implantation due to detoxication of culture medium and secretion of embryotrophic substances (cytokines, growth factors, steroid hormones and interleukins) [11, 12]. Feeder cells ensure significant enrichment of culture medium in vitro, thereby providing embryonic development in the enriched culture environment [13]. In 1996, Quinn P. et al. were the first to conduct prospective study, which showed the difference in embryonic development between classical embryo culture and co-culture with cumulus cells [14]. In detailed study of influence mechanisms of cumulus cells on embryo implantation potential, Benkhalifa M. et al. showed that cumulus cells act as exogenous source of leukemia inhibitory factor and platelet activating factor [15]. These growth factors influence embryonic development, blastulation as well as implantation due to impact on protein phosphorylation, including tyrosine kinase, as has been shown in animal models [16]. In addition, the effect of woman's age on the quality of cumulus cells and metabolic profiling was studied [17]. The authors reported the effects of age-related molecular changes in cumulus cells on meiotic maturation, function and chromosomal divergence, cytoplasmic maturation of female gametes [17]. It should be noted that co-culture of embryos and feeder cells in the clinical practice is a modern method of improving infertility treatment, which is especially effective in women with recurrent failed attempts of ART [18, 19]. Several studies showed that differential expression of a number of genes in cumulus cells correlates with a high probability of successful embryonic development and implantation [20–23].
Cell culture in nutrient media and metabolomics assessment is a promising method to study cell metabolism, including cumulus cells. This approach was successfully used for spent culture media and found a significant difference in the biochemical profile of spent media after different quality embryo culture and implantation results [24], in pathologies of female reproductive system [25], as well as using different culture media [26]. Among variety of methods for assessment of metabolism of cells and tissues, chromatographic separation of metabolites with subsequent mass spectrometric detection is currently one of the most widely used technique. This technique has high sensitivity and specificity, as well simple sample preparation simplifies its introduction into routine clinical practice.
The purpose of the study was to assess the effects of autologous embryo–cumulus cells co-culture on blastocyst morphology and probability of their implantation versus the classical method using conventional culture, as well as metabolomics analysis both of cumulus cells and culture media after 5 days of cumulus cells culture.
Materials and methods
The study included the patients, who were admitted to Prof. B.V. Leonov Department of Assisted Technologies in Infertility Treatment of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation to undergo infertility treatment with ART. The clinical effect of embryo–cumulus cells co-culture was evaluated in 127 couple participating in the study, and metabolomic profile of culture media and cumulus cells were evaluated in 90 women. Informed consent was obtained from all patients involved. The study was approved by the Bioethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. The inclusion criteria were: patient age 18–40 years, regular menstrual cycle, preserved ovarian reserve (the basal levels of follicle-stimulating hormone (FSH) less than 12 mIU /mL, anti-Müllerian hormone (AMH) not less than 1.2 ng/ mL); fertilization throught intracytoplasmic sperm injection (ICSI); at least 2 failed IVF attempts in history (at least two programs of embryo transfer into the uterine cavity, including transfer of thawed embryos of good quality into the uterine cavity). Non-inclusion criteria were: contraindications for ART programs, including extragenital pathology and oncological diseases; obtaining of less than 2 mature oocytes on the day of transvaginal ovarian puncture (depletion of the ovarian reserve); laparoscopic and/or sonographic confirmation of genital endometriosis stage III–IV; interstitial and/or subserosal uterine fibroids of more than 4 cm, submucosal fibroids that distort the uterine cavity; endometrial pathology; genital anomalies; pronounced pathozoospermia.
In vitro fertilization program and methods of embryo culture
All patients in the study underwent mandatory medical examination before IVF cycle in accordance with the Order No. 803n of July, 2020 of the Ministry of Health of the Russian Federation “About the procedure for the use of assisted reproductive technologies, contraindications and limitations to the use". Both clinical examination and special examination tests were used depending on the clinical situation. Ovarian stimulation was performed according to standard protocol using recombinant FSH and gonadotropin-releasing hormone antagonist (GnRHa). Human chorionic gonadotropin (HCG) was administered at the dose of 10,000 IU to trigger final oocyte maturation. Follicle puncture was performed with atraumatic aspiration needles (VitroLife, Sweden) under general anesthesia in a small operating room. The obtained cumulus–oocyte complexes were collected from HEPESs-buffered culture media (Gamete Buffer, СООК, Ireland), and the biological material was subsequently placed in bicarbonate-buffered cell culture media (Irvine Sc., USA). The ejaculate produced by masturbation with sexual abstinence of 3–4 days was processed by density gradient centrifugation followed by swim up. ICSI was used for fertilization. Embryos were cultured for 5 days in culture incubator in 5% О2, 6% СО2 atmosphere at 37°C. Embryo quality was evaluated according to the Russian Association for Human Reproduction.
In the clinical part of the study, all obtained zigotes of 127 women (2PN2PB, a total of 625 zigotes) were divided into 2 groups: group 1 was embryo-cumulus cells co-culture (n=236 zigotes), group 2 (comparison group) was conventional cell culture (n=389 zigotes). The standard method was used for embryo culture. The embryos were cultured individually in 25 μl drops in culture medium (Irvine CSC, USA) covered with oil. Co-culturing included the following procedure: through transvaginal follicle puncture, a small number of cumulus cells (2×2 mm) were separated from cumulus-oocyte complex under oil using sterile scalpel and were stored separately from oocytes for 24 hours. On the day of zigote evaluation and separation of the control group, each of these cumulus cells was placed in 25 μl drop under oil, where normal fertilized zygote was placed as well. Cumulus cells were added to each zygote in the drop and cultured together up to 5 days without changing culture medium.
On day 5 of culture, morphological assessment of the obtained embryos in two groups with further transfer of the best quality embryo (from any group) was performed. Depending on the transferred embryo, the women were divided into 2 groups: embryo transfer after co-culture (group 1), embryo transfer after classical co-culture (group 2). Preparation of the endometrium for embryo transfer was performed by progesterone administration according to the manufacturer’s instruction.
Cumulus Cell Cultivation and Metabolome Evaluation by High Performance Liquid Chromatography with Tandem Mass Spectrometry (HPLC-MS/MS)
To perform metabolome analysis of spent culture media, after transvaginal puncture cumulus cells (3×3 mm) were cut with sterile scalpel with no blood leakage and degeneration in medium that contained HEPES buffer (Gamete Buffer, СООК, Ireland). Cumulus cells were washed in medium with bicarbonate buffer (Irvine Sc., USA) and placed in 25 μl drops under culture oil (Irvine CSC, USA) for 5 days without subsequent change of culture medium. Control medium was a drop of medium without cumulus cells. Petri dishes were placed in CO2 incubator (37°С, 6% СО2, 5% О2). Equal volumes (20 μl) of spent media were collected on day 5. Cumulus cells were collected separately. All selected samples were marked and frozen at -80° С. To prepare the probes for HPLC-MS/MS analysis, metabolites were extracted by adding three volumes of methanol to each medium sample or directly to the pellet of cumulus cells for protein precipitation, after that they were centrifuged (13 000g) for 15 min. The supernatant was transferred to clean vials. For HPLC-MS/MS, 2 µl of internal standard with a final concentration of 5 µm was added to 18 µl of the extract in each sample. The samples were separated on Atlantis T3 C18 column (3 µm, length 15 cm, inner diameter 1 mm) (Waters, USA) with Ultimate 3000 Nano LC System (Thermo Scientific, США).
Isocratic elution in reversed-phase chromatography was used to elute sample components in mobile phase B ( 0.1% formic acid in acetonitrile) gradient: 5% B and phase A (1.0% formic acid in water) gradient: 95% A in 15 minutes, then gradient of 5–95% in mobile phase B in 10 minutes with flow rate of 40 µL/min. After that, the samples were washed for 5 minutes (95% B), then the concentration of phase B returned to the initial concentration (5%) for 1 minute and column equilibration was for 3 minutes. The total time for chromatography separation for one sample was 34 minutes. Two-dimensional metabolites detection in each specimen was performed with Hybrid Quadrupole mass spectrometer Bruker MaXis Impact (Bruker Daltoniks, Germany). Mass spectra were obtained with mass resolving power 50,000 in the range 50–1500 m/z in the positive ion mode. XCMS software program was used for pick detection, grouping and retention time correction. Peak detection was performed with the Centwave algorithm with parameters m/z = 15 ppm, minimum peak width = 10 seconds and maximum peak = 50 seconds, respectively. Peak grouping in all samples was performed by Peak Density with selection of default parameters. The Human Metabolome Database (HMDB) (www.hmdb.ca) was used for initial identification of metabolites with corresponding molecular masses.
Statistical analysis
The data were evaluated using LibreOffice Calc and IBM SPSS Statistics Version 23.0 (USA) and Microsoft Excel tables. The type of data distribution was detected for quantitative data analysis in the groups using the Kolmogorov–Smirnov test and the Shapiro–Wilk test. When distribution differed from normal, the variables were presented as median (Me) and interquartile range [Q1; Q3]. Mann–Whitney U test for pairwise comparison was used in statistical analysis. For normal distribution of variables arithmetic mean (M) and standard deviation (SD): m(SD) was used. Parametric Student’s t-test was used for data analysis. The percentage of the total number of patients in group P and absolute number n P% (n) were used to describe categorical binary data (clinical and anamnestic data and outcomes of ART programs (implantation and childbirth). Chi-square test and Fisher’s exact test were used to analyze nominal data. Nominal data between the groups was compared in the prospective study. Relative risk (RR) was calculated with 95% confidence interval for comparison of probability of outcomes (implantation, childbirth) depending of the presence of the factor (autologous embryo-cumulus cells co-culture). The significance threshold was at p=0.05.
The multivariate statistical method (orthogonal partial least squares discriminant analysis (OPLS-DA)) was used for metabolome analysis of culture media for differences between the samples in visualization of search results. Student’s t-test was used for statistical significance of difference in relative concentrations (average values of integrated peak square) between the groups for certain metabolites. Considering correction for multiple hypothesis testing (the FDR method), variations were statistically significant at p˂0.05. The additional selection criteria for potential biomarkers were changes in multiplicity and concentration at least 2 times between groups. Fisher's exact test was used to assess statistical significance of the found metabolic pathways. The multiplicity of changes, as well as one-dimensional and multi-dimentional statistics were calculated using Metaboanalyst V5.0.
Increased blastulation rate was the criterion for the effectiveness of co-culture of zygotes with autologous cumulus cells. Primary outcomes coincided with the criterion of effectiveness – improvement of indicator scores at the embryonic stage. Secondary outcomes were increased effectiveness of ART programs (high clinical pregnancy (implantation) and live birth rates).
Results
The clinical and anamnestic data of the patients participating in the study were assessed. Statistical analysis of clinical characteristics and embryonic stage in the groups is shown in Table 1. The characteristics of ovarian reserve and menstrual function in women are presented in Table 2. According to the results of the Kolmogorov–Smirnov test, distribution of qualitative characteristics significantly differed from normal distribution (р<0.05) with the exception of the FSH index (normal distribution, the data are presented as M (SD)).
The average total dose of gonadotropin was 1575 IU [1262; 2025], duration of ovarian stimulation was 9 days [8; 10]. Table 3 shows the characteristics of oogenesis and spermatogenesis, as well as embryological characteristics of the analyzed ART cycles. As can be seen from the presented results, married couples had clinical characteristics suggesting that the result of infertility treatment could be positive. However, all patients had at least 2 failed IVF protocols in anamnesis. In all women, strictly a single embryo was transferred into the uterine cavity. Out of 127 women, embryo transfer was performed in 105 women. In 22 women, embryo transfer was cancelled due to unsatisfactory blastocyst quality or endometrial pathology, that was detected by ultrasound on the day of embryo transfer. The embryos were cryopreserved in these cases.
On day 5 of culture, the embryos in the blastocyst stage were evaluated in both groups. The total number of blastocysts in the group of co-culture was 236, and 389 was in the comparison group. The comparison of the rates of embryo quality (high- quality, good-quality, fair and poor) depending on embryo culture conditions showed that there was statistically significant differences (p=0,01). The differences were due to a higher percentage of high-quality embryos – 36.9% (87/236) versus 24.9% (97/389) (р=0.002) and lower percentage of poor-quality embryos – 43.2% (102/236) versus 50,6% (197/389) in embryo-cumulus cells co-culure (р=0.07) versus classical embryo culture.
The comparison showed that autologous embryo-cumulus cells co-culture significantly increased the quality of blastocysts that were obtained on day 5 of culture. This made it possible both elective embryo transfer into the uterine cavity and to increase the number of frozen embryos for subsequent cryo-embryo transfer. A total of 47 embryo transfers were performed after classical culture and 58 transfers from the group of embryo-cumulus cells co-culture. The clinical results are presented in Table 5.
Clinical data showed that autologous embryo–cumulus cells co-culture was not effective in the general cohort of patients. For this reason, at the next stage, age-stratified women were divided into 2 subgroups: ≤35 and 36+ years of age. The clinical results are shown in table 6.
As can be seen from the presented data, with autologous embryo–cumulus cells co-culture, there is only a trend towards reduced frequency of pregnancy in patients of late reproductive age. More data are necessary to prove a negative effect of co-culture for women aged over 36 years.
Metabolome analysis of spent culture media and cumulus cells
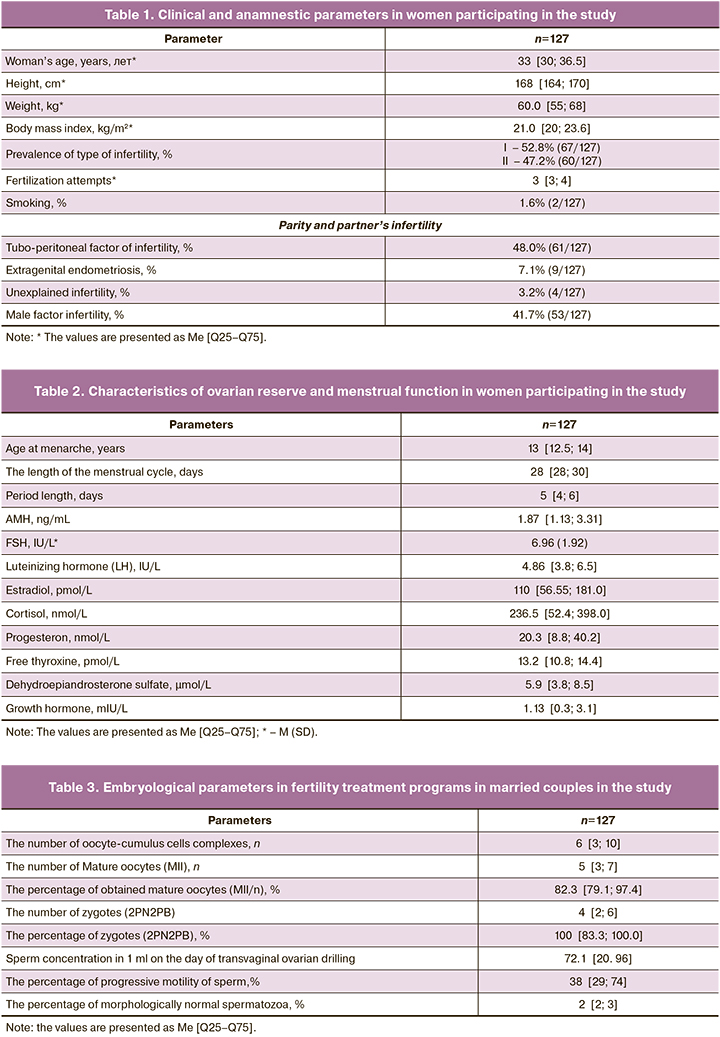
To analyze the properties of cumulus cells metabolism, their possible influence on embryonic development and implantation ability, we studied the metabolome of both cumulus cells and culture media, where they were cultured for 5 days. A total of 190 samples were analyzed. Out of them 90 samples of spent culture media from cumulus cells culture, 90 samples of cumulus cells and 10 control samples of culture medium without cumulus cells. After peak detection, 1722 molecular ions in the range from 84 до 1007 m/z were found. To identify sample clusters, the multivariate statistical method (OPLS-DA) was used, that revealed a clear clustering of samples when spent culture media from cumulus cells was compared with the control culture media (Figure).
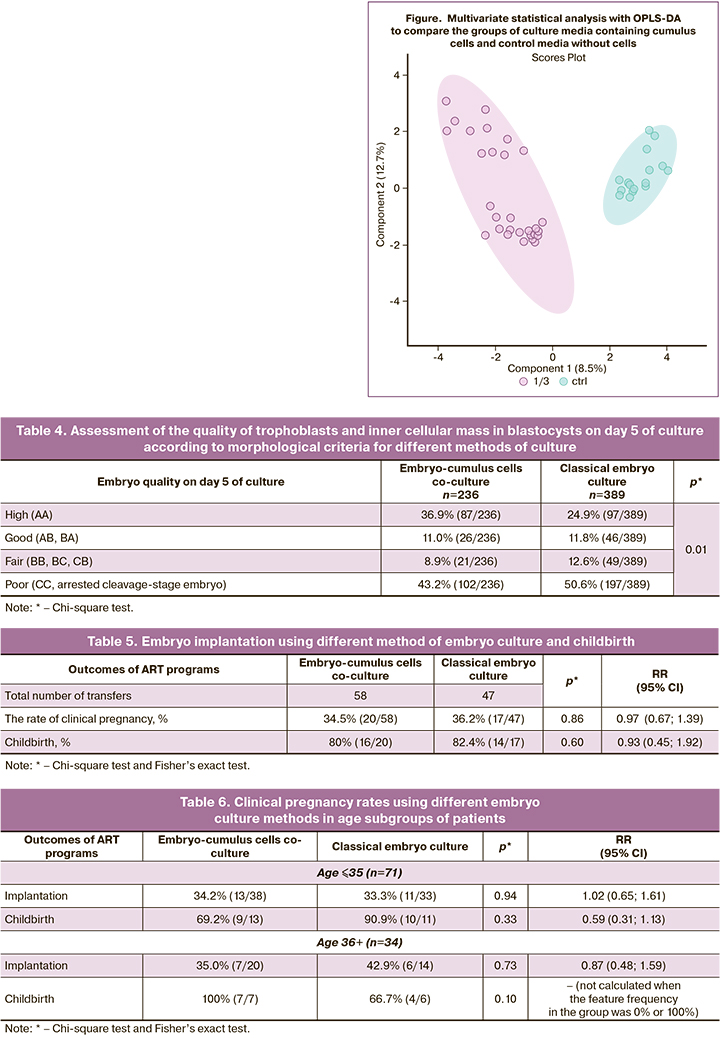
One-dimensional non-parametric statistics expectedly identified 113 molecular ions, in which the concentration of masses changed significantly FDR p<0.05). The results of the analysis of the multiplicity and direction of changes, the concentration of 65 molecular ions out of 113 changed by 2 or more times between the groups of media containing cumulus cells and control media. These ions were initially identified using the HMDB database. Identification results, which present a list of potential biomarkers, are shown in Table 7. It shows that a number of amino acids (such as leucine, valine, serine) and amino acids-based dipeptides were detected, and their concentration in medium with cumulus cells increased by several times. At the same time, the concentration of the majority of lipids and their derivatives that were found in medium, decreased (Table 8).
The list of molecular ions was used to analyze the representation of metabolic pathways that determined the differences between groups. Table 8 shows the list of the most important metabolic pathways.
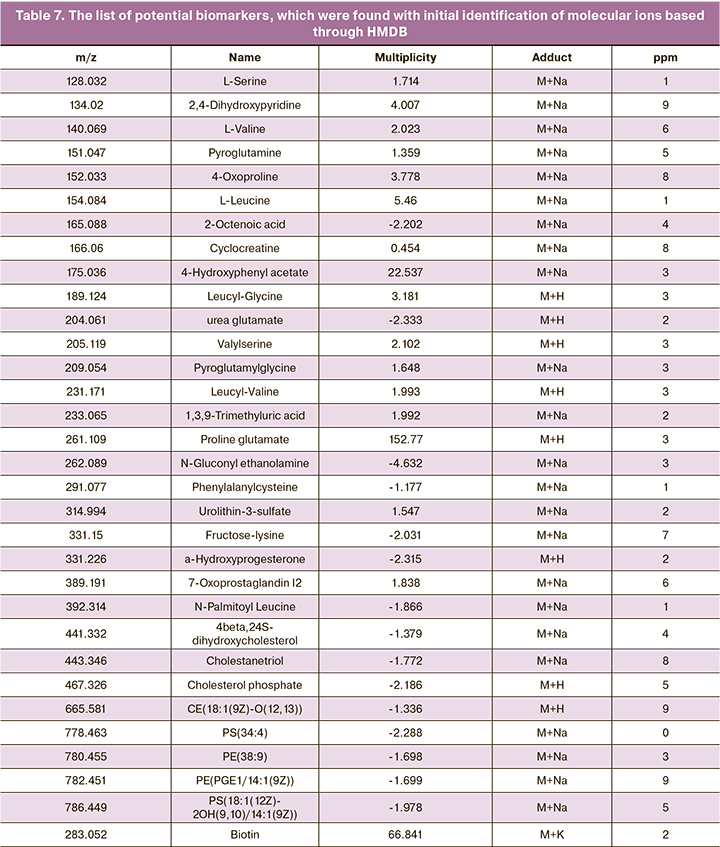
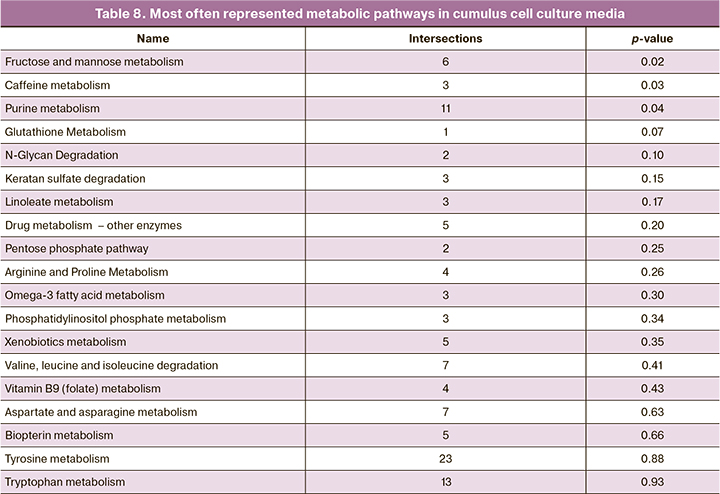
It is notable that purine metabolism was highly represented and indicated the importance of this pathway for the function of cumulus cells. The pathway for caffeine metabolism also indicated this importance, and apart from caffeine includes a number of other purine derivatives. Moreover, there is a large number of pathways that determine metabolism of amino acids and sugar, that is consistent with the physiological function of cumulus cells. We also analyzed the representation of metabolic pathways in cumulus cells based on the peaks of molecular ions and retention time that was detected in the studied samples. The results are shown in Table 9.
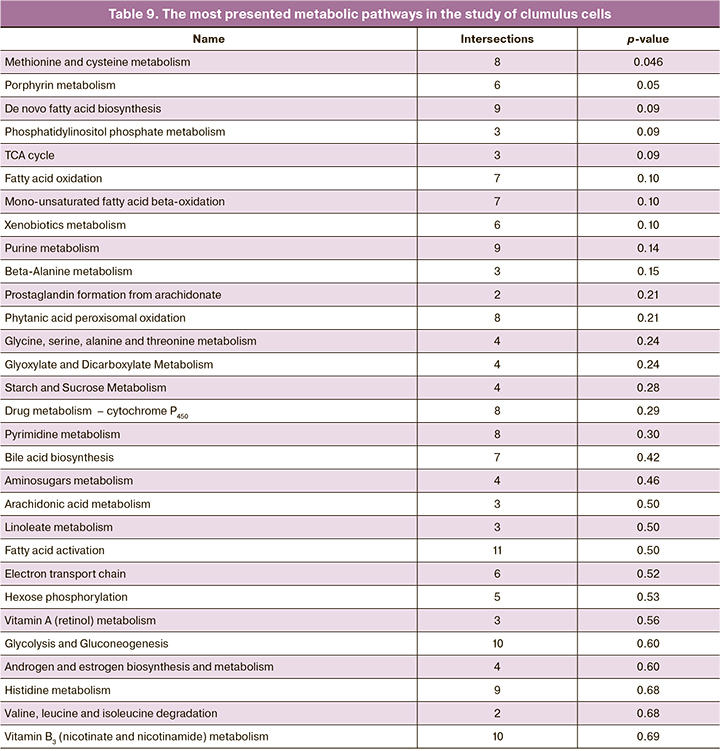
It should be noted that most pathways differ from those that were found for culture media. There are both common and interrelated metabolic pathways such as metabolism of purines, phosphatidylinositol phosphate and valine, leucine/isoleucine. Also, it is noteworthy to mention increased number of metabolic pathways for the regulation of lipids and their derivatives and reduced role of sugar metabolism.
Discussion
Autologous embryo-cumulus cells co-culture is a method for treatment of repeated implantation failures, since a number of studies have previously showed the difference in embryo growth rate and development, as well as embryo morphology between the groups with classical cultivation and co-cultivation with cumulus cells [17, 27]. At the same time, there are conflicting data in literature on the results of embryo-cumulus cells co-culture and the likelihood of implantation and getting pregnant [28, 29]. Some authors pointed to the increased frequency of implantation and pregnancy during embryo transfer on day 3 of culture [30, 31], others demonstrated that there were no statistically significant differences between implantation and pregnancy frequency in the groups with classical embryo culture and embryo-cumulus cells co-culture both on day 3 and day 5–6 of cuture [32].
Our study did not find statistically significant differences between pregnancy rates and probability of successful delivery in comparison of the group with co-culture and the group with classical cultivation without cumulus cells. However, a more detailed analysis of the groups and age stratification found increased frequency of pregnancy with classical cultivation in women over 36. At the same time, in the general cohort, high-quality blastocysts were registered significantly more often with co-culture – 40,2% versus 26.9% of all embryos, and the number of fair and poor-quality blastoicysts reduced (10.5% and 38% versus 14.2% and 47.8%, respectively). The results of our study confirmed the findings in literature that cumulus cell co-culture improves embryo maturation and makes it possible to obtain embryos of higher quality. It has been previously shown, that specific changes in the gene expression profile of oocytes in co-culture with cumulus cells, that improve oocyte quality could be suggested as one of the mechanisms of beneficial effects of embryo- cumulus cells co-culture on the development of oocytes and embryos. In the study by Virant-Klun I. et al., comparison between the groups of oocytes with classical in vitro culture and in vitro co-culture found that gene expression profile of oocytes in co-culture is most similar to oocyte maturation in vivo, imitating the natural process. Moreover, these two groups of samples had higher number of intersections of expressed genes [32]. At the same time, classical culture was characterized by significant fluctuations in gene expression with regard to natural maturation. The greatest difference was demonstrated by proteins associated with transcriptional regulation, embryogenesis and epigenetics, as well as the cell cycle.
However, there are single published studies on co-culture of oocytes and embryo-cumulus cells. These studies were mainly carried out on animals. Practically, there are no studies on the characteristics of metabolism in oocytes and embryos in co-culture. This is due to the fact that among other things, there is ethical difficulty in conducting this kind of studies with human oocytes and embryos. One of the ways to circumvent these limitations is to study embryo culture media. Metabolite consumption levels from culture medium and excretion of waste products into culture medium by embryos directly correlate with their metabolic activity. Thus, by cell culture media profiling we can get an idea of active metabolic processes in embryos. There is a large number of published studies on culture media, where in particular, concentrations of various proteins [33], amino acids [34], glucose, human chorionic gonadotropin [35] and other metabolites were studied. The methods that simultaneously allow to analyze a large number of components of culture media are most suitable for such studies. Previously, infrared spectrometry and Raman spectroscopy [36, 37], nuclear magnetic resonance [38], and mass spectrometry in combination with chromatography for the separation of complex biological mixtures were used to study the metabolome of culture media and its effect on the quality of embryos, as well as their ability to implant. Thus, human embryonic metabolome characteristics depending on the quality, karyotype, composition of cultivation media, and the influence of external factors were previously identified by mass-spectrometry.
In this study we analyzed metabolomic profiles of cumulus cell culture media after 5 days of culture, as well as the cumulus cells themselves. As a control, 5-day culture medium was analyzed. Univariate and multivariate statistical analysis showed that a number of metabolites significantly changed their concentration in comparison with control media. These metabolites can be directly associated with metabolic activity in cumulus cells and include amino acids and their derivatives, various classes of lipids, dipeptides and vitamins. It should be noted that the number of phenylalanine derivatives (4-hydroxyphenylacetate) in medium increased, and could indicate active phenylalanine metabolism in cumulus cells and embryo [24]. In addition, the concentration of proline-glutamate dipeptide increased significantly. In the study by Morris M. et al., the experiments conducted on mice showed that proline and glutamate in culture medium had a beneficial effect on preimplantation development and embryo growth [39]. The identified metabolites were then used to find the most active metabolic pathways. Glucose and other hexoses metabolism is critical for the active embryonic development, while glutathione acts as an antioxidant, protecting against reactive oxygen species [40]. Since early embryonic development is associated with significantly high nucleic acids concentration, purine/pyrimidine imetabolism is also extremely important for embryo development [41].
In addition to culture media, we extracted metabolites directly from cumulus cells for subsequent profiling. In this case, the study of active metabolic pathways in cumulus cells and their comparison with the previously identified pathways in culture media were of greatest interest.
According to the data in literature, cumulus cells play an important role in delivery of various amino acids to the oocyte, with the exception of branched-chain amino acids (valine, leucine, and isoleucine), which are probably regulated in a different way. This can be conditioned by enrichment of a large number of amino acid metabolic pathways. At the same time, sulfur-containing amino acids, such as methionine and cysteine, are most important, since they are necessary for correct imprinting (methylation status of imprinting), protein biosynthesis, and their deficiency leads to deprivation syndrome and the launch of a caspase-independent pathways of cell death [42]. A large number of lipid metabolism pathways is natural due to their active role not only as energy source, but also as regulators of various cellular processes [43].
Cumulus cells are actively involved in lipid metabolism, initiating but not limited to fatty acid β-oxidation that is necessary for normal oocyte and embryo development, and inhibition leads to serious impairments in normal development [44, 45].
Conclusion
Thus, our study showed that autologous embryo-cumulus cells co-culture does not lead to increased rates of successful implantation and childbirth. However, it significantly increases the number of high-quality embryos. Profiling identifies the components of metabolic pathways in cumulus cells that are critical for physiological embryo growth and development. The use of co-culture in ART protocols can increase the likelihood of successful blastocyst maturation and improve their quality, that is a decisive factor for selecting embryo to transfer. Co-culture seems to be especially relevant in case of a small number or poor quality of the initial oocytes. This method, in combination with other non-invasive methods for assessment of embryo quality, such as measurement of glucose intake in embryos, can be used for a dual assessment of embryo quality or as an additional criterion for selecting embryo to transfer into the uterine cavity based on morphological and biochemical parameters. Taking into account widespread non-adoption of multiple embryo transfer, such selection may increase the likelihood of successful ART programs and lead to increasing number of childbirths.
References
- Loutradis D., Theofanakis C., Anagnostou E., Mavrogianni D., Partsinevelos G.A. Genetic profile of SNP(s) and ovulation induction. Curr. Pharm. Biotechnol. 2012; 13(3): 417-25. https://dx.doi.org/10.2174/138920112799361954.
- Gunby J., Daya S. Assisted reproductive technologies (ART) in Canada: 2002 results from the Canadian ART Register. Fertil. Steril. 2006; 86(5): 1356-64. https://dx.doi.org/10.1016/j.fertnstert.2006.04.030.
- Borght M.V., Wyns C. Fertility and infertility: definition and epidemiology. Clin. Biochem. 2018; 62: 2-10. https://dx.doi.org/10.1016/j.clinbiochem.2018.03.012
- Boomsma C.M., Kavelaars A., Eijkemans M.J., Lentjes E.G., Fauser B.C, Heijnen C.J., Macklon N.S. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum. Reprod. 2009; 24(6): 1427-35. https://dx.doi.org/10.1093/humrep/dep011.
- Richani D., Dunning K.R., Thompson J.G., Gilchrist R.B. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum. Reprod. Update. 2021; 27(1): 27-47. https://dx.doi.org/10.1093/humupd/dmaa043.
- Крылова Ю.С., Кветной И.М., Айламазян Э.К. Рецептивность эндометрия: молекулярные механизмы регуляции имплантации. Журнал акушерства и женских болезней. 2013; 62(2): 63-74. [Krylova Yu.S., Kvetnoy I.M., Aylamazyan E.K. Endometrial receptivity: the molecularmechanisms regulation of implantation. Journal of obstetrics and women's diseases. 2013; 62(2): 63-74. (in Russian)]. https://dx.doi.org/10.17816/JOWD622.
- Nikoloff N. The key role of cumulus cells in oocytes in vitro maturation protocols. Fertil. Steril. 2021; 116(6): 1663. https://dx.doi.org/10.1016/j.fertnstert.2021.10.010.
- Turathum B., Gao E.M., Chian R.C. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells. 2021; 10(9): 2292. https://dx.doi.org/10.3390/cells10092292.
- Gao E.M., Turathum B., Wang L., Zhang D., Liu Y.B., Tang R.X., Chian R.C. The differential metabolomes in cumulus and mural granulosa cells from human preovulatory follicles. Reprod. Sci. 2022; 29(4): 1343-56.https://dx.doi.org/10.1007/s43032-021-00691-3.
- Kattal N., Cohen J., Barmat L.I. Role of coculture in human in vitro fertilization: a meta-analysis. Fertil. Steril. 2008; 90(4): 1069-76. https://dx.doi.org/10.1016/j.fertnstert.2007.07.1349.
- Parikh F.R., Nadkarni S.G., Naik N.J., Naik D.J., Uttamchandani S.A. Cumulus coculture and cumulus-aided embryo transfer increases pregnancy rates in patients undergoing in vitro fertilization. Fertil. Steril. 2006; 86(4): 839-47. https://dx.doi.org/10.1016/j.fertnstert.2006.03.028.
- Lin Y.H., Hwang J.L., Seow K.M., Huang L.W., Chen H.J., Tzeng C.R. Effects of growth factors and granulosa cell co-culture on in-vitro maturation of oocytes. Reprod. Biomed. Online. 2009; 19(2): 165-70. https://dx.doi.org/10.1016/s1472-6483(10)60068-5.
- von Mengden L., De Bastiani M.A., Grun L.K., Barbé-Tuana F., Adriaenssens T., Smitz J. et al. Bioinformatic analysis of human cumulus cells to unravel cellular's processes that could be used to establish oocyte quality biomarkers with clinical application. Reprod. Sci. 2022 Jul 26. https://dx.doi.org/10.1007/s43032-022-01046-2.
- Quinn P., Margalit R. Beneficial effects of coculture with cumulus cells on blastocyst formation in a prospective trial with supernumerary human embryos. J. Assist. Reprod. Genet. 1996; 13(1): 9-12. https://dx.doi.org/10.1007/BF02068862.
- Benkhalifa M., Demirol A., Sari T., Balashova E., Tsouroupaki M., Giakoumakis Y., Gurgan T. Autologous embryo-cumulus cells co-culture and blastocyst transfer in repeated implantation failures: a collaborative prospective randomized study. Zygote. 2012; 20(2): 173-80. https://dx.doi.org/10.1017/S0967199411000062.
- Vendrell-Flotats M., García-Martínez T., Martínez-Rodero I., López-Béjar M., LaMarre J., Yeste M., Mogas T. In vitro maturation in the presence of Leukemia Inhibitory Factor modulates gene and miRNA expression in bovine oocytes and embryos. Sci. Rep. 2020; 10(1): 17777. https://dx.doi.org/10.1038/s41598-020-74961-6.
- Babayev E., Duncan F.E. Age-associated changes in cumulus cells and follicular fluid: the local oocyte microenvironment as a determinant of gamete quality. Biol. Reprod. 2022; 106(2): 351-65. https://dx.doi.org/10.1093/biolre/ioab241.
- Carles M., Lefranc E., Bosquet D., Capelle S., Scheffler F., Copin H., Cabry R., Benkhalifa M. In vitro maturation of oocytes from stimulated IVF-ICSI cycles using autologous cumulus cell co-culture: A preliminary study. Morphologie. 2022 Jun 25: S1286-0115(22)00028-5. https://dx.doi.org/10.1016/j.morpho.2022.02.002.
- Kattal N., Cohen J., Barmat L.I. Role of coculture in human in vitro fertilization: a meta-analysis. Fertil. Steril. 2008; 90(4): 1069-76. https://dx.doi.org/10.1016/j.fertnstert.2007.07.1349.
- Kumar K., Venturas M., Needleman D.J., Racowsky C., Wells D. Extensive analysis of mitochondrial DNA quantity and sequence variation in human cumulus cells and assisted reproduction outcomes. Hum. Reprod. 2021; 37(1): 66-79. https://dx.doi.org/10.1093/humrep/deab231.
- Cadenas J., Pors S.E., Nikiforov D., Zheng M., Subiran C., Bøtkjær J.A. et al. Validating reference gene expression stability in human ovarian Follicles, Oocytes, Cumulus Cells, Ovarian Medulla, and Ovarian cortex tissue. Int. J. Mol. Sci. 2022; ;23(2): 886. https://dx.doi.org/10.3390/ijms23020886.
- Caponnetto A., Battaglia R., Ferrara C., Vento M.E., Borzì P., Paradiso M. et al.; Italian Society of Embryology, Reproduction, Research (SIERR). Down-regulation of long non-coding RNAs in reproductive aging and analysis of the lncRNA-miRNA-mRNA networks in human cumulus cells. J. Assist. Reprod. Genet. 2022; 39(4): 919-31. https://dx.doi.org/10.1007/s10815-022-02446-8.
- Ekart J., McNatty K., Hutton J., Pitman J. Ranking and selection of MII oocytes in human ICSI cycles using gene expression levels from associated cumulus cells. Hum. Reprod. 2013; 28(11): 2930-42. https://dx.doi.org/10.1093/humrep/det357.
- Зорина И.М., Эльдаров Ч.М., Ярыгина С.А., Макарова Н.П., Трофимов Д.Ю., Смольникова В.Ю., Калинина Е.А., Бобров М.Ю. Профилирование метаболитов в питательных средах пятидневных эмбрионов человека. Биомедицинская химия. 2017; 63(5): 385-91. [Zorina I.M., Eldarov C.M., Yarigina S.A., Makarova N.P., Trofimov D.Yu., Smolnikova V.Yu., Kalinina E.A., Bobrov M.Yu. Metabolomic profiling in culture media of day-5 human embryos. Biomedical Chemistry. 2017; 63(5): 385-91. (in Russian)].
- Ибрагимова Л.К., Смольникова В.Ю., Эльдаров Ч.М., Бобров М.Ю., Агаджанян Д.С., Романов Е.А., Калинина Е.А. Особенности метаболомного профиля фолликулярной жидкости и сред культивирования эмбрионов пациенток с наружным генитальным эндометриозом. Акушерство и гинекология. 2021; 11: 114-24. [Ibragimova L.K., Smol'nikova V.Yu.,El'darov Ch.M., Bobrov M.Yu., Agadzhanyan D.S., Romanov E.A., Kalinina E.A. Metabolomic profile of follicular fluid and embryo culture media in patients with extragenital endometriosis. Obstetrics and Gynecology. 2021; (11): 114-24. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.114-124.
- Ярыгина С.А., Смольникова В.Ю., Бобров М.Ю., Эльдаров Ч.М., Макарова Н.П. Культивирование эмбрионов в среде, содержащей в своем составе гранулоцитарно-макрофагальный колониестимулирующий фактор в программах ВРТ. Акушерство и гинекология. 2019; 1: 50-4. [Iarygina S.A., Smolnikova V.Yu., Bobrov M.Yu., Eldarov Ch.M., Makarova N.P. Embryo cultivation in the medium containing granulocyte-macrophage colony-stimulating factor in the ART programs. Obstetrics and Gynecology. 2019; (1): 50-4. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.1.50-54.
- Bhadarka H.K., Patel N.H., Patel N.H., Patel M., Patel K.B., Sodagar N.R. et al. Impact of embryo co-culture with cumulus cells on pregnancy & implantation rate in patients undergoing in vitro fertilization using donor oocyte. Indian J. Med. Res. 2017; 146(3): 341-5. https://dx.doi.org/10.4103/ijmr.IJMR_1702_15.
- Saito H., Hirayama T., Koike K., Saito T., Nohara M., Hiroi M. Cumulus mass maintains embryo quality. Fertil. Steril. 1994; 62(3): 555-8.
- Carrell D.T., Peterson C.M., Jones K.P., Hatasaka H.H., Udoff L.C., Cornwell C.E. et al. A simplified coculture system using homologous, attached cumulus tissue results in improved human embryo morphology and pregnancy rates during in vitro fertilization. J. Assist. Reprod. Genet. 1999; 16(7): 344-9.https://dx.doi.org/10.1023/a:1020533711711.
- Kim M.J., Kim Y.S., Kim Y.J., Lee H.R., Choi K.H., Park E.A. et al. Upregulation of low-density lipoprotein receptor of the steroidogenesis pathway in the cumulus cells is associated with the maturation of oocytes and achievement of pregnancy. Cells. 2021; 10(9): 2389. https://dx.doi.org/10.3390/cells10092389.
- Benkhalifa M., Demirol A., Sari T., Balashova E., Tsouroupaki M., Giakoumakis Y., Gurgan T. Autologous embryo-cumulus cells co-culture and blastocyst transfer in repeated implantation failures: a collaborative prospective randomized study. Zygote. 2012, 20(2): 173-80. https://dx.doi.org/10.1017/S0967199411000062.
- Virant-Klun I., Bauer C., Ståhlberg A., Kubista M., Skutella T. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod. Biomed. Online. 2018; 36(5): 508-23.https://dx.doi.org/10.1016/j.rbmo.2018.01.011.
- Domínguez F., Gadea B., Esteban F.J., Horcajadas J.A., Pellicer A., Simón C. Comparative protein-profile analysis of implanted versus non-implanted human blastocysts. Hum. Reprod. 2008; 23(9): 1993-2000. https://dx.doi.org/10.1093/humrep/den205.
- Houghton F.D., Hawkhead J.A., Humpherson P.G., Hogg J.E., Balen A.H., Rutherford A.J., Leese H.J. Non-invasive amino acid turnover predicts human embryo developmental capacity. Hum. Reprod. 2002; 17(4): 999-1005.https://dx.doi.org/10.1093/humrep/17.4.999. Erratum in Hum. Reprod. 2003; 18: 1756-7.
- Chen C.Y., Hwu Y.M., Weng Y.W., Lu C.H., Chen Y.J., Sun F.J. Clinical application of immunomagnetic reduction for quantitative analysis of beta-subunit of human chorionic gonadotropin in blastocyst culture media to differentiate embryo quality. Clin. Chim. Acta. 2019; 491: 46-51.https://dx.doi.org/10.1016/j.cca.2019.01.012.
- Lundin K., Ahlström A. Quality control and standardization of embryo morphology scoring and viability markers. Reprod. Biomed. Online. 2015; 31(4): 459-71. https://dx.doi.org/10.1016/j.rbmo.2015.06.026.
- Seli E., Sakkas D., Scott R., Kwok S.C., Rosendahl S.M., Burns D.H. Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil. Steril. 2007; 88(5): 1350-7.https://dx.doi.org/10.1016/j.fertnstert.2007.07.1390.
- Wallace M., Cottell E., Cullinane J., McAuliffe F.M., Wingfield M., Brennan L. 1H NMR based metabolic profiling of day 2 spent embryo media correlates with implantation potential. Syst. Biol. Reprod. Med. 2014; 60: 58-63.
- Morris M.B., Ozsoy S., Zada M., Zada M., Zamfirescu R.C., Todorova M.G., Day M.L. Selected amino acids promote mouse pre-implantation embryo development in a growth factor-like manner. Front. Physiol. 2020; 11: 140. https://dx.doi.org/10.3389/fphys.2020.00140.
- Ménézo Y., Lichtblau I., Elder K. New insights into human pre-implantation metabolism in vivo and in vitro. J. Assist. Reprod. Genet. 2013; 30(3): 293-303. https://dx.doi.org/10.1007/s10815-013-9953-9.
- Alexiou M., Leese H.J. Purine utilisation, de novo synthesis and degradation in mouse preimplantation embryos. Development. 1992; 114(1): 185-92.https://dx.doi.org/10.1242/dev.114.1.185.
- Ménézo Y., Lichtblau I., Lu S., Hoestje S.M., Choo E., Epner D.E. Induction of caspase dependant and independant apoptosis in response to methionine restriction. Int. J. Oncol. 2003; 22(2): 415-20.
- Брусенцев Е.Ю., Мокроусова В.И., Игонина Т.Н., Рожкова И.Н., Амстиславский С.Я. Роль липидных гранул в развитии ооцитов и преимплантационных эмбрионов млекопитающих. Онтогенез. 2019; 50(5): 297-305. [Brusentsev E.Y., Mokrousova V.I., Igonina T.N., Rozhkova I.N., Amstislavsky S.Ya. Role of lipid droplets in the development of oocytes and preimplantation embryos in mammals. Russ. J. Dev. Biol. 2019; 50(5): 297-305. (in Russian)].
- Dunning K.R., Russell D.L., Robker R.L. Lipids and oocyte developmental competence: the role of fatty acids and β-oxidation. Reproduction. 2014; 148(1): R15-27. https://dx.doi.org/10.1530/REP-13-0251.
- McKeegan P.J., Sturmey R.G. The role of fatty acids in oocyte and early embryo development. Reprod. Fertil. Dev. 2011; 24(1): 59-67.https://dx.doi.org/10.1071/RD11907.
Received 20.12.2022
Accepted 06.04.2023
About the Authors
Gunay R. Asfarova, postgraduate student, B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(926)262-11-13, asfarovag@gmail.com,117997, Russia, Moscow, Academician Oparin str., 4.
Veronika Yu. Smolnikova, Dr. Med. Sci., Leading Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, v_smolnikova@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.
Natalya P. Makarova, Dr. Bio. Sci., Leading Researcher, B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, np_makarova@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.
Mikhail Yu. Bobrov, PhD, Head of Molecular Pathophysiology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, m_bobrov@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Chupalav M. Eldarov, PhD, Senior Researcher at Molecular Pathophysiology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, ch_eldarov@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Boris V. Zingerenko, Junior Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, b_zingerenko@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, e_kalinina@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.



