Assisted reproductive technology in late reproductive age women with polycystic ovarian syndrome
Objective: To compare the efficiency of frozen–thawed embryo transfer after preimplantation genetic testing for aneuploidy (PGT-A) in late reproductive age women with polycystic ovarian syndrome (PCOS) and those with tubal-peritoneal factor infertility.Mikhailova N.D., Aksenenko A.A., Durinyan E.R., Gavisova A.A.
Materials and methods: The study included 63 infertile women of late reproductive age undergoing IVF/ICSI with gonadotropin-releasing hormone (GnRH) antagonists followed by PGT-A. The patients were divided into Group 1 including women with tubal-peritoneal infertility (n=30) and Group 2 including women with PCOS (n=33). Then, all the patients included in this study underwent frozen–thawed embryo transfer, providing the availability of embryos recommended for transfer by genetic testing. The effectiveness of the ART program was evaluated by the rates of achieved pregnancy, biochemical pregnancy rate, ectopic pregnancy rate, spontaneous miscarriage rate, missed miscarriage rate, and live birth rates.
Results: There were no differences in IVF/ICSI efficacy between the two study groups. A total of 9/27 (33.3%) pregnancies were recorded in the tubal-peritoneal factor group versus 8/25 (32%) in the PCOS group (p=0.919), a difference that was not statistically significant [OR 1.042 (CI 0.48; 2.28), p=0.919]. Of them, 5/27 (18.5%) and 4/25 (16%) ended in birth [OR 1.157 (CI 0.35; 3.83), p=0.811] and 6/27 (22.2%) and 4/25 (16%) resulted in miscarriage [OR 1.39 (CI 0.44; 4.35), p=0.569]. There was only one miscarriage (3.7%) in the group of patients with tubal-peritoneal infertility factor.
Conclusion: In late reproductive age, patients with both PCOS and tubal-peritoneal infertility factor, IVF/ICSI and PGT-A programs followed by frozen–thawed embryo transfer demonstrated similar efficacy. Despite the higher ovarian reserve, the efficacy of ART programs in patients with PCOS also decreases with age, requiring PGT-A in older patients, regardless of the infertility factor. Further studies of this problem on a larger sample of patients are needed.
Keywords
Key clinical features of polycystic ovarian syndrome (PCOS) include polycystic ovary transformation combined with endocrine and metabolic disorders. Identification of various combinations of symptoms and endocrine and metabolic disorders allows the diagnosis of one of the PCOS phenotypes presented in the classification. [1]. Polycystic ovary transformation adversely affects ovarian stimulation during in vitro fertilization (IVF). Ovarian stimulation in this category of patients is associated with a high risk of ovarian hyperstimulation syndrome (OHSS), a low percentage of mature oocytes, and disrupting embryogenesis stages [2–4]. To avoid OHSS in patients with PCOS, replacement of ovulation trigger and cycle segmentation with cryopreservation of all obtained embryos are often used [5, 6]. The need for preimplantation genetic testing for aneuploidy (PGT-A) of embryos in this category of patients is debatable. Some researchers believe that, based on the pathogenesis of the disease and its possible genetic determination, PGT-A will improve treatment outcomes (7, 8). Others see no need for it, focusing on the young age of the patients and the sufficient number of embryos obtained [7, 8].
An interesting question is the effect of patients' age on the quality of embryos and the outcomes of IVF programs in patients with PCOS. It is known that the parameters of the ovarian reserve in PCOS do not coincide with the age values of the general population and significantly exceed them at a later reproductive age, making it possible to obtain more oocytes [9]. This suggests that if the hormonal background is normalized in older reproductive age, better outcomes of assisted reproductive technology (ART) could be expected in these patients compared to older reproductive age patients with other infertility factors. However, the issue of the quality of oocytes obtained in late reproductive age patients with PCOS is not adequately addressed in the literature.
Research evidence is available on the effectiveness of PGT-A in patients with PCOS of younger age (up to 35 years) [10], but the issue remains debatable. A recent study by Mikhailova N.D. et al. [11] showed the clinical relevance of PGT-A in women, but the effect of patient age on the outcomes of ART programs was not investigated in this study. Therefore, this study aimed to compare the efficiency of frozen–thawed embryo transfer after PGT-A in late reproductive age women with PCOS and those with tubal peritoneal factor infertility.
Materials and methods
This nonrandomized clinical trial was conducted at the 1st Gynecology Department of V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia from September 2020 to April 2022. The study included 63 women aged 38-42 years who were treated for infertility with IVF/intracytoplasmic sperm-to-oocyte injection (ICSI) programs using a protocol with gonadotropin-releasing hormone (GnRH) antagonists followed by PGT-A and then in the frozen–thawed embryo transfer programs if an embryo was recommended for transfer.
Patients were followed up until one of the possible outcomes, including a negative blood test for serum β-subunit of human chorionic gonadotropin, biochemical pregnancy, miscarriage, ectopic pregnancy, missed miscarriage, or live birth.
The first group (n=30) consisted of women with tubal peritoneal factor infertility. The second group (n=33) included women with PCOS (diagnosis based on the Rotterdam criteria) who were treated in IVF/ICSI programs as a 3rd line therapy when 1st and 2nd line therapy (clomiphene citrate/letrozole and gonadotropin ovulation stimulation/laparoscopic ovarian drilling, respectively) for anovulatory infertility caused by PCOS was ineffective [12, 13].
Participants in this study were not blinded to allocation to study conditions.
Before the IVF/ICSI programs, all patients underwent a standard clinical and laboratory examination according to the Russian Ministry of Health Order No. 803n.
The exclusion criteria were contraindications to ART, premature ovarian insufficiency, severe male factor, genital malformations, and immunodeficiency conditions.
All patients underwent ovarian stimulation starting on days 2-3 of the menstrual cycle using recombinant follicle stimulating hormone (FSH) and human menopausal gonadotropins containing equal amounts of FSH and luteinizing hormone (LH). To inhibit the premature LH peak, GnRH antagonist was used. Triptorelin acetate at a dose of 0.2 mg was used as an ovulation trigger in all cycles.
Fertilization of aspirated oocytes was performed by ICSI, and blastocyst trophectoderm biopsy was performed on days 5–6 of culture (provided that there were morphologically suitable embryos for genetic testing according to the Gardner classification system). All embryos of appropriate quality were vitrified.
Subsequent PGT-A was performed on 46 chromosomes at the Molecular Genetic Methods Laboratory by next generation sequencing (NGS).
Provided there were embryos recommended for transfer based on PGT-A results, patients entered the program of frozen-thawed embryo transfer on the background of hormone replacement therapy from days 4–5 of the next menstrual cycle. The endometrium was prepared using oral estradiol valerate 8 mg/day; micronized progesterone or didrogesterone 30 mg was added on days 15–16 of the menstrual cycle. The embryo was transferred to the uterine cavity on day 6 of progesterone therapy.
The effectiveness of the ART program was evaluated by conception rates, biochemical pregnancy rate, the ectopic pregnancy rate, the spontaneous miscarriage rate missed miscarriage rate, and the live birth rates.
Statistical analysis
Statistical analysis was performed using Statistica 8.0 software (StatSoft Inc., USA). Quantitative variables that showed normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, medians (Me) with the interquartile range (Q1; Q3) were reported. The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. Categorical variables were described as counts with percentages. Normally distributed continuous variables were compared with a Student’s t test. Variables not meeting normality assumptions were compared by the nonparametric Mann–Whitney U-test. The critical level of significance when testing statistical hypotheses was considered at p<0.05. Relative risk (RR) with 95% confidence interval (CI) was calculated using the Woolf method. For binary outcomes, the effect size was defined as the risk ratio and the absolute risk reduction with 95% CI. The study hypothesis was superior efficacy in achieving and carrying a pregnancy that ends in live birth. The sample size calculation was based on the frequency of clinical pregnancy and live birth.
Results
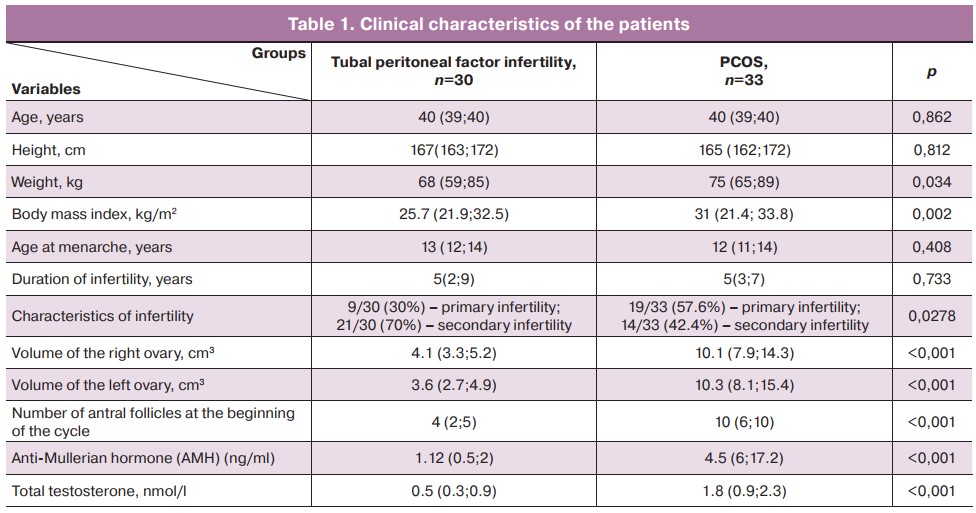
There were statistically significant differences between the study groups in the main clinical characteristics (Table 1), including body weight and BMI, ovarian reserve, and androgen profile (total testosterone). Signs of hyperandrogenism were identified in 6/33 (18.2%) women in the group of women with PCOS. Apart from PCOS, other infertility factors were detected in some patients; however, the endocrine factor was the leading one in all cases. Tubal and uterine factors were found in 7/33 (21.2%) and 12/33 (36.4%) women, respectively. Infertility due to the male factor was noted in 27/33 (81.8%) patients.
In the tubal peritoneal factor, 29 pregnancies were recorded in women with secondary infertility, including 6/29 (20.7%) deliveries, 6/29 (20.7%) ectopic pregnancies, 2/29 (6.9%) abortions, 12/29 (41.4%) missed miscarriages, 4/29 (13.8%) miscarriages. Nine (31.03%) pregnancies resulted from IVF, of which 3/9 (33.3%) ended in childbirth, 4/9 (44.4%) had missed miscarriages, and 2/9 (22.2%) had miscarriages.
There were 14 pregnancies in the PCOS patient group. Of these, 2/14 (14.3%) resulted in labor, 0/14 had ectopic pregnancies, 1/14 (7.1%) had abortions, 8/14 (57.1%) had missed miscarriage, and 3/14 (21.4%) had miscarried. 10/14 (71.4%) pregnancies were due to IVF, of which 2/10 (20%) ended in delivery, 6/10 (60%) were missed miscarriages, and 2/10 (25%) were miscarriages.
In this group of patients, 2/33 (6.1%) women had a history of ovarian drilling.
Statistically significant differences were found between the groups in the parameters of folliculogenesis, oogenesis, and embryogenesis (Table 2). In the PCOS group, more antral follicles were observed and more mature oocytes, blastocysts and blastocysts suitable for cryopreservation were obtained compared to the group of patients with tubal peritoneal factor infertility.
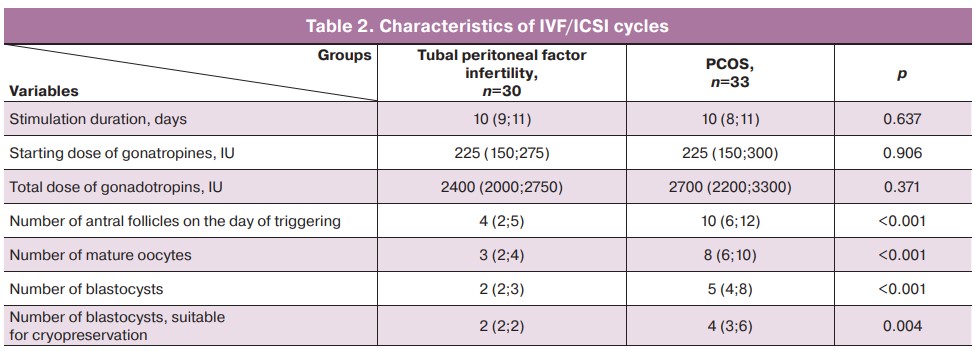
One patient in each group did not have blastocysts of appropriate quality after IVF/ICSI; therefore, no trophectoderm biopsy or vitrification were performed. These patients were excluded from the study at this stage.
In the group of patients with tubal peritoneal factor, 75 embryos were biopsied from the trophectoderm. Of these, 32 were recommended for transfer, five were mosaic, 15/38 (39.5%) embryos were found to be trisomic, 12/38 (31.5%) were monosomic, and another 7/38 (18.4%) embryos had other aneuploidy/compound changes. Four embryos were excluded from the study due to lack of signal passage; two patients in this group dropped out of the study because they did not have embryos recommended for transfer by PGT-A results. As a result, 27 women with tubal peritoneal infertility factor entered the frozen-thawed embryo transfer program.
In the group of patients with PCOS, 119 embryos underwent a trophectoderm biopsy; of these, 45 were recommended for transfer, six were mosaic. Twenty nine (42.6%) embryos had trisomy, 27/68 (39.7%) monosomy, and 6/68 (8.8%) had other aneuploidy/combined changes. Six embryos were excluded from the study due to lack of signal passage. In this group, seven women dropped out of the study at this stage because they did not have embryos recommended for transfer based on PGT-A results. Thus, 25 women with PCOS entered the frozen-thawed embryo transfer program.
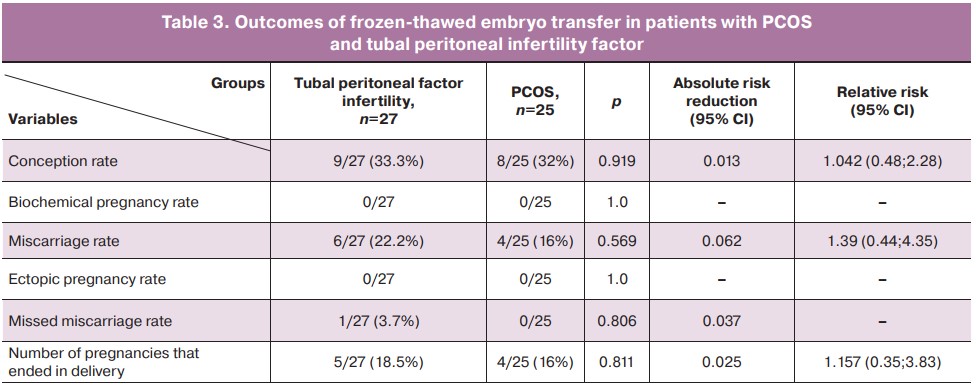
The results (Table 3) showed no statistically significant differences in the effectiveness of ART programs between the two study groups despite higher rates in the group of patients with PCOS at the embryological stage. Nine (33.3%) pregnancies were recorded in the tubal peritoneal infertility factor group versus 8/25 (32%) in the PCOS group (p=0.919), the difference was not statistically significant (OR 1.042 (CI 0.48; 2.28), p=0.919). Of them, 5/27 (18.5%) and 4/25 (16%) ended in labor, difference not statistically significant (OR 1.157 (CI 0.35; 3.83), p=0.811); 6/27 (22.2%) and 4/25 (16%) were miscarriages, difference not statistically significant, (OR 1.39 (CI 0.44; 4.35), p=0.569). Only 1/27 (3.7%) patient with tubal peritoneal factor infertility had missed miscarriage.
Discussion
A recent study by Gordon C.E. et al. concluded that women over 40 years with less than four 2pn zygotes should consider the transfer of one or more untested embryos either on day 3 or on day 5. Despite the discussed feasibility of PGT-A embryos in patients of older reproductive age, this can be said only if embryos are available for diagnosis [14]. In our study, we observed two cases (one in each group) of patients dropping out of the study due to the absence of embryos of adequate quality.
Older women of reproductive age with PCOS, even with satisfactory ovarian reserve parameters are less likely to produce euploid embryos than women with a normal menstrual cycle, and the pregnancy rate decreases with age. Again, this can be partially explained by the fact that oocyte quality can suffer in PCOS despite high AMH values (3, 4). However, there is evidence showing that the efficacy of ART programs in patients with PCOS remains high up to and including 38 years of age [15]. There is also evidence that the effectiveness of ART programs in this category of patients generally declines more slowly with age [16]. However, in our study, 6/33 (18.2%) patients in the PCOS group dropped out of the study because all embryos given for PGT-A were aneuploid.
Patients of older reproductive age used higher doses of gonadotropins (compared with patients of younger age), which is explained by a decrease in ovarian reserve and risk of OHSS and does not contradict the literature [15].
Failures in ART programs in patients with PCOS can be explained in general by the increased risk of pregnancy failure in this category of patients [17], the higher risk of chromosomal abnormalities [18], and the fact that the PGT-A result cannot be an absolute guarantee of euploid blastocyst transfer. The uterine factor should also be considered, as there is evidence of possible endometrial resistance to progesterone in PCOS [19].
A large proportion of women with PCOS over 35 experience normalization of the cycle, a decrease in the number of follicles, and a decrease in AMH to normal values. However, there is a decrease in the level of androgens, which are one of the regulators of folliculogenesis, which may have a negative impact on the embryological stage.
At the same time, according to the literature, hyperandrogenism in patients with PCOS can have adverse impact on the outcomes of ART programs. For example, there is evidence of a negative effect of hyperandrogenism on follicular growth, blastocyst formation, and implantation [6, 15]. There is also evidence of an increased risk of pregnancy failure in patients with PCOS and hyperandrogenism during embryo transfer in an induced cycle, for which reason, cycle segmentation is also preferable [20].
Therefore, in terms of the presence of hyperandrogenism, one would expect, on the contrary, an improvement in ART outcomes in patients with PCOS with increasing age. However, it is also necessary to take into account other factors that can influence the results and change the clinical picture.
It should also be kept in mind that the results of our study may differ from the classically expected clinical picture in PCOS patients because most women with PCOS achieve pregnancy in natural fertility cycles, and ART programs are the third-line infertility therapy for PCOS [2]. Therefore, all women who participated in this study had an indication for ART programs, which means a more complicated history than other women with PCOS and possibly a combined effect of other factors of infertility.
PCOS patients are also characterized by an increase in body mass index, which can adversely affect the outcomes of ART programs. This is confirmed by research evidence of an increased risk of pregnancy failure after frozen-thawed embryo transfer in obese women with PCOS [15, 21]. Therefore, even despite the negative attitudes of the patients themselves, weight normalization before entering ART programs is necessary.
Conclusion
In late reproductive age, patients with both PCOS and tubal peritoneal infertility factor, IVF/ICSI and PGT-A programs followed by frozen–thawed embryo transfer demonstrated similar efficacy. Despite the higher ovarian reserve, the efficacy of ART programs in patients with PCOS also decreases with age, requiring PGT-A in older patients, regardless of the infertility factor. Further studies of this problem with a larger sample of patients are needed.
References
- Lizneva D., Suturina L., Walker W., Brakta S., Gavrilova-Jordan L., Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Steril. 2016; 106(1): 6-15. https://dx.doi.org/10.1016/j.fertnstert.2016.05.003.
- Nazarenko T.A., Korneeva I.E., Mishieva N.G. Clinical guidelines. Ovarian hyperstimulation syndrome: diagnosis, treatment, prevention, intensive care. Moscow: Russian Society of Obstetricians and Gynecologists; 2018. (in Russian).
- Dumesic D.A., Padmanabhan V., Abbott D.H. Polycystic ovary syndrome and oocyte developmental competence. Obstet. Gynecol. Surv. 2008; 63(1): 39-48. https://dx.doi.org/10.1097/OGX.0b013e31815e85fc.
- Qiao J., Feng H.L. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Reprod. Update. 2011; 17(1): 17-33. https://dx.doi.org/10.1093/humupd/dmq032.10.
- Martazanova B.A., Mishieva N.G., Abubakirov A.N. Ovulation trigger replacement as a method for preventing ovarian hyperstimulation syndrome. Obstetrics and Gynecology. 2014; 5: 15-8. (in Russian).
- Martazanova B.A., Mishieva N.G., Levkov L.A., Gracheva A.M., Bogatyreva Kh.A., Eapen S.M., Lapina V.S., Abubakirov A.N. Optimization of in vitro fertilization programs by replacing an ovulation trigger. Obstetrics and Gynecology. 2015; 10: 73-80. (in Russian).
- Yang W., Yang R., Yang S., Li J., Tu B., Gao C. et al. Infertile polycystic ovary syndrome patients undergoing in vitro fertilization with the gonadotropin-releasing hormone-antagonist protocol: role of hyperandrogenism. Gynecol. Endocrinol. 2018; 34(8): 715-8. https://dx.doi.org/10.1080/2018.1431773.
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Steril. 2018; 109(3): 429-36. https://dx.doi.org/10.1016/j.fertnstert.2018.01.002.
-
Mishieva N.G., Nazarenko T.A., Durinyan E.R., Chechurova T.N., Smirnova A.A. Endocrine and metabolic disorders in PCOS and their significance for the health of patients. Polycystic ovary syndrome (modern approaches to the diagnosis and treatment of infertility). Moscow:
MEDpress-inform; 2005: 83-91. (in Russian).
- Pearson H., Abittan B., Goldman R.H., Mullin C. Preimplantation genetic testing for aneuploidy confers greater benefit to young patients with polycystic ovarian syndrome. Steril. 2020; 114(3): e422-3. https://dx.doi.org/10.1016/j.fertnstert.2020.08.1229.
- Mikhailova N.D., Aksenenko A.A., Ibragimova M.Kh., Ekimov A.N., Gavisova A.A. Efficiency of assisted reproductive technology in women with polycystic ovarian syndrome who undergo preimplantation genetic testing for aneuploidy. Obstetrics and Gynecology. 2022; 7: 60-7. (in Rusian). https://dx.doi.org/10.18565/aig.2022.7.60-67.
- Legro R.S., Arslanian S.A., Ehrmann D.A., Hoeger K.M., Murad M.H., Pasquali R. et al. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013; 98(12): 4565-9. https://dx.doi.org/10.1210/jc.2013-2350.
- Costello M.F., Garad R.M., Hart R., Homer H., Johnson L., Jordan C. et al. A review of second- and third-line infertility treatments and supporting evidence in women with polycystic ovary syndrome. Med. Sci. (Basel). 2019; 7(7): 75. https://dx.doi.org/10.3390/medsci7070075.
- Gordon C.E., Keefe K.W., Ginsburg E.S., Racowsky C., Lanes A. Embryo attrition in planned PGT-A: predicting the number of available blastocysts for transfer. J. Assist. Reprod. Genet. 2022; 39(1): 173-81. https://dx.doi.org/10.1007/s10815-021-02365-0.
- Koloda Yu.A., Podzolkova N.M., Petrichenko Yu.G. Prediction of ART outcomes and treatment of choice in PCOS patients. Obstetrics and Gynecology. 2021; 2: 84-9. (in Russian). https://dx.doi.org/10.18565/aig.2021.2.84-89.
- Li J., Liu X., Hu L., Zhang F., Wang F., Kong H. et al. A slower age-related decline in treatment outcomes after the first ovarian stimulation for in vitro fertilization in women with polycystic ovary syndrome. Front. Endocrinol. (Lausanne). 2019; 10: 834. https://dx.doi.org/10.3389/fendo.2019.00834.
- Luo L., Gu F., Jie H., Ding C., Zhao Q., Wang Q. et al. Early miscarriage rate in lean polycystic ovary syndrome women after euploid embryo transfer - a matched-pair study. Reprod. Biomed. Online. 2017; 35(5): 576-82. https://dx.doi.org/10.1016/j.rbmo.2017.07.010.
- Li Y., Wang L., Xu J., Niu W., Shi H., Hu L. et al. Higher chromosomal aberration rate in miscarried conceptus from polycystic ovary syndrome women undergoing assisted reproductive treatment. Fertil. Steril. 2019; 111(5): 936-43.e2. https://dx.doi.org/10.1016/j.fertnstert.2019.01.026.
- Savaris R.F., Groll J.M., Young S.L., DeMayo F.J., Jeong J.W., Hamilton E. et al. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J. Clin. Endocrinol. Metab. 2011; 96(6): 1737-46. https://dx.doi.org/10.1210/jc.2010-2600.
- Roque M., Haahr T., Geber S., Esteves S.C., Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum. Reprod. Update. 2019; 25(1): 2-14. https://dx.doi.org/10.1093/humupd/dmy033.
- Qiu M., Tao Y., Kuang Y., Wang Y. Effect of body mass index on pregnancy outcomes with the freeze-all strategy in women with polycystic ovarian syndrome. Steril. 2019; 112(6): 1172-9. https://dx.doi.org/10.1016/j.fertnstert.2019.08.009.
Received 27.05.2022
Accepted 25.08.2022
About the Authors
Nina D. Mikhailova, Ph.D. Student at the 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(915)456-81-51, mihailnina@mail.ru, 117997, Russia, Moscow, Akademika Oparina str., 4.Artem A. Aksenenko, Gynecologist at the 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(926)354-98-60, a_axenenko@oparina4.ru, 117997, Russia, Moscow, Akademika Oparina str., 4.
Evelina R. Durinyan, Ph.D., Clinical Care Supervisor at the 1st Gynecological Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(916)612-99-30, e_durinyan@oparina4.ru, 117997, Russia, Moscow, Akademika Oparina str., 4.
Alla A. Gavisova, Ph.D., Senior Researcher at the 1st Gynecological Department. Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(916)829-05-90, gavialla@yandex.ru, 117997, Russia, Moscow, Akademika Oparina str., 4.
Authors' contributions: Gavisova A.A., Aksenenko A.A., Durinyan E.R. – conception and design of the study;
Mikhailova N.D. – data collection and analysis, manuscript drafting; Mikhailova N.D., Aksenenko A.A. – statistical analysis; Gavisova A.A., Aksenenko A.A. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study, which was conducted as part of the dissertation work of Nina D. Mikhailova.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Mikhailova N.D., Aksenenko A.A., Durinyan E.R., Gavisova A.A.
Assisted reproductive technology in late reproductive age women with polycystic ovarian syndrome.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 9: 87-93 (in Russian)
https://dx.doi.org/10.18565/aig.2022.9.87-93



