The results of infertility treatment with assisted reproductive technology and sperm selection via cumulus‑oocyte complexes
During in vivo fertilization, human spermatozoa need to pass through cumulus-oocyte complex (COC), to cross the zona pellucida, and then penetrate into the oocyte for completion of fertilization. For this reason, COC plays an important role in physiological selection of functionally competent male gametes.Chistyakova A.V., Makarova N.P., Nepsha O.S., Smolnikova V.Yu., Kalinina E.A.
Objective: Assessment of the clinical effectiveness of using cumulus cells for sperm selection during intracytoplasmic sperm injection into the oocyte (ICSI) in cases of impaired spermatogenesis in the population of the Russian married couples, who underwent infertility treatment using assisted reproductive technology (ART).
Materials and methods: The study included 267 married couples, who underwent infertility treatment using ART. The group under study comprised 130 patients (the COC group) with the sperm selection via COC and subsequent ICSI procedure. The comparison group consisted of 137 patients, who underwent standard ICSI procedure with sperm selection according to morphological characteristics (the ICSI group). The clinical and embryological parameters of the ART program were assessed.
Results: The clinical results of the study showed that there was no significant increase in the frequency of implantation (38/5% in the COC group versus 35.9% in the ICSI group) in the general cohort of patients with sperm selection via COC. The frequency of successful delivery was similar (77.8% in the COC group versus 73.8% in the ICSI group). There were no significant improvements in the embryonic stage in the general cohort of patients. However, based on sperm morphology assessment, men with sperm morphology scores 0–1% and 2–3% were stratified into groups with mild and expressed teratozoospermia. The percentage of fertilization significantly increased (100% in the COC group and 77.78% in the ICSI group, p=0.001). The percentage of good and high quality growing blastocysts was also significantly higher in the group with sperm selection via COC (60% in the COC group versus 50% in the ICSI group, p=0.004).
Conclusion: The use of cumulus cells for sperm selection in ART programs for infertility treatment may improve the outcomes in patients with impaired spermatogenesis in the form of decreasing the percentage of morphologically normal spermatozoa in the ejaculate. In these married couples, fertilization rates can be improved, embryos can reach the blastocyst stage and higher number of embryos can be suitable for transfer to the uterus. This makes possible to reduce the cost of ovarian stimulation and to achieve pregnancy by frozen thawed embryo transfer in a cryo-cycle.
Authors’ contributions: Chistyakova A.V. – clinical data collection and analysis, processing of the initial material, article writing; Makarova N.P. – collection and analysis of embryological data, analysis of the results; Nepsha O.S. – analysis of the results, statistical data processing, clinical interpretation; Smolnikova V.Yu. – article editing, critical analysis; Kalinina E.A. – approval of the manuscript.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: Financial support for the study was provided by the State Assignment of the Ministry of Health of the Russian Federation No. №121040600410-7 “Solution of the problem of infertility in modern conditions by developing clinical and diagnostic pathway for infertile married couples and using innovations in the programs of assisted reproduction technology”.
Ethical Approval: The study was approved by the local Ethics Committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of the Russian Federation.
Patients’ Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Chistyakova A.V., Makarova N.P., Nepsha O.S., Smolnikova V.Yu., Kalinina E.A. The results of infertility treatment with assisted reproductive technology and sperm selection via cumulus-oocyte complexes.
Akusherstvo i Gynecologia/Obsterics and Gynecology. 2023; (5): 92-99 (in Russian)
https://dx.doi.org/10.18565/aig.2023.53
Keywords
Sperm selection in the female reproductive tract is extremely complex and very important for fertilization and embryo development. Currently, the molecular biological mechanism of human gamete interaction at a distance and in close proximity has not been fully studied due to the unavailability of the material for the ethical reasons. However, development of assisted reproductive technology (ART) techniques makes it possible to imitate many processes in vivo in the Petri dish, observing moral and ethical principles, and thus, contributes to understanding the causes of infertility, especially at the cellular level.
Development of the intracytoplasmic sperm injection (ICSI) technique that involves sperm injection directly into the oocyte made it possible for many couples with impaired spermatogenesis in men to have a genetically related, healthy child. During this procedure, the ejaculate is processed by density gradient technique, and the clinical embryologist injects motile, morphologically normal sperm. With this method of fertilization, the selected male gamete is not physiologically selected. ICSI bypasses the multiple barriers that are in the female reproductive tract. In particular, neither chemotaxis nor rheotaxis (selection by molecular characterization of membranes and molecular movement patterns) is used. Moreover, in case of teratozoospermia (the presence of morphological abnormalities of spermatozoa in the ejaculate), the risk of using “poor-quality” male material for ICSI increases, leading to the absence of fertilization, early embryonic arrest and multiple failed ART attempts. The data in literature confirm that with pronounced male factor infertility (high sperm DNA fragmentation, teratozoospermia) and ICSI, the rates of clinical and embryonic stages are low in fertility treatment programs (reduced rates of fertilization, embryo cleavage, blastulation, euploidy and implantation into the uterine cavity) [1–3]. For this reason, currently, there is an active search for possibilities to imitate the selection of male gametes for ICSI, which should be maximally close to the conditions in the female reproductive tract. This will help to improve embryo development in vitro and increase the effectiveness of infertility treatment.
It was shown that high-quality male germ cells during distant interaction of gametes should respond to chemoattractants secreted from cumulus cells that surround the oocyte. During natural fertilization, only those spermatozoa that pass through the cumulus-oocyte complex (COC) are able to reach and penetrate the zona pellucida and fertilize the egg. In the absence of cumulus cells, the ovulated oocyte remains unfertilized. The main component in the structure of COC is hyaluronic acid (HA), which is synthesized by cumulus cells after increased luteinizing hormone (LH) level, and as it is believed, plays a key role in the selection of healthy sperm. PICSI technique is based on interaction between HA and sperm (sperm selection based on their binding affinity to HA in the Petri dish). Some researchers showed that spermatozoa that pass through cumulus-oocyte complex have better morphology and can initiate the acrosome reaction [4]. Moreover, sperm has a higher ability to bind to the zona pellucida and is more likely to be DNA intact sperm. For this reason, several groups of scientists have recently proposed to use COC for sperm selection for ICSI [5–7]. The authors showed improvement of embryo parameters in fertility treatment programs due to impaired spermatogenesis. However, in their studies, different design of Petri dishes and different methods of using COC for male gamete selection were used. Due to this fact, it is difficult to compare the results of these researches.
The purpose of our study was assessment of the clinical effectiveness of using COC for sperm selection in intracytoplasmic sperm injection into the oocyte (ICSI) in cases of impaired spermatogenesis in the population of the Russian married couples, who underwent infertility treatment with assisted reproductive technologies (ART).
Materials and methods
The study included married couples who underwent treatment from January 2020 to December 2023 in B.V. Leonov Department of Assisted Reproduction Technologies for Infertility Treatment, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of Russia. Inclusion criteria were the following parameters: women aged 18–38 years, signed informed consent to participate in the study; anti-Müllerian hormone (AMH) serum levels in women greater than or equal to 1 ng /mL on the second – third day of the period; at least 50% of live spermatozoa in the male partner's ejaculate; progressive sperm motility not less than 12% in native ejaculates; sperm count at least 1 million sperm per milliliter (mL) of semen. A total of 267 married couples were included in the study. The analyzed group consisted of 130 patients (the COC group). In these patients, sperm was selected with subsequent ICSI. The comparison group consisted of 137 patients, who underwent standard ICSI procedure with sperm selection based on morphological characteristics (the ICSI group).
All married couples were comprehensively tested before undergoing infertility treatment using ART according to the regulatory documents: Articles 32, 27, 55 of the Federal Law of the Russian Federation No 323-FZ of November 21, 2011 “On the Fundamentals of Citizens' Health Protection in the Russian Federation”; the Order of the Ministry of Health of Russia No. 107n of August 30, 2012 “On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use”; the Order of the Ministry of Health of Russia No 556n of October 30, 2012 “On approval of the standard of medical care for infertility using assisted reproductive technology”. The ART program was performed using the standard method. Ovarian stimulation was performed on day 2–3 of the menstrual cycle using preparations of recombinant follicle-stimulating hormone or human menopausal gonadotropin. To prevent premature LH surge when the follicle diameter reached 14 mm, a gonadotropin-releasing hormone antagonist preparation was administered subcutaneously at a dose of 0.25 mg/day. For final oocyte maturation, when follicles reached ≥17 mm in diameter, human chorionic gonadotropin (hCG) (10,000 IU) was given to trigger ovulation. 36–37 hours after trigger shot injection, transvaginal puncture of follicles was performed under intravenous anesthesia in the operating room to collect follicular fluids and subsequently assess oocyte quality.
To collect COCs, cumulus cells were partially removed using the optical microscope for mechanical cut off with sterile needle of insulin syringe. COCs were preliminary washed from blood cells and follicular fluid in HEPES buffer (Gamet Buffer, COOK, Ireland). Then the obtained COCs and separate cumulus cells were placed in culture medium (G-IVF, Vitrolife, Sweden) and incubated at 37°C in 6.2% CO2, 5% О2 atmosphere individually in 4-well plates. In the comparison group, cumulus cells were not cut off.
Semen samples were collected by masturbation after 3–5 days of ejaculatory abstinence. Spermatozoa were isolated by density gradient centrifugation followed by subsequent washing in sperm medium according to the manufacturer's instructions (Spermwash, IrvineSc., USA). After 2–3 hours of incubation, cumulus cells were separated from the oocytes with the Pasteur pipette using hyaluronidase solution (IrvineSc., USA) before standard ICSI procedure. After final denudation, MII stage oocytes that were suitable for ICSI, were selected. Fertilization in the comparison group was carried out by conventional method used in the laboratory.
Sperm selection with the use of COC
In all cases of fertilization, the patients’ own COCs were used. One hour before ICSI procedure Petri dish was prepared, as is shown in Figure. HEPES-buffered medium (Gamet Buffer, COOK, Ireland) was used. The collected COCs were placed in the center of the drop, and the processed sperm was placed in the leftmost drop (Figure).
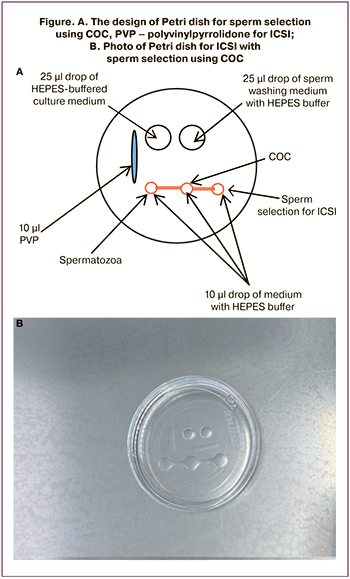
We expected that sperm would float from the leftmost droplet through the central drop that contained cumulus cells, and gather in the rightmost droplet. From there, male germ cells were selected with ICSI micro-instruments, and fertilization was carried out. The prepared Petri dish with COC and sperm were kept in incubator for 1 hour in order sperm could cross cumulus cells.
All ICSI procedures were performed 39–40 hours after hCG was injected by well-trained embryologist with more than 5 years of experience. Spermatozoa with “ideal” morphology were selected for injection using an inverted microscope equipped with micromanipulators. Best-looking spermatozoa with normal morphological parameters were selected for injection using inverted microscope equipped with micromanipulators. After injection, the oocytes were transferred to separate culture dishes containing fertilization medium (G-IVF, Vitrolife, Sweden) for observation of pronuclei. Further culture procedure was conducted for 5 days in single-step media (G-TL, Vitrolife, Швеция) up to the moment of embryo transfer into the uterine cavity. The remaining blastocysts suitable for using were vitrified. Embryo evaluation was according to recommendations of the Russian Association of Human Reproduction RAHR).
Progesterone preparation was administered for luteal phase support. On day 12–14 after embryo transfer, blood samples were obtained from all patients for β-hCG test to confirm pregnancy. The results of pregnancy cause on the suggested date of 12 weeks of embryo development and live birth outcomes on the date of delivery were obtained by telephone survey.
Statistical analysis
Analysis of the results was performed using software program IBM SPSS Statistics версии 23.0 (USA), as well as Microsoft Excel tables. The type of data distribution was detected for quantitative data analysis in the groups using the Kolmogorov–Smirnov test. Continuous variables were presented as median (Me) and interquartile values (Q1; Q3) based on sample distribution. For statistical analysis, Mann–Whitney U test was used for pairwise comparison, when distribution differed from normal. The categorical binary data (clinical and anamnestic data and outcomes of ART programs (implantation and childbirth)) were shown as the percentage of the total number of patients in group P and absolute number N, P% (N). Analysis of nominal data was performed using chi-squared test (χ2) and Fisher’s exact test. To compare the nominal characteristics between the groups, relative risk (RR) was calculated with 95 % confidence interval (CI) for probability of the outcome (implantation, birth) depending on the presence of the factor (sperm selection using cumulus-oocyte complex). The Hodges-Lehman estimator was used to estimate the value of the clinical effect of major effectiveness criteria for primary outcomes (fertilization and blastulation rates). For continuous variables the difference in medians with 95% CI was used. Significance threshold was at p =0.05. The strength of the relationship between binary values was calculated using the odds ratio (OR). Additionally, assessment of statistical significance of OR was based on the values of 95% confidence interval (CI).
Quantitative variables included woman's age, the number of COCs, MII oocytes, fertilized cells (2PN), the number of good-quality and high-quality blastocysts, the percentage of mature cells, fertilization rate, blastulation rate, the number of IVF/ICSI attempts in anamnesis. Clinical and anamnestic data were encoded in binary format, that included tubo-peritoneal factor infertility, endometriosis stage I-II or endometrial cysts, primary or secondary infertility, uterine factor infertility (fibroids, division of the synechiae, adenomyosis, uterine scar), as well as belonging to the studied groups (selection of spermatozoa for OCC or the standard procedure for ICSI), as well as attribution to the group under study (sperm selection using COC or standard ICSI procedure. We considered blastulation as the percentage of good-quality and high-quality blastocysts that were suitable for transfer.
Results
Statistical analysis of the clinical characteristics and embryonic stage in the groups under study is shown in Table 1. According to the results of the Kolmogorov–Smirnov test, distribution of quantitative characteristics significantly differed from normal (р<0.05).
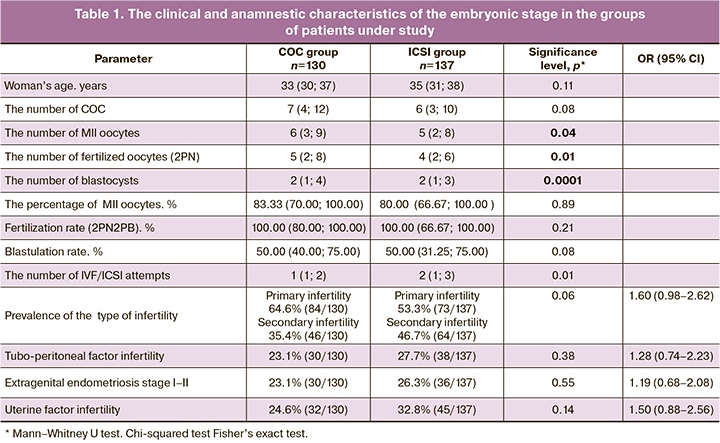
According to the data in Table 1, the analysis showed that there were no differences between the groups in patients’ age and clinical and anamnestic characteristics, including the prevalence of the type of infertility (primary or secondary), the presence of tubo-peritoneal factor, endometriosis and/or endometrial cysts, as well as uterine factors (fibroids, adenomyosis, division of the synechiae) in medical history. In the groups of patients who underwent standard ICSI procedure, the number of couples with multiple IVF/ICSI attempts prevailed.
Statistically, the absolute quantitative values of the embryonic stage (the number of mature oocytes, fertilized cells, good-quality and high-quality blastocysts) were significantly higher in the group of sperm selection using COC. However, assessment of these characteristics in relative units showed, that these differences were not confirmed/remained as they were. The trend toward higher blastulation rate was observed in the COC group (р=0,08).
Taking into account the results of the embryonic stage, the outcomes of ART programs depended on many factors, and one of them was ejaculate quality (normal sperm morphology), we took a decision to assess separately these values in the COC group with 0–1% and 2–3% of sperm morphology and compare them with similar subgroups in the group of patients who underwent standard ICSI procedure. Table 2 shows the number of patients in the subgroups of the comparison groups and assessment results of embryonic stage.
The results of sperm selection using COC with subsequent performance of ICSI procedure in the cohort of patients with 0–1% of sperm morphology showed that statistically, fertilization and blastulation rates were significantly higher versus the subgroup of patients in whom sperm selection was based only on the morphological parameters. In the cohort of patients with 2–3% of sperm morphology, the differences were not found. Thus, sperm selection using COC is a promising method to improve significant characteristics of the embryonic stage.
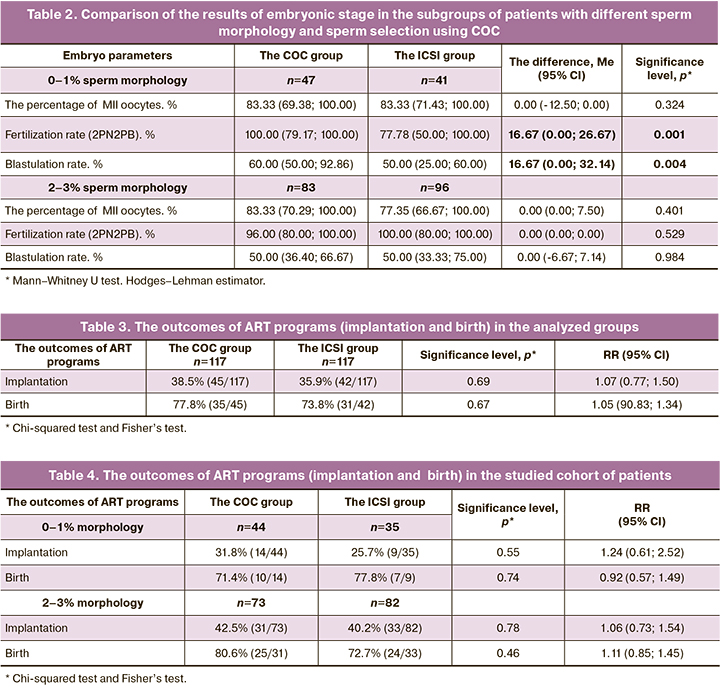
The outcomes of ART programs in the groups and subgroups under study is shown in Table 3 and Table 4. The obtained data showed that, neither the chance for getting pregnant, nor birth rate depends on sperm selection using COC both among the patients with 0–1% morphology and with 2–3% morphology for native embryo transfer in ovarian stimulation phase.
Discussion
In every sexual intercourse, several million sperm enter the vagina, but only about 1000 male gametes reach the fallopian tube potentially to fertilize an egg. The increasing decline in sperm count reaching the fallopian tube is the result of selection during in vivo fertilization. Before being selected, spermatozoa must be capable to interact with the female reproductive tract and pass through cumulus cells and the zona pellucida [8]. According to some estimates, 15% of couples of reproductive age (15–49 years) are infertile worldwide, and 20–70% of overall infertility is male infertility. ART techniques make it possible effectively to overcome infertility, however, about 10% of married couples experience fertilization failures, embryo arrest and lack of suitable embryos for transfer into the uterine cavity. ICSI made it possible partially to overcome male factor infertility. However, until present, the efficiency of conception in case of impaired spermatogenesis remains lower versus tubal-peritoneal factor of infertility.
The researchers try to improve sperm selection in the era of ICSI applying different methods [9, 10]. One of these methods is the use of cumulus cells, and its effectiveness was assessed in our study. According to the clinical results of the conducted study, the implantation rate (38.5% in the COC group versus 35.9% the ICSI group) in the general cohort of patients significantly did not increase with sperm selection using cumulus cells. Also, the rate of successful births remained the same (77.8% in the COC group versus 73.8% in the ICSI group).
. Embryo stage in the general cohort of patients did not improve significantly. The trend toward higher number of high-quality and good-quality blastocysts was observed, when egg was fertilized by sperm that passed through cumulus cells. As a result, a higher number of embryos were suitable for transfer into the uterine cavity and cryopreservation.
However, in identification of the group of patients with 0–1% and 2–3% sperm morphology, that is stratification of teratozoospermia as mild and severe, it was observed that fertilization rate significantly increased (100% в in the COC group and 77.78% in the ICSI group, p=0.001). Also, high-quality and good-quality blastocyst development rate was higher in the group of sperm selection using COC (60% in the COC group versus 50% in the ICSI group, p=0.004). The obtained results indicated the appropriateness of using this method of sperm selection in cases of teratozoospermia with 0–1% sperm morphology. It is especially important to use additional criteria for sperm selection in cases of 100 % teratozoospermia (0% of normal sperm morphology). Indeed, when the patients were stratified into groups based on sperm morphology assessment, we failed to show significantly high effectiveness of infertility treatment programs using ART techniques. In the group with 0–1% sperm morphology, implantation rate with sperm selection using cumulus cells was 31.8%, and 25.7% using ICSI (р=0.62). However, a positive trend was observed. It is probable that with larger sample size, it would be possible to find a significant difference. Also, birth rates in cases of teratozoospermia (0–1%) significantly increased after sperm selection using clumulus cells: 71.4% versus 77.8% in the ICSI group. However female factors were not taken into account in pregnancy.
The important result of our study was that the number of good-quality and high-quality embryos with 0–1% teratozoospermia increased with fertilization based on sperm selection using cumulus cells. This gives a chance to a couple to undergo the program of embryo transfer after thawing without repeated ovarian stimulation and transvaginal follicle puncture, and to achieve long-awaited pregnancy. In addition, sperm selection using cumulus cells in this cohort of patients makes it possible to reduce government expenditure on the birth of 1 child, since the total cost of IVF program is 3 times higher than cryoprotocol.
The published data by foreign authors correlated with the results obtained in our study [5–7]. According to some researches, the effective approach, that is based on sperm selection using COC, provides safe sperm selection on ICSI dish [7]. Naknam W. et al. analyzed 857 sibling oocytes at MII stage, which were randomly and equally divided for fertilization using COC-ICSI (n=429) and standard ICSI procedure (n=428). Fertilization rate of eggs that were fertilized by sperm selection using cumulus cells was higher compared to the group with standard ICSI procedure (85.31% versus 74.77%; p<0.05). There was no significant difference in the cleavage rates (98.09% versus 98.13%; р>0.05) and the percentage of obtained embryos without day-3 fragmentation (63.23% versus 58.92%; р>0.05) between COC-ICSI and standard ICSI procedure. However, observation of further development showed higher blastocyst formation rate on day 5 (46.52% versus 38.85%; р<0,05), as well as higher rate of high-quality blastocysts suitable for transfer into the uterine cavity and cryopreservation (38.72% versus 24.20%; р<0,05) in COC-ICSI group compared to standard ICSI group. The cumulative implantation rate with COC-ICSI and standard ICSI procedure was 64.29% and 53.85%, respectively.
Conclusion
The results of our study showed that the use of COC for sperm selection in ART programs for infertility treatment can be considered as recommended treatment for the patients with impaired spermatogenesis (low percentage of morphologically normal sperm in the ejaculate). In these couples, it is possible to achieve high fertilization rate and embryo development to blastocyst stage. This allows to reduce the cost of ovarian stimulation and achieve desired pregnancy with cryo-embryo transfer. Also, a significant advantage of sperm selection using cumulus cells is the use of autologous biological material, in contrast to PICSI technique, where synthesized HA is placed in the Petri dishes.
To improve the effectiveness of infertility treatment, based on the data obtained by us and in published literature, it is advisable to recommend physiological sperm selection using cumulus cells when sperm with normal morphology is less than 1% in the ejaculate.
References
- Liu K., Mao X., Pan F., Chen Y., An R. Correlation analysis of sperm DNA fragmentation index with semen parameters and the effect of sperm DFI on outcomes of ART. Sci. Rep. 2023; 13(1): 2717. https://dx.doi.org/10.1038/s41598-023-28765-z.
- Yang H., Liu Y., Niu W., Yang Z., Wang Y., Jin H., Li G. Correlation study of male semen parameters and embryo aneuploidy in preimplantation genetic testing for aneuploidy. Front. Endocrinol. (Lausanne). 2023; 13: 1072176.https://dx.doi.org/10.3389/fendo.2022.1072176.
- Макарова Н.П., Лобанова Н.Н., Кулакова Е.В., Непша О.С., Екимов А.Н., Калинина Е.А. Влияние преимплантационного генетического тестирования на результаты программ вспомогательных репродуктивных технологий у супружеских пар с мужским фактором бесплодия. Акушерство и гинекология. 2021; 11: 154-64. [Makarova N.P., Lobanova N.N., Kulakova E.V., Nepsha O.S., Ekimov A.N., Kalinina E.A. Impact of preimplantation genetic testing on assisted reproductive technology outcomes in couples with male factor infertility. Obstetrics and Gynecology. 2021; (11): 154-64. (in Russian)].https://dx.doi.org/10.18565/aig.2021.11.154-164.
- West R., Coomarasamy A., Frew L., Hutton R., Kirkman-Brown J., Lawlor M. et al. Sperm selection with hyaluronic acid improved live birth outcomes among older couples and was connected to sperm DNA quality, potentially affecting all treatment outcomes. Hum. Reprod. 2022; 37(6): 1106-25.https://dx.doi.org/10.1093/humrep/deac058.
- Wang C., Feng G., Shu J., Zhou H., Zhang B., Chen H. et al. Cumulus oophorus complexes favor physiologic selection of spermatozoa for intracytoplasmic sperm injection. Fertil. Steril. 2018; 109(5): 823-31. https://dx.doi.org/10.1016/j.fertnstert.2017.12.026.
- Hamze J.G., Jiménez-Movilla M., Romar R. Sperm-binding assay using an in vitro 3D model of the mammalian cumulus-oocyte complex. Curr. Protoc. Toxicol. 2020; 86(1): e100. https://dx.doi.org/10.1002/cptx.100.
- Naknam W., Salang L., Sothornwit J., Amnatbuddee S., Seejorn K., Pongsritasana T., Sukkasame S. Effect of sperm selection method by cumulus oophorus complexes and conventional sperm preparation method on sperm quality and DNA fragmentation for assisted reproduction techonology. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 243: 46-50. https://dx.doi.org/10.1016/j.ejogrb.2019.10.004.
- Leung E.T.Y., Lee C.L., Tian X., Lam K.K.W., Li R.H.W., Ng E.H.Y. et al. Simulating nature in sperm selection for assisted reproduction. Nat. Rev.Urol. 2022; 19(1): 16-36. https://dx.doi.org/10.1038/s41585-021-00530-9.
- Гамидова П.С., Смольникова В.Ю., Макарова Н.П., Лобанова Н.Н. Методы улучшения исходов программ вспомогательных репродуктивных технологий путем инновационных подходов к селекции мужских половых клеток. Акушерство и гинекология. 2022; 7: 34-42. [Gamidova P.S., Smolnikova V.Yu., Makarova N.P., Lobanova N.N. Methods for improving the outcomes of assisted reproductive technology programs through innovative approaches to selecting male germ cells. Obstetrics and Gynecology. 2022; (7): 34-42. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.7.34-42.
- Чистякова А.В., Макарова Н.П., Лобанова Н.Н., Смольникова В.Ю. Возможности повышения эффективности программ вспомогательных репродуктивных технологий с помощью селекции сперматозоидов на ооцит-кумулюсных комплексах. Акушерство и гинекология. 2022; 5: 30-4. [Chistyakova A.V., Makarova N.P., Lobanova N.N., Smolnikova V.Yu. Possibilities for increasing the effectiveness of assisted reproductive technology programs using cumulus-oocyte complex-induced sperm selection. Obstetrics and Gynecology. 2022; (5): 30-4. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.5.30-34.
Received 01.03.2023
Accepted 31.03.2023
About the Authors
Alina V. Chistyakova, PhD Student, B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, alinadubinina_07@mail.ru, 117997, Russia, Moscow, Academician Oparin str., 4.Natalya P. Makarova, Dr. Bio. Sci., Leading Researcher, B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, np_makarova@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.
Oksana S. Nepsha, Ph.D., Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, o_nepsha@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Veronika Yu. Smolnikova, Dr. Med. Sci., Leading Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, v_smolnikova@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, e_kalinina@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.



