Influence of ovulation inducing drugs and the woman’s age on the results of infertility treatment using assisted reproductive technologies with sperm selection by oocyte-cumulus complexes
Objective: To investigate the effect of ovulation-inducing drugs and woman’s age on the outcomes of infertility treatment using ART with spermatozoa selection by cumulus cells.Chistyakova A.V., Makarova N.P., Nepsha O.S., Smolnikova V.Yu., Kalinina E.A.
Materials and methods: This study included 267 couples undergoing ART for infertility treatment. Clinical and embryological parameters of the ART cycle were assessed. The study group included 130 patients (CC group) with spermatozoa selected by CC and subsequent ICSI procedures. The control group consisted of 137 patients who underwent a standard ICSI procedure with spermatozoa selection based on morphological characteristics (ICSI group). Each group was divided into women under the age of 35 years and those aged 36 years and above. To assess the effect of ovarian stimulation, groups of women were categorized according to the type of trigger for final follicle maturation: human chorionic gonadotropin (hCG) (n=182) and gonadotropin-releasing hormone agonist (GnRH agonist) (n=52). Some women did not undergo embryo transfer (n=33). VitroLife medium (Sweden) was used during the embryological stage. Only one embryo was transferred to the uterine cavity. Statistical analysis was performed using SPSS Statistics software (USA), and included both parametric and non-parametric methods.
Results: Among women aged < 35 years, the absolute number of blastocysts obtained on the 5th day of culture was significantly higher in the CC group than that in the ICSI group (Me 2 (1; 3) and 3 (1; 5), p=0.02). Patients of late reproductive age (≥ 36 years) also showed a significantly higher number of blastocysts on the 5th day of culture when spermatozoa were selected using CC (p=0.03). When evaluating the effect of the trigger of the final follicle maturation, it was found that the embryo implantation rate was significantly higher when hCG was used and sperm were selected by CC (CC 44.9%, ICSI 17.9%, p=0.01); however, the birth rate did not differ significantly, regardless of the age of the woman.
Conclusion: This study shows that the technology of spermatozoa selection using CC in infertility treatment with ART can be successfully implemented in different age groups of women, which will increase the number of embryos at the blastocyst stage suitable for transfer to the uterine cavity and cryopreservation. At the same time, the implantation rate can only be increased in women in whom exogenous hCG is used to trigger the final maturation of oocytes. The data obtained in this study provide hope for the development of more advanced in vitro sperm selection methods that would mimic the physiological conditions in the female reproductive tract.
Authors’ contributions: Chistyakova A.V. – collection and analysis of clinical data, analysis of initial material and manuscript drafting; Makarova N.P. – collection and analysis of embryological data, analysis of results; Nepsha O.S. – analysis of results, statistical analysis, clinical interpretation; Smolnikova V.Yu. – manuscript editing, critical analysis; Kalinina E.A. – manuscript approval.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The work was supported by the state task of the Ministry of Health of the Russian Federation No. 121040600410-7 «Solving the problem of infertility in modern conditions by developing a clinical diagnostic model of infertile marriage and using innovative technologies in assisted reproduction programs».
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Chistyakova A.V., Makarova N.P., Nepsha O.S., Smolnikova V.Yu., Kalinina E.A.
Influence of ovulation inducing drugs and the woman’s age on the results of infertility treatment using assisted reproductive technologies with sperm selection by oocyte-cumulus complexes.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (6): 99-106 (in Russian)
https://dx.doi.org/10.18565/aig.2023.78
Keywords
In human ovaries, oocytes are located inside follicles filled with follicular fluid, the cavity of which is lined by somatic granulosa cells. Within the antral follicle, the female gamete is surrounded by cumulus cells (CC), a specialized subgroup of granulosa cells. CCs differentiate from mural granulosa cells by oocyte-secreted factors and ovarian hormones. The innermost layers of the CC, called the corona radiata, are in direct contact with the oocyte through transzonal projections. These processes of granulosa cells penetrate the zona pellucida and form specialized connections with the oolemma. All the indicated groups of cells are involved not only in maintaining the viability of the oocyte but also in the distant interaction of gametes during fertilization. Spermatozoa can move in the direction of secreted CC chemo attractants [1, 2]. Currently, CCs are being actively studied using modern molecular biology methods to deepen our understanding of fertilization processes in humans and improve the effectiveness of infertility treatment using assisted reproductive technologies (ART) [3]. Research has shown that only a small proportion of spermatozoa can overcome multiple obstacles in the female genital tract and reach the fertilization site. CCs serve as a barrier for sperm without an acrosome. Only male germ cells with intact acrosomes can bind to the zona pellucida and penetrate the cell. In routine in vitro fertilization (IVF) practice, simply obtaining a patient's CCs and using them in a Petri dish is sufficient to improve the embryological stage of ART programs and enhance the outcomes of infertility treatment programs.
In our previous work, we showed that the use of spermatozoa selection by CC is associated with improved embryological parameters of ART, including higher fertilization and blastulation rates, especially in couples with a pronounced male infertility factor [4].
The purpose of this study was to evaluate the contribution of a woman's age and the effect of an ovulation-triggering drug on the outcomes of fertility treatment programs using ART in the selection of spermatozoa for CC. Women’s age is a critical factor that largely determines the outcomes of treatment programs [5]. Patients of advanced reproductive age are more likely to experience a decrease in the ovarian reserve, an increase in the total dose of gonadotropins, the absence of oocyte fertilization, and a halt in early embryogenesis. Therefore, it is advisable to enhance the embryological stage of ART programs for women of advanced reproductive age by introducing new methods for spermatozoa selection and optimizing culture conditions.
Materials and methods
The study included men and women who underwent ART programs in the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, from January 2020 to December 2022. The inclusion criteria were a woman’s age from 18 to 45 years; signed informed consent to participate in the study; the level of anti-Müllerian hormone in the blood of a woman was greater than or equal to 1 ng/ml on the 2nd–3rd day of the menstrual cycle; the presence of at least 50% live spermatozoa in the ejaculate of a partner; progressive sperm motility in native ejaculate PR not less than 12%; and spermatozoa concentration of at least 1 million/ml. In total, 267 married couples were included in this study. The study group consisted of 130 patients (CC group) who underwent spermatozoa sampling for CC followed by the ICSI procedure. The control group consisted of 137 patients who underwent a standard ICSI procedure with spermatozoa selection based on morphological characteristics (ICSI group). Each group was divided into women under the age of 35 years and those aged 36 years and above. To assess the impact of ovulation trigger during ovarian stimulation on the outcomes of ART programs, women were divided into groups according to the type of trigger for final follicle maturation: human chorionic gonadotropin (hCG) (n=182) and gonadotropin-releasing hormone agonist (GnRH agonist) (n=52).
Some women who did not undergo embryo transfer due to unsatisfactory quality of the embryo, inadequate growth of the endometrium, and somatic disease were excluded. In total, 234 women were included in the analysis.
Before undergoing ART, all married couples underwent a complete examination in accordance with current regulatory documents. ART was performed according to a generally accepted methodology. Stimulation of ovarian function was performed from days 2–3 of the menstrual cycle with GnRH antagonists and recombinant follicle-stimulating hormone or human menopausal gonadotropin. To prevent premature LH surge, cetrorelix acetate was administered subcutaneously at a dose of 0.25 mg/day when the follicle reached a diameter of 14 mm. For the final oocyte maturation, ΧHCh at a dose of 10,000 μE or the GnRH agonist triptorelin (0.1 mg was used when the follicle diameter reached ≥17 mm. The use of GnRH agonist drugs as a trigger is associated with a high risk of ovarian hyperstimulation syndrome. In the operating theatre, a transvaginal follicular puncture was performed under intravenous anesthesia 36–37 hours after the ovulation trigger was administered, with collection of follicular fluid and subsequent assessment of oocyte quality.
Semen samples were collected by masturbation after 3–5 days of abstinence from ejaculation. Spermatozoa were isolated by density gradient centrifugation followed by washing in spermatozoa medium, according to the manufacturer's instructions (Spermwash, Irvine, CA, USA). After incubation for 2–3 h, CCs were separated from oocytes with a hyaluronidase solution using a Pasteur pipette before ICSI using standard methods (IrvineSc., USA). After the final denudation, MII stage oocytes suitable for ICSI were selected. In the CC group, spermatozoa were selected by a previously described method [4], in the comparison group, according to the standard operating procedures of the embryology laboratory. Briefly, using a culture medium containing HEPES, three drops were prepared in a petri dish and interconnected. The collected autologous CC was placed in a Petri dish in the central drop. Spermatozoa were placed in the first drop, from which they moved through the central drop containing CCs, collecting in the rightmost drop. Male germ cells were collected with ICSI microtools, and fertilization was performed. The prepared work dish with CCs and sperm was kept in an incubator for 1 h to allow the spermatozoa to cross the CC. The resulting embryos were cultured at 37°C, 6.2% CO2, and 5% O2 in separate wells of a Petri dish under mineral oil for up to 5–6 days. Blastocyst transfer into the uterine cavity was performed under ultrasound guidance using COOK catheters (Ireland). Only 1 embryo was transferred. The luteal phase was supported by progesterone preparations according to the manufacturer's instructions. When using a GnRH agonist as a trigger for the final maturation of follicles, a modified scheme for supporting the luteal phase has been used [5]. Pregnancy was determined based on the level of hCG in the venous blood 14 days after the transfer, and the fetal egg was fixed in the uterine cavity after 21 days. Information about childbirth was collected via telephone calls.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 23.0 (USA) and Microsoft Excel spreadsheets. The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. Continuous variables were reported as median (Me) and interquartile range (Q1; Q3) based on the distribution of the sample. Statistical analysis was performed using the Mann–Whitney test in pairwise comparisons when the distribution did not meet the normality assumption. Quantitative variables showing a normal distribution were expressed as mean (M) and standard deviation (SD). Parametric methods (Student's t-test) were used for analysis. Frequencies and percentages were reported for categorical variables, such as clinical and demographic data and ART outcomes (implantation and childbirth). Categorical variables were analyzed using the Chi-square (χ2) test and Fisher's exact test. To compare groups according to categorical variables, in a prospective study, the relative risk (RR) was calculated with a 95% confidence interval (CI) to compare the probability of outcome (implantation, childbirth) depending on the presence of the factor. The difference between medians with a 95% CI (Hodges–Lehman estimate) was used to estimate the effect size for the primary outcome measures (fertilization and blastulation rates) for continuous data. The significance threshold was set at p<0.05. Fertilization and blastulation rates were used as criteria for the effectiveness of spermatozoa selection by CCs. Primary outcomes coincide with the criterion of effectiveness, that is, improvement in the indicators of the embryological stage. The secondary outcomes are the effectiveness of ART programs (clinical pregnancy rate (implantation) and live birth rate).
Quantitative variables included age, number of oocyte-cumulus complexes received, number of mature MII oocytes, number of fertilized cells (2PN), number of good and excellent quality blastocysts, percentage of mature cells, percentage of fertilization, percentage of blastulation, and number of previous IVF/ICSI attempts. As clinical and anamnestic data, coded in binary form, we considered the presence of a tubal-peritoneal factor, the presence of endometriosis I and II stages of spread, or endometrioid cysts, primary infertility (PI) or secondary infertility (SI), the presence of a uterine factor (fibroids, synechia, adenomyosis, uterine scar), as well as belonging to the studied groups (selection of spermatozoa by CCs or standard ICSI procedure). The blastulation rate was defined as the proportion of good- and excellent-quality blastocysts suitable for transfer in the total cohort of received blastocysts. The quality of the embryos on day 5 of culture was determined according to the recommendations of the Russian Association of Human Reproduction (RAHR, 2020). Morphologically distinct and good blastocysts were categorized as AA, AB, BA, or BB.
Results
The demographic and clinical data, as well as the characteristics of the embryological stage of the study groups are shown in Table 1 (for women ≤35 years old) and in Table 2 (for women >36 years old). Evaluation of data distribution using the Kolmogorov–Smirnov test showed a non-normal distribution of the study dataset in the group of women ≤35 years old (p<0.05). While in the group of patients aged 36+ years, the variable "percentage of progressively motile spermatozoa" had a normal distribution.
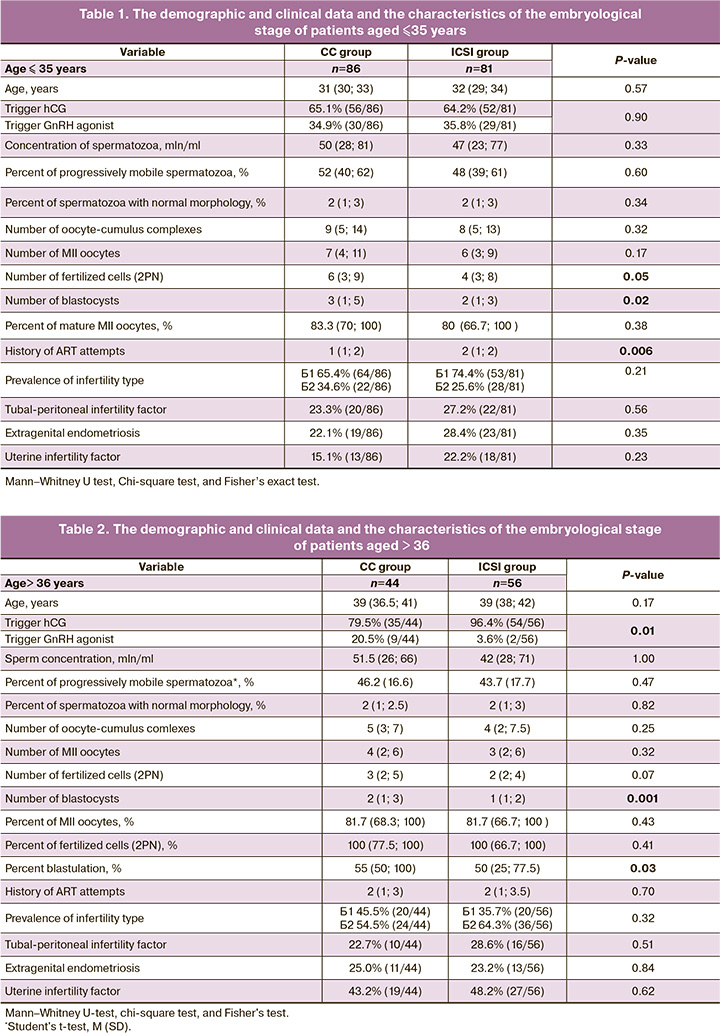
Despite the fact that embryological parameters in absolute values (the number of fertilized cells and the number of blastocysts) in the CC group were significantly different from those in the ICSI group and were higher when comparing the corresponding relative values of these values, these differences were not confirmed when analyzing the results of the embryological stage in a cohort of women aged < 35 years.
Notably, there was a significant difference between groups in the frequency of trigger use in stimulation protocols: in the ICSI group, hCG was used more often than GnRH agonists.
It is worth noting that the results of comparing the embryological stage parameters in the group of patients older than 36 years showed a statistically significant increase in the percentage of blastulation in the subgroup of patients who underwent spermatozoa selection using CCs (p=0.03). The results are presented in Table 3. The outcomes of the ART programs in the study groups and subgroups are presented in Table 4. Based on the results, neither the pregnancy rate nor the birth rate was affected by sperm selection using CCs, both among young patients (≤35 years), and among women of advanced reproductive age (36+ years)
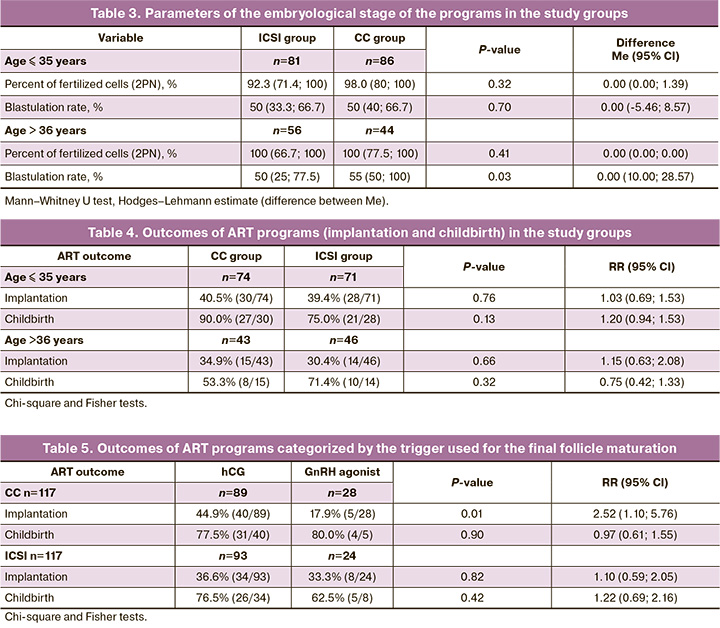
An analysis of the treatment results of two groups of patients (experimental and control), regardless of age, showed that in the group with spermatozoa selection using CCs, the use of hCG as a trigger was associated with a significantly higher embryo implantation rate than the use of GnRH agonist in the stimulation protocols. In the ICSI group, no difference was found in either implantation or delivery rates according to the trigger (Table 5).
Discussion
The contribution of the spermatozoon to early embryogenesis is currently under active investigation because up to 50% of infertility cases are associated with paternal factors [6]. Traditional methods used in ART infertility treatment programs mainly rely on motility and viability parameters to select and evaluate sperm quality. However, other characteristics of spermatozoa, such as DNA integrity, membrane conditions, and chromatin packaging, are equally important for successful live birth.
In natural fertilization, sperm travels through the male and female reproductive tracts to reach the oocyte and fertilize it. During this journey, sperm cells encounter numerous obstacles that significantly reduce the number of sperm reaching the fertilization site. In humans, the number of spermatozoa in the fallopian tube decreases from tens of millions in the ejaculate to hundreds in the fallopian tube [6]. Fallopian tubes play a key role in fertility by modulating sperm transport, viability, and maturation, providing male germ cells suitable for fertilization at the right time. Therefore, it is necessary to develop methods for selecting spermatozoa during in vitro fertilization that mimic physiological conditions.
In the present study, the method of selecting spermatozoa using autologous CCs from spouses was applied. The results showed that the age of the woman is of critical importance for any method of fertilization, both with and without CCs. In the group of patients aged < 35 years, the number of blastocysts obtained on day 5 of culture was significantly higher in the group with sperm selected by CCs than in the traditional ICSI group (Me 2 (1; 3) and 3 (1; 5), p=0.02). However, this may be because of the large number of oocytes obtained at the MII stage (p=0.05). At the same time, patients in the conventional ICSI group had a significantly higher rate of failed previous ART attempts (p=0.006). The groups did not differ in terms of the causes of infertility among married couples.
Patients of advanced reproductive age also showed a significantly higher number of blastocysts on day 5 of culture when spermatozoa were selected using CCs (p=0.03). The groups did not differ in the other parameters analyzed. These results indicate the feasibility of using spermatozoa selection by CC to improve the embryological stage, namely, to increase the number of blastocysts. However, we did not investigate the genetic status of the resulting embryos. For a group of women under 35 years of age, a larger number of blastocysts received, due to the low risk of aneuploidy in the embryos, will allow a long-awaited pregnancy and the birth of a healthy child with one stimulation and several cryopreserved transfers. For a group of women aged over 36 years, when the risk of chromosomal abnormalities in embryos increases, an improvement in embryological parameters in the form of an increase in the number of blastocysts suitable for transfer may result in the birth of a child with genetic disorders and multiple unsuccessful attempts at thawed embryo transfer. For women aged > 36 years, the recommendation to conduct preimplantation genetic testing of embryos for aneuploidy is relevant to reduce the risk of having sick offspring [7].
When analyzing the results of infertility treatment using ART with different types of sperm selection, there were no statistically significant differences in pregnancy and delivery rates across all age groups.
As the trigger for final follicular maturation mainly affects CCs, the patients were divided according to the type of drug (hCG and GnRH agonist). Women who did not undergo embryo transfer due to the unavailability of the endometrium or the risk of developing ovarian hyperstimulation syndrome were excluded from the analysis. There were 117 patients in the CC group and 117 patients in the ICSI group. When assessing the effect of the trigger on final follicular maturation, it was found that the use of hCG and selection of spermatozoa by CCs was associated with a significantly higher implantation rate (CC 44.9%, ICSI 17.9%, p=0.01), while birth rates did not differ significantly. In the ICSI group, these patterns were not observed in the ICSI group.
Consequently, the selection of spermatozoa using CCs increases the effectiveness of infertility treatment programs using hCG as a trigger for final follicular maturation. This can be explained by the effect of hCG on CCs. In a natural menstrual cycle with normal ovulation, the rupture of the dominant follicle and the release of oocytes is initiated by a sharp rise in luteinizing hormone (LH) levels in the middle of the cycle. Ovarian stimulation programs use GnRH agonists to prevent premature LH rise and hCG as a substitute for the endogenous LH surge. Exogenous hCG also supports the luteal phase due to its long half-life; however, it increases the likelihood of developing ovarian hyperstimulation syndrome. Prolonged exposure of CCs to hCG allows the oocyte-cumulus complex to form fewer tight bonds on the hyaluronic matrix, thereby facilitating better sperm selection. When a GnRH agonist is used as a trigger, CCs do not have this 'fluffy' matrix, resulting in less efficient sperm selection [8]. The expansion of CCs, determined by the type of trigger, can be said to affect the efficiency of in vitro sperm selection in infertility treatment with ART.
Conclusion
This study demonstrates the successful implementation of CC-based spermatozoa selection technology in infertility treatment with ART for women of different age groups, resulting in an increased number of blastocyst stage embryos suitable for transfer and cryopreservation. However, the implantation rate can only be increased in women who receive exogenous hCG for triggering the final maturation of oocytes. These findings offer hope for the development of more advanced in vitro sperm selection methods that mimic the physiological conditions in the female reproductive tract, ultimately leading to an increase in the number of healthy children born after ART programs.
References
- Martínez-Moro Á., González-Brusi L., Lamas-Toranzo I., González-Dosal P., Rodríguez-Juárez F., Bermejo-Álvarez P. The human cumulus cell transcriptome provides poor predictive value for embryo transfer outcome. Reprod. Biomed. Online. 2023 Jan 20: S1472-6483(23)00046-9. https://dx.doi.org/10.1016/j.rbmo.2023.01.012.
- Soto-Heras S., Sakkas D., Miller D.J. Sperm selection by the oviduct: perspectives for male fertility and assisted reproductive technologies. Biol. Reprod. 2023 Jan 10: ioac224. https://dx.doi.org/10.1093/biolre/ioac224.
- Cadenas J., Poulsen L.C., Nikiforov D., Grøndahl M.L., Kumar A., Bahnu K. et al. Regulation of human oocyte maturation in vivo during the final maturation of follicles. Hum. Reprod. 2023 Feb 9: dead024. https://dx.doi.org/10.1093/humrep/dead024.
- Чистякова А.В., Макарова Н.П., Непша О.С., Смольникова В.Ю., Калинина Е.А. Результаты лечения бесплодия методами вспомогательных репродуктивных технологий с использованием селекции сперматозоидов на ооцит-кумулюсных комплексах. Акушерство и гинекология. 2023; 5: 92-9. [Chistyakova A.V., Makarova N.P., Nepsha O.S., Smolnikova V.Yu., Kalinina E.A. The results of infertility treatment with assisted reproductive technology and sperm selection via cumulus-oocyte complexes. Obstetrics and Gynecology. 2023; (5): 92-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.53.
- Ипен С.М., Павлович С.В., Мишиева Н.Г., Абубакиров А.Н., Мартазанова Б.А. Сравнительный анализ эффективности программ вспомогательных репродуктивных технологий в зависимости от триггера овуляции. Акушерство и гинекология. 2017; 6: 99-103. [Eapen S.M., Pavlovich S.V., Mishieva N.G., Abubakirov A.N., Martazanova B.A. Comparative analysis of the effectiveness of an assisted reproductive technology program depending on an ovulation trigger. Obstetrics and Gynecology. 2017; (6): 99-103. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.6.99-103.
- Soto-Heras S., Sakkas D., Miller D.J. Sperm selection by the oviduct: perspectives for male fertility and assisted reproductive technologies. Biol. Reprod. 2023 Jan 10: ioac224. https://dx.doi.org/10.1093/biolre/ioac224.
- Lang M., Zhou M., Lei R., Li W. Comparison of pregnancy outcomes between IVF-ET pregnancies and spontaneous pregnancies in women of advanced maternal age. J. Matern. Fetal Neonatal Med. 2023; 36(1): 2183761.https://dx.doi.org/10.1080/14767058.2023.2183761.
- Cadenas J., la Cour Poulsen L., Mamsen L.S., Andersen C.Y. Future potential of in vitro maturation including fertility preservation. Fertil. Steril. 2023; 119(4): 550-9. https://dx.doi.org/10.1016/j.fertnstert.2023.01.027.
Received 27.03.2023
Accepted 29.05.2023
About the Authors
Alina V. Chistyakova, PhD Student, B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, alinadubinina_07@mail.ru, 117997, Russia, Moscow, Academician Oparin str., 4.Natalya P. Makarova, Dr. Bio. Sci., Leading Researcher, B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, np_makarova@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.
Oksana S. Nepsha, PhD, Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, o_nepsha@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Veronika Yu. Smolnikova, Dr. Med. Sci., Leading Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility,
Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, v_smolnikova@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov
National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, e_kalinina@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.



