Relationship between the severity of respiratory and cardiovascular disorders in preterm infants and the degree and size of maternal abnormal placentation
Balakina A.D., Balashova E.N., Ionov O.V., Kirtbaya A.R., Mikheeva A.A., Vasilchenko O.N., Zubkov V.V., Shmakov R.G., Degtyarev D.N.
Objective: To assess the severity of respiratory and cardiovascular disorders in preterm infants based on the degree and anatomical and topographic type of placenta accreta spectrum disorders as well as the diameter of the placental hernia in their mothers.
Materials and methods: A retrospective cohort study was conducted involving 288 preterm infants with a gestational age (GA) of 330–366 weeks born to mothers with placenta accreta spectrum disorders. The clinical data were analyzed in three stages. In Stage I, patients were divided into three groups based on the type of placenta accreta spectrum (accreta, increta, and percreta). In Stage II, infants were classified into five groups according to the anatomical and topographic types of the placenta accreta spectrum, following H. Palacius's classification. In Stage III, the infants were divided into two groups based on the placental hernia diameter. In the study groups, GA, anthropometric parameters, sex, Apgar scores at 1 and 5 min after birth, and the correspondence of body weight and length to GA were compared. The severity and duration of respiratory disorders were assessed using indirect criteria: frequency and duration of respiratory therapy, including mechanical ventilation (MV) and high-frequency oscillatory ventilation (HFOV), maximum required mean airway pressure (MAP), frequency and duration of additional oxygen supplementation, and frequency of surfactant replacement therapy. The severity of acute cardiovascular disorders was evaluated based on the frequency of cardiotonic and vasopressor therapies, including the use of dopamine and dobutamine, and the maximum vasoactive inotropic index (VII). Integral indicators of the severity of preterm infants' conditions after birth included the length of stay in the NICU and total duration of hospitalization in days.
Results: The analysis revealed no significant differences in the severity of respiratory and cardiovascular disorders among newborns based on the degree of placental invasion or type of accreta. However, a significant increase in hospitalization duration was observed in newborns of mothers with placenta percreta compared to those born to mothers with placenta accreta, likely due to the lower GA of infants born to mothers with placenta percreta. Additionally, an increase in the duration of HFOV and the need for higher HFOV parameters, particularly MAP, were noted in preterm infants born to mothers with a placental hernia diameter exceeding 7 cm.
Conclusion: The severity of respiratory and cardiovascular disorders in preterm infants was not influenced by the degree of abnormal placental invasion or the anatomical and topographic type of placental implantation. However, a correlation was found between the severity of respiratory disorders and the placental hernia diameter.
Authors' contributions: Balashova E.N., Balakina A.D., Vasilchenko O.N. – conception and design of the study; Balashova E.N., Balakina A.D., Mikheeva A.A., Kirtbaya A.R., Vasilchenko O.N. – database creation, material acquisition and processing; Balashova E.N., Balakina A.D. – statistical analysis; Balakina A.D., Balashova E.N. – drafting of the manuscript; Ionov O.V., Zubkov V.V., Shmakov R.G., Degtyarev D.N. – general supervision, acquisition of information, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted under the State Order on Planned Research and Development 25-A19 "Prognosis, Diagnosis and Treatment of Patients with Placenta Accreta Spectrum" No. R&D AAAA-A19-119021490133-6.
Ethical approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No: 11 of 11.11.2021).
Patient consent for publication: All patients provided informed consent for the publication of their data.
Authors' data sharing statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Balakina A.D., Balashova E.N., Ionov O.V., Kirtbaya A.R., Mikheeva A.A., Vasilchenko O.N., Zubkov V.V., Shmakov R.G., Degtyarev D.N. Relationship between the severity of respiratory and cardiovascular disorders
in preterm infants and the degree and size of maternal abnormal placentation.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (3): 72-83 (in Russian)
https://dx.doi.org/10.18565/aig.2024.282
Keywords
Placenta accreta spectrum is a pathological process characterized by abnormal trophoblast invasion into the uterine wall [1].
The incidence of placenta accreta spectrum varies across different countries, with estimates ranging from 1.7 to 14.1 cases per 10,000 deliveries according to various authors [2, 3]. As of 2016, the incidence of pregnancies complicated by placenta accreta spectrum was reported in one case out of 272 [4].
Globally, the incidence of abnormal placentation, particularly placenta accreta spectrum, shows a steady upward trend. The primary factors contributing to this increase include a rise in the number of deliveries via cesarean section and a demographic shift marked by an increase in the average maternal age [1, 5].
In the Russian Federation, data from Rosstat indicate that from 2005 to 2020, the number of cesarean section deliveries rose from 17.9 to 30.3 per 100 deliveries, and this trend continues to grow [6].
To evaluate the depth of placental invasion, the International Federation of Obstetrics and Gynecology (FIGO) classification is used, which integrates clinical and morphological criteria. According to this classification, the placenta accreta spectrum encompasses three levels of invasion: placenta accreta, characterized by superficial adhesion of the placenta to the myometrium; placenta increta, where villi penetrate deeply into the myometrium up to the uterine serosa without signs of extravaginal spread; and placenta percreta, where invasive villous tissue penetrates through the uterine serosa and may extend to the surrounding pelvic tissues, vessels, and organs [7]. Since 2020, the V.I. Kulakov NMRC for OG&P has implemented and actively uses an intraoperative anatomical and topographic classification proposed by H. Palacius [8]. This classification categorizes abnormal placentation as follows: type 0, where the placenta reaches the serosa through a uterine wall defect, adhesive but without true placental invasion or newly-formed vascularization, this group is considered a “false PAS”; type 1, where the placenta reaches or exceeds the serosa, with newly-formed vessels between the uterus, placenta, and bladder, associated with the upper posterior bladder; type 2, where the placenta reaches or exceeds the serosa with placental parametrial invasion; type 3, where the placenta invades the posteroinferior area of the bladder; and type 4, which shares similar features with type 3 but includes intensive fibrosis between the bladder and uterus.
Data on the condition of newborns born to mothers with placenta accreta spectrum in the literature are extremely limited. Typically, these studies focus on the degree of prematurity and birth length and weight features, highlighting the number of newborns with intrauterine growth restriction [9, 10].
Several clinical studies have demonstrated that placenta accreta is associated with an increased incidence of respiratory distress syndrome and a prolonged need for respiratory support in newborns, including the provision of continuous positive airway pressure [11, 12].
Preliminary analyses indicated a correlation between the severity of respiratory and cardiovascular disorders in preterm infants and the presence of placental invasion in their mothers in comparison to similar children born to mothers without abnormal placentation. This correlation is reflected in a more severe course during the early neonatal period [13]. Further investigation into the impact of placenta accreta on early neonatal characteristics led to the hypothesis that the degree of invasion may influence the severity of respiratory and cardiovascular disorders in newborns during this critical period.
This study aimed to assess the severity of respiratory and cardiovascular disorders in preterm infants of gestational age (GA) 330–366 weeks, based on the degree and anatomical and topographic type of placenta accreta spectrum disorders as well as the diameter of the placental hernia in their mothers.
Materials and methods
The study was conducted as a retrospective cohort analysis. From January 2019 to January 2023, 326 newborns whose mothers were diagnosed with placenta accreta spectrum were observed in the neonatal units of the Institute of Neonatology and Pediatrics of the V.I. Kulakov NMRC for OG&P of the Ministry of Health of the Russian Federation.
Informed consent to participate in the study was obtained from legal representatives of the newborns. The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P (Ref. No: 11 of 11.11.2021).
The inclusion criteria for the study were singleton pregnancy, GA of the newborn at 330–366 weeks, and clinically and/or histologically confirmed placenta accreta in the mother.
Infants with a GA of 330–366 weeks were included in the study because most deliveries of women with placenta accreta occur between 330–366 weeks, according to the protocol for the management of pregnant women with placenta accreta.
Exclusion criteria were congenital malformations (CM), including congenital heart disease (CHD), multiple gestations, and neonatal GA mismatch.
The study was divided into three stages. The study design is illustrated in Figure 1.
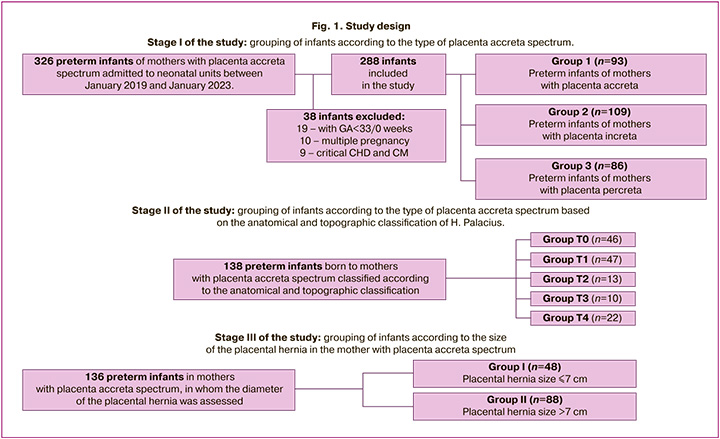
At stage I of the study, division into groups was carried out depending on the type of placenta accreta spectrum, considering the clinical and histological criteria described in the FIGO classification [7].
The study included 288 newborns. Preterm newborns were divided into three groups based on the maternal type of placenta accreta spectrum. Ninety-three women were diagnosed with placenta accreta, 109 with placenta increta, and 86 with severe placenta percreta. Figure 1 shows the sequence of the patient sample formation.
In stage II of the study, 138 women with placenta accreta spectrum were divided into five groups based on the intraoperative anatomical and topographic classification of H. Palacius [8]. Group T0 included children born to mothers with placenta accreta without invasion of the bladder wall; groups T1–T4 corresponded to types 1–4 of the placenta accreta spectrum according to the intraoperative anatomical and topographic classification. In stage III of the study, the children were divided into two groups (group I and group II) based on the size (diameter) of the placental hernia according to the prenatal ultrasound examination (US) in 136 pregnant women with placenta accreta spectrum before delivery. Group I included preterm newborns born to mothers with a placental hernia diameter of 7 cm or less (n=48), and group II included a placental hernia with a diameter of more than 7 cm (n=88). Children whose mothers did not have a hernial protrusion according to ultrasound data, which was predominantly observed with placenta accreta, were not included in the analysis.
In the study groups, GA, weight-length indicators, sex, Apgar scores at 1 and 5 min after birth, and the correspondence of body weight and length to GA were compared. Small infants were defined as those with a birth weight below the 10th percentile of the INTERGROWTH-21 scale, and large infants were defined as those with a birth weight above the 90th percentile. Infants with a birth weight and length below the 10th percentile were defined as small for GA. The severity and duration of respiratory distress were assessed using the following surrogates: frequency and duration of respiratory therapy, including mechanical ventilation (MV) and high-frequency oscillatory ventilation (HFOV); maximal required mean airway pressure (MAP); frequency and duration of supplemental oxygen; and frequency of surfactant replacement therapy. The severity of acute cardiovascular events was assessed by the frequency of cardiotonic and vasopressor therapy, including the frequency of dopamine and dobutamine use and the maximum vasoactive inotropic index (VII).
VII was calculated using the formula: VII = dopamine dose (mcg/kg/min) + dobutamine dose (mcg/kg/min) + 100×adrenaline dose (mcg/kg/min) + 50×levosimendan dose (mcg/kg/min) [14, 15]. The analysis included the maximum VII value in newborn children during the observation period.
The integral indicators of the severity of the condition of preterm infants after birth were the length of stay in the neonatal intensive care unit (NICU) and total duration of hospitalization of the newborn in days.
Statistical analysis
Statistical analysis was performed using StatTech v.3.0.9 and IBM SPSS Statistics, version 26.0. Owing to the lack of similar studies in the literature, the sample size was not calculated. The distribution of continuous variables was tested for normality using the Shapiro–Wilk and Kolmogorov–Smirnov tests. For non-normal data distribution, non-parametric tests were used, and data are presented as median (Me) and interquartile range (IQR: 25%–75%). Categorical variables were presented as counts and percentages.
Comparisons of continuous variables between two groups were analyzed using the non-parametric Mann–Whitney test in three or more groups using the Kruskal–Wallis test, followed by pairwise comparison using the Mann–Whitney test with Bonferroni correction for multiple comparisons. Bonferroni correction for multiple comparisons was performed by multiplying each obtained significance level, p, by the number of comparisons. The number of comparisons was calculated using the formula m=n(n-1)/2, where n is the number of groups. When comparing categorical variables, the Pearson χ2 test (expected frequency >10), Pearson χ2 with Yates correction (expected frequency >5 but <10), Fisher's exact test (expected frequency <5) were used. Relative risks (RR) with 95% confidence intervals (CI) were calculated to assess the influence of the risk factors. Differences were considered statistically significant at p<0.05.
Results
The characteristics of the newborns included in this study are summarized in Table 1.
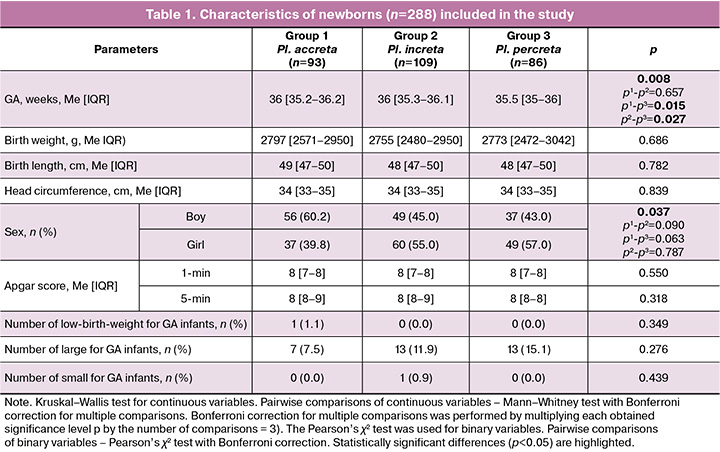
The GA of infants born to mothers with placenta percreta was significantly lower than that of infants in the accreta and increta groups. The study groups were comparable in anthropometric parameters and Apgar scores at 1 and 5 minutes. No significant differences were found in the number of low birth weight, high birth weight, and small-for-gestational-age infants. No significant differences were found in the pairwise comparisons of sexes between the study groups. The study included a comparative analysis of the frequency of surfactant replacement therapy, respiratory support, invasive respiratory therapy and HFOV, supplemental oxygen therapy, duration of respiratory support, duration of HFOV, and supplemental oxygen therapy. A comparison was also made of the maximum required parameters of respiratory therapy, in particular the supplemental oxygen concentration and MAP. The results are presented in Table 2.

Analysis of the data showed that there were no statistically significant differences in the frequency of use and duration of respiratory therapy, HFOV, surfactant therapy, or additional oxygen supplementation according to the degree of placental invasion. Respiratory therapy parameters, such as the maximum required MAP and oxygen concentration, were also not significantly different between the groups.
To compare the severity of cardiovascular disturbances in the studied preterm infants, the frequency and duration of cardiotonic and/or vasopressor therapy, frequency of administration of individual vasopressors and cardiotonic drugs (dopamine and dobutamine), maximum required doses of dopamine and dobutamine, and maximum VII dose were analyzed. The results are presented in Table 3.
A comparative analysis showed that there were no statistically significant differences in the frequency of use and duration of cardiotonic and vasopressor therapy in premature newborns born to mothers with placental ingrowth, depending on the degree of ingrowth. When comparing the frequency of use and the maximum doses of cardiotonic and vasopressor drugs, no significant difference was also found.
We compared the length of stay in NICU and the total length of hospital admission of premature newborns depending on the degree of placenta ingrowth. Comparison results are presented in Figures 2 and 3.
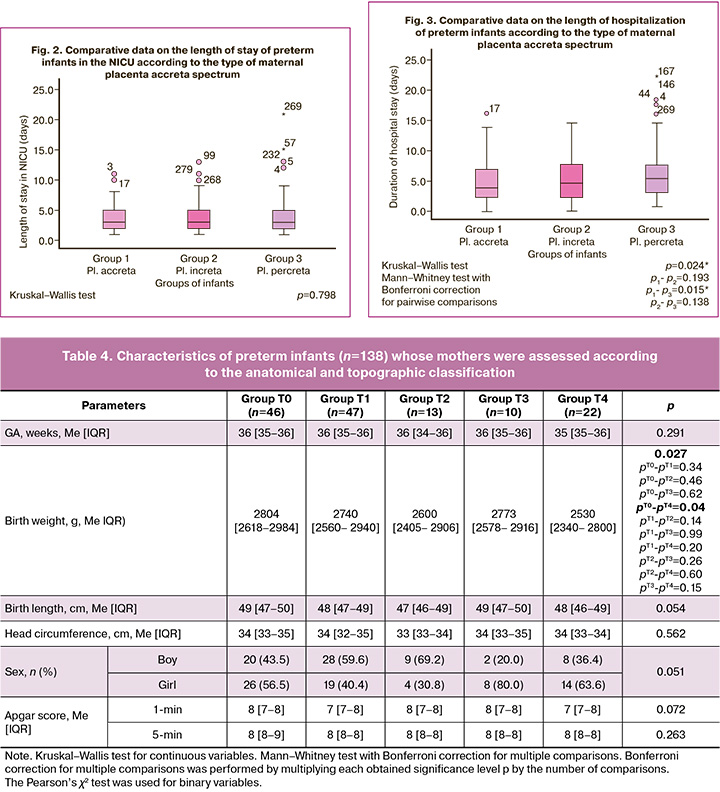
Based on the results of the comparative analysis, statistically significant differences were found in the length of hospitalization of preterm babies born to mothers with placenta accreta. Preterm infants born to mothers with placenta percreta had a longer hospital stay than preterm infants born to mothers with placenta accreta did. However, the length of hospital stay in the NICU did not differ significantly between the groups.
To assess the association between the severity of respiratory and cardiovascular disorders in preterm infants and different types of placenta accreta spectrum, a comparison of the studied parameters was made according to the topographic and anatomical types of placenta accreta spectrum. The comparability of groups by GA, anthropometric parameters, sex, and Apgar scores is presented in Table 4.
Statistically significant differences (p<0.05) are highlighted. The study groups were comparable in GA, but the children from the T4 placenta accreta group had significantly lower body weight than those from the T0 placenta accreta group. The Apgar score did not differ between the groups, similar to the sex distribution. To assess the dependence of the severity of respiratory and cardiovascular dysfunction in preterm infants on the anatomical and topographic type of placenta accreta spectrum in the mother, a comparative analysis was performed on the frequency of respiratory support, invasive respiratory therapy, HFOV, supplemental oxygen, surfactant replacement therapy, cardiotonic and vasopressor therapy, total duration of respiratory therapy, duration of HFOV, supplemental oxygen and cardiotonic therapy. A comparison was also made of the maximum required parameters of respiratory therapy, in particular, the concentration of additional oxygen and MAP. Table 5 presents the results of the comparative analysis.
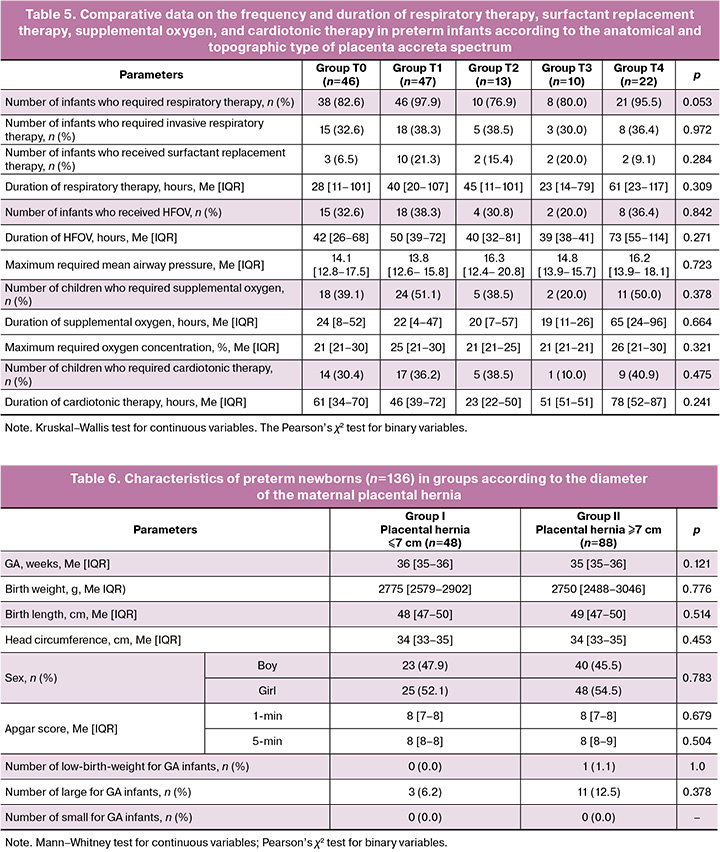
The comparative analysis showed that there were no statistically significant differences between the groups in the frequency and duration of respiratory support, supplemental oxygen, surfactant therapy, and cardiotonic therapy.
A total of 136 infants were included in the analysis of the dependence of the severity of respiratory and cardiovascular disorders in preterm infants on the diameter of the placental hernia in the mother. The comparability of the groups of children according to the diameter of the placental hernia is shown in Table 6.
The groups were comparable for GA, anthropometric indicators, Apgar score, and sex. There was no significant difference in the number of low birth weight, high birth weight and small for gestational age infants.
A comparison of the above indicators of respiratory and cardiotonic therapy was made according to the diameter of the placental hernia. The results are shown in Table 7.
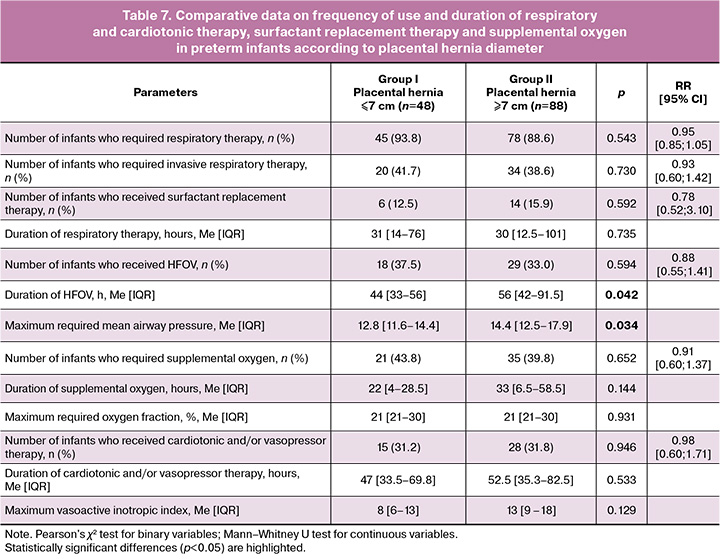
A comparative analysis of respiratory and cardiotonic therapy parameters showed a longer use of HFOV and a higher required MAP in preterm infants born to mothers with a placental hernia diameter >7 cm.
No association was found between the frequency and duration of cardiotonic or vasopressor therapy and placental hernia diameter in the study groups.
Discussion
Despite the increasing incidence of placenta accreta spectrum, its impact on newborn outcomes remains poorly understood. Published studies primarily assessed maternal and neonatal outcomes related to placenta accreta spectrum, focusing on the proportions of low birth weight and preterm infants, Apgar scores, and neonatal mortality rates. Several studies indicate an increase in low birth weight among newborns of mothers with placenta accreta spectrum [9, 10].
Only a limited number of researchers have analyzed the neonatal period in children born to mothers with placenta accreta spectrum.
Recent studies have suggested that placenta accreta spectrum is associated with a heightened incidence of respiratory diseases in newborns. For instance, Spillane N. et al. (2018) [11] demonstrated an increased occurrence of respiratory distress syndrome among newborns of mothers with placenta accreta spectrum. These infants also required respiratory therapy and continuous positive airway pressure for a longer duration.
A 2022 study indicated that infants born to mothers with placenta accreta spectrum more frequently require respiratory support for extended periods, with a high incidence of using high-flow cannulas and intubation [12].
Our previous study revealed that the severity of respiratory and cardiovascular disorders correlates with placenta accreta spectrum, demonstrating a more severe early neonatal course in infants born to mothers with this condition [13].
Few studies have assessed the neonatal outcomes based on the degree of placental invasion. Seet E.L. et al. (2012) [16] was the first to compare indicators such as prematurity, neonatal mortality, NICU transfers, Apgar scores, and the percentages of low birth weight and very low birth weight infants across different degrees of placental invasion. No statistically significant differences were found, which aligns with the findings of this study. The number of low-birthweight infants was similar across the three groups, and no differences in Apgar scores were noted based on the degree of placental invasion. However, in this study, preterm infants born to mothers with placenta percreta had a lower GA than those born to mothers with placenta accreta and placenta increta.
A recent study by Detlefs S.E. et al. (2023) also compared the incidence of low birth weight and large birth weight infants across subgroups depending on the degree of placental invasion and found no significant differences [17]. Additionally, a study by Palacios-Jaraquemada et al. [18], published in 2022, compared Apgar scores, birth weights, and MV use based on the type of abnormal placentation according to an intraoperative anatomical and topographic classification in mothers who underwent conservative reconstructive surgery for placenta accreta. This study found that invasion of the lower third of the lower uterine segment (type 3 according to the classification) was linked to an increased incidence of neonatal complications, including the need for resuscitation and MV. However, in their analysis, infants in this group had a lower GA of 300–336 weeks, which was associated with a higher incidence of complications. Our study included only preterm infants with GAs of 330–366 weeks, and we found no significant differences in MV use based on the anatomical and topographic type of abnormal placental invasion.
We have not identified any studies comparing the severity of newborn conditions based on the degree of abnormal placental invasion in the domestic and international literature. Furthermore, no studies in the global literature have analyzed the dependence of neonatal outcomes on the placental hernia diameter, underscoring the uniqueness of our study.
Our study did not reveal any differences in the severity of respiratory and cardiovascular disorders in preterm infants during the early neonatal period based on the degree of abnormal placental invasion.
We found statistically significant differences in the duration of hospitalization for newborns of mothers with placenta percreta compared to those of mothers with placenta accreta.
Additional analysis indicated that a longer hospitalization duration for these preterm infants was associated with lower GA in the placenta percreta group. The GA of infants born to mothers with placenta percreta was significantly lower than that of infants with placenta accreta and placenta increta.
These differences are likely due to the planned delivery time being shifted to 340 weeks in cases of more severe placenta accreta due to the increased risk of uterine bleeding [19]. No other factors influencing longer hospitalization were identified.
We established an increase in the duration of HFOV and the need for higher HFOV parameters, particularly MAP, in preterm infants born to mothers with a placental hernia diameter greater than 7 cm.
The identified differences suggest that the area of damage in placenta accreta has a more significant impact on the clinical condition of the child than the depth of placental invasion. This may be explained by the more pronounced placental disorders associated with a more extensive placentation area.
A limitation of this study was the small number of newborns with GAs of 34–35 weeks in the placenta accreta and placenta increta groups, which affected the differences in hospital stay duration compared to the placenta percreta group. Additionally, the sample size for analyzing the dependence of respiratory and cardiovascular disorders on the diameter of the placental hernia was reduced owing to incomplete data on the hernia size for all mothers included in the study.
Conclusion
The study findings demonstrated that the severity of respiratory and cardiovascular disorders in preterm infants born to mothers with placenta accreta spectrum was not influenced by the degree of abnormal placentation or the anatomical and topographic type of placental implantation. However, an increase in placental hernia diameter is associated with more severe manifestations of respiratory disorders in preterm infants.
References
- De Mucio B., Serruya S., Alemán A., Castellano G., Sosa C.G. A systematic review and meta-analysis of cesarean delivery and other uterine surgery as risk factors for placenta accreta. Int. J. Gynaecol. Obstet. 2019; 147(3): 281-91. https://dx.doi.org/10.1002/ijgo.12948.
- Higgins M.F., Monteith C., Foley M., O'Herlihy C. Real increasing incidence of hysterectomy for placenta accreta following previous caesarean section. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013; 171(1): 54-6. https://dx.doi.org/10.1016/j.ejogrb.2013.08.030.
- Mehrabadi A., Hutcheon J.A., Liu S., Bartholomew S., Kramer M.S., Liston R.M. et al.; Maternal Health Study Group of the Canadian Perinatal Surveillance System (Public Health Agency of Canada). Contribution of placenta accreta to the incidence of postpartum hemorrhage and severe postpartum hemorrhage. Obstet. Gynecol. 2015; 125(4): 814-21. https://dx.doi.org/10.1097/AOG.0000000000000722.
- Cahill A.G., Beigi R., Heine R.P., Silver R.M., Wax J.R.; Society of Gynecologic Oncology; American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine. Placenta accreta spectrum. Am. J. Obstet. Gynecol. 2018; 219(6): B2-B16. https://dx.doi.org/10.1016/j.ajog.2018.09.042.
- Balayla J., Bondarenko H.D. Placenta accreta and the risk of adverse maternal and neonatal outcomes. J. Perinat. Med. 2013; 41(2): 141-9. https://dx.doi.org/10.1515/jpm-2012-0219.
- Федеральная служба государственной статистики. Здравоохранение в России 2021. Статистический сборник. Росстат; 2021. 171 с. [Federal State Statistics Service. Healthcare in Russia 2021. Statistical compilation. Rosstat; 2021. 171 p. (in Russian)].
- Jauniaux E., Ayres-de-Campos D., Langhoff-Roos J., Fox K.A., Collins S.; FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int. J. Gynaecol. Obstet. 2019; 146(1): 20-4. https://dx.doi.org/10.1002/ijgo.12761.
- Palacios-Jaraquemada J.M., Fiorillo A., Hamer J., Martínez M., Bruno C. Placenta accreta spectrum: a hysterectomy can be prevented in almost 80% of cases using a resective-reconstructive technique. J. Matern. Fetal Neonatal Med. 2022; 35(2): 275-82. https://dx.doi.org/10.1080/14767058.2020.1716715.
- Moeini R., Dalili H., Kavyani Z., Shariat M., Charousaei H., Akhondzadeh A. et al. Maternal and neonatal outcomes of abnormal placentation: a case-control study. J. Matern. Fetal Neonatal Med. 2021; 34(19): 3097-103. https://dx.doi.org/10.1080/14767058.2019.1678128.
- Gielchinsky Y., Mankuta D., Rojansky N., Laufer N., Gielchinsky I., Ezra Y. Perinatal outcome of pregnancies complicated by placenta accreta. Obstet. Gynecol. 2004; 104(3): 527-30. https://dx.doi.org/10.1097/01.AOG.0000136084.92846.95.
- Spillane N.T., Zamudio S., Alvarez-Perez J., Andrews T., Nyirenda T., Alvarez M. et al. Increased incidence of respiratory distress syndrome in neonates of mothers with abnormally invasive placentation. PLoS One. 2018; 13(7): e0201266. https://dx.doi.org/10.1371/journal.pone.0201266.
- Munoz J.L, Kimura AM., Julia J., Tunnell C., Hernandez B., Curbelo J. et al. Impact of placenta accreta spectrum (PAS) pathology on neonatal respiratory outcomes in cesarean hysterectomies. J. Matern. Fetal Neonatal Med. 2022; 35(26): 10692-7. https://dx.doi.org/10.1080/14767058.2022.2157716.
- Балашова Е.Н., Ионов О.В., Киртбая А.Р., Никонец А.Д., Михеева А.А., Васильченко О.Н., Зубков В.В., Шмаков Р.Г., Дегтярев Д.Н. Особенности дыхательных и сердечно-сосудистых нарушений у недоношенных детей, рожденных у матерей с врастанием плаценты. Акушерство и гинекология. 2021; 5: 85-93. [Balashova E.N., Ionov O.V., Kirtbaya A.R., Nikonets A.D., Mikheeva A.A., Vasilchenko O.N., Zubkov V.V., Shmakov R.G., Degtyarev D.N. The features of respiratory and cardiovascular disorders in preterm infants born to mothers with abnormally invasive placenta. Obstetrics and Gynecology. 2021; (5): 85-93 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.5.85-93.
- Belletti A., Lerose C.C., Zangrillo A., Landoni G. Vasoactive-inotropic score: evolution, clinical utility, and pitfalls. J. Cardiothorac. Vasc. Anesth. 2021; 35(10): 3067-77. https://dx.doi.org/10.1053/j.jvca.2020.09.117.
- Favia I., Vitale V., Ricci Z. The vasoactive-inotropic score and levosimendan: time for LVIS? J. Cardiothorac. Vasc. Anesth. 2013; 27(2): e15-6. https://dx.doi.org/10.1053/j.jvca.2012.11.009.
- Seet E.L., Kay H.H., Wu S., Terplan M. Placenta accreta: depth of invasion and neonatal outcomes. J. Matern. Fetal Neonatal Med. 2012; 25(10): 2042-5. https://dx.doi.org/10.3109/14767058.2012.678429.
- Detlefs S.E., Carusi D.A., Modest A.M., Einerson B.D., Lyell D., Grace M.R. et al. The association between placenta accreta spectrum severity and incidence of small for gestational age neonates. Am. J. Perinatol. 2023; 40(1): 9-14. https://dx.doi.org/10.1055/s-0042-1757261.
- Palacios-Jaraquemada J.M., Basanta N., Fiorillo A., Labrousse C., Martínez M. Neonatal outcome after conservative-reconstructive surgery for placenta accreta spectrum disorders. J. Matern. Fetal Neonatal Med. 2022; 35(25): 4994-6. https://dx.doi.org/10.1080/14767058.2021.1873944.
- Munoz J.L., Pfeiffer A.F., Ramsey P.S. Correlation of clinical outcomes with the application of the 2020 consensus panel on histological classification for Placenta Accreta Spectrum (PAS). J. Matern. Fetal Neonatal Med. 2022; 35(25): 10044-8. https://dx.doi.org/10.1080/14767058.2022.2086797.
Received 06.11.2024
Accepted 26.02.2025
About the Authors
Anastasia D. Balakina, anesthesiologist-resuscitator of the NICU named after Prof. A.G. Antonov of the Institute of Neonatology and Pediatrics, V.I. Kulakov NMRCfor OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4, +7(495)438-22-77, nikon.na@yandex.ru, https://orcid.org/0000-0002-4717-1865
Ekaterina N. Balashova, Dr. Med. Sci., Leading Researcher at the NICU named after Prof. A.G. Antonov of the Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC
for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4; Associate Professor at Neonatology Department of the Faculty of Pediatrics,
I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), +7(495)438-22-77, e_balashova@oparina4.ru, https://orcid.org/0000-0002-3741-0770
Oleg V. Ionov, Dr. Med. Sci., Head of the NICU named after Prof. A.G. Antonov of the Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4; Professor at Neonatology Department of the Faculty of Pediatrics, I.M. Sechenov First MSMU,
Ministry of Health of Russia (Sechenov University); Chief Researcher, Research Clinical Institute of Childhood, Ministry of Health of the Moscow Region, +7(495)438-22-77, o_ionov@oparina4.ru, https://orcid.org/0000-0002-4153-133X
Аnna R. Kirtbaya, Dr. Med. Sci., Head of the Clinical Work, NICU named after Prof. A.G. Antonov of the Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4; Professor at Neonatology Department of the Faculty of Pediatrics, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), +7(495)438-22-77, a_kirtbaya@oparina4.ru, https://orcid.org/0000-0002-7628-8157
Alexandra A. Mikheeva, obstetrician-gynecologist, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4, shuratora@mail.ru
Oksana N. Vasilchenko, PhD, Senior Researcher at the Department of Innovative Technologies, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Oparin str., 4, +7(495)438-30-47, o_vasilchenko@oparina4.ru, https://orcid.org/0000-0001-9434-0011
Victor V. Zubkov, Dr. Med. Sci., Professor, Director of the Institute of Neonatology and Pediatrics, Head of Neonatology Department of the Department of Professional Education, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4; Professor at Neonatology Department of the Faculty of Pediatrics, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), +7(495)438-22-66, v_zubkov@oparina4.ru, https://orcid.org/0000-0001-8366-5208
Roman G. Shmakov, Dr. Med. Sci., Director of Academician V.I. Krasnopolsky Moscow Regional Research Institute of Obstetrics and Gynecology, 101000, Russia, Moscow, Pokrovka str., 22a; Professor at the Department of Obstetrics and Gynecology, Faculty of Pediatrics, Pirogov Russian National Research Medical University, Ministry of Health of Russia; Chief Freelance Obstetrics Specialist of the Ministry of Health of the Russian Federation, Mdshmakov@mail.ru, https://orcid.org/0000-0002-2206-1002
Dmitriy N. Degtyarev, Dr. Med. Sci., Professor, Deputy Director, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4; Professor at Neonatology Department, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), +7(495)438-23-88, d_degtiarev@oparina4.ru,
https://orcid.org/0000-0001-8975-2425



