Changes in placental growth factor levels in patients with different pregnancy complications
Khodzhaeva Z.S., Muminova K.T., Poluektova A.A., Avdeeva A.M., Tokareva A.O., Kukaev E.N., Baranov I.I., Starodubtseva N.L.
Objective: To study changes in placental growth factor (PlGF) levels at 11–14 weeks of gestation and before delivery (at 37–40 weeks) in relation to various pregnancy complications, including gestational diabetes mellitus, fetal macrosomia, premature birth, and abnormal placentation.
Materials and methods: The study included 3,274 pregnant women who underwent first-trimester screening at V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Serum PlGF levels were measured at various gestational ages. The Mann–Whitney U test and χ² Pearson test were used for the analysis, with statistical significance set at p<0.05.
Results: No statistical differences were found in PlGF levels at 11-14 weeks between the groups, in contrast to the PAPP-A levels. However, there were distinct patterns of PlGF level changes associated with various pregnancy complications. Gestational diabetes was characterized by a decline in PlGF levels as pregnancy progressed, with the most pronounced reduction observed in patients receiving insulin therapy (p<0.001). In contrast, elevated PlGF levels were detected in the third trimester in cases of fetal macrosomia (p=0,004). In cases of abnormal placentation (particularly placenta previa), a significant increase in PlGF level was detected prior to delivery (p=0,01). In cases of preterm birth, the changes in PlGF levels did not reach statistical significance.
Conclusion: The findings of the study highlight the potential usefulness of evaluating PAPP-A and PlGF levels across various stages of pregnancy to assist in risk stratification for complicated pregnancies. Lower PAPP-A levels during the first trimester are linked to an increased risk of gestational diabetes mellitus (GDM) and preterm birth, while PlGF levels in the third trimester area are associated with the severity of metabolic and placental complications.
Authors' contributions: Khodzhaeva Z.S., Baranov I.I. – conception and design of the study, analysis of study results, drafting of the manuscript; Muminova K.T., Poluektova A.A., Avdeeva A.M. – clinical examination of pregnant women, collection of material, systematization and analysis of the obtained data, drafting of the manuscript; Tokareva A.O., Kukaev E.N., Starodubtseva N.L. – conception and design of the study, organization and conduct of laboratory studies, systematization and analysis of the obtained data, drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was supported by a grant from the Russian Science Foundation (No. 24-14-00140): https://rscf.ru/project/24-14-00140/
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Khodzhaeva Z.S., Muminova K.T., Poluektova A.A., Avdeeva A.M., Tokareva A.O., Kukaev E.N., Baranov I.I., Starodubtseva N.L Changes in placental growth factor levels in patients
with different pregnancy complications.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (5): 86-96 (in Russian)
https://dx.doi.org/10.18565/aig.2025.95
Keywords
Placental growth factor (PlGF) is a protein in the vascular endothelial growth factor (VEGF) family, primarily expressed in the placenta but also found in other tissues. It plays a crucial role in angiogenesis, particularly during embryonic development [1]. In healthy pregnancies, PlGF concentrations progressively increase, peaking at 28–30 weeks, which correlates with the enhanced vasodilation and proangiogenic effects of VEGF and the maintenance of systemic vascular relaxation.
Recently, attention has been focused on the potential of PlGF to predict various pregnancy complications, especially gestational diabetes mellitus (GDM). Experimental studies on pregnant mice have indicated that insufficient PlGF expression in pancreatic β-cells results in decreased proliferation, mass, and glucose tolerance [2]. Analysis of placental samples from women with GDM confirms that hyperglycemia negatively affects the placenta, causing angiogenesis disorders and trophoblast dysfunction; however, its effect on PlGF expression remains unclear [3]. Evidence suggests an increase in PlGF levels in trophoblasts isolated from women with GDM [4]. Furthermore, hyperglycemia has been shown to induce pyroptosis in trophoblasts, whereas PlGF therapy appears to reduce pyroptosis levels, indicating its potential as a therapeutic target for correcting placental dysfunction in GDM [5]. Earlier studies have reported elevated PlGF levels in the blood serum during the first trimester among patients who later developed GDM. However, recent studies have failed to corroborate these findings. Several studies have found no differences in PlGF levels between women in the GDM and control groups [6, 7], while a retrospective cohort study revealed that lower PlGF concentrations in blood serum are associated with the development of GDM [8].
However, data on PlGF levels in the second and third trimesters of pregnancy and their clinical significance remain limited and inconsistent. Some studies have reported increased PlGF levels in the blood serum of pregnant women with GDM compared to controls [9], whereas others found no statistically significant intergroup differences [10, 11].
The association between PlGF and the birth of large-for-gestational-age infants (fetal weight >90th percentile) has not been sufficiently investigated, especially in women without metabolic disorders. Nevertheless, in patients with prediabetes, a significant association was found between elevated PlGF levels in the late third trimester and the risk of delivering a large fetus (weighing >4000 g) (OR, 1.72) [12].
Factors expressed by trophoblasts, including PlGF, play vital roles in neoangiogenesis and invasion. Various clinical studies have suggested that elevated PlGF levels identified during first-trimester screenings are associated with placenta accreta and could potentially serve as a biomarker for the early diagnosis of this condition [13, 14]. PlGF levels were significantly higher in the placenta accreta spectrum (PAS) group compared to the control group and the group with placenta previa [15–17]. Consequently, measuring these molecules in the blood enhances the preoperative diagnosis of placentation anomalies along with ultrasound examinations [18]. Recently, the role of PlGF in predicting preterm birth (PB) has garnered research interest, because it reflects placental dysfunction, a contributing factor to this complication. Studies have indicated that decreased PlGF levels during the second and third trimesters correlate with an increased risk of PB. A multicenter cohort study (n=9037) conducted in the United States from 2020 to 2024 found that low PlGF levels (<100 pg/ml) detected during the second screening were associated with PB in 40% of cases [19].
Despite the limitations and inconsistencies of existing studies regarding the prognostic and diagnostic roles of PlGF in identifying the aforementioned pregnancy complications, the available data suggest that this biomarker holds promise. However, larger studies are needed to confirm its clinical significance, establish reference values, and determine the optimal time intervals for implementing PlGF in diagnosing and predicting various pregnancy complications. Notably, a significant advantage of our study is the dynamic assessment of PlGF levels beginning from early pregnancy, specifically from to 11–14 weeks, which enables timely initiation of preventive measures.
This study aimed to evaluate the changes in PlGF levels at 11–14 weeks of gestation and prior to delivery (at 37–40 weeks) in patients with GDM, fetal macrosomia, premature birth, and abnormal placentation.
Materials and methods
This retrospective longitudinal case-control study included 3,274 patients and was conducted at V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia (hereinafter referred to as the Center) in accordance with the principles of the Helsinki Declaration of the World Medical Association. All the patients provided informed consent to participate in the study. The study focused on women who underwent first-trimester screening at the center and delivered at the same institution, ensuring standardization of diagnostic methods and data collection.
The inclusion criteria were as follows: gestation period of 11–14 weeks at the time of screening, availability of data on the concentration of PlGF and other biochemical markers (e.g., pregnancy-associated plasma protein A [PAPP-A]), and clinical information on the course of pregnancy and its outcomes. Women with multiple pregnancies, severe extragenital diseases (e.g., oncological or autoimmune pathologies), and incomplete or unreliable data that could distort the analysis results were excluded. Additionally, patients with congenital fetal anomalies or chromosomal abnormalities detected during screening were excluded because these conditions could independently affect PlGF levels. During antenatal hospitalization (within a week before delivery), the blood concentrations of angiogenic markers were re-determined in 242 (7.4 %) patients.
The high risk of PE was determined using a specialized calculator (https://www.fetalmedicine.org/research/assess/preeclampsia/first-trimester) based on the results of first-trimester screening. Pregnant women with a result of 1:100 or higher were included in this group. The following groups were formed: women with uncomplicated pregnancies who carried to term (control group) (n=2,098); pregnant women with GDM (n=771), further divided into subgroups based on diet or insulin therapy (n=477/n=109); patients whose pregnancies resulted in the birth of a large child (>4,000 g) (macrosomia) (n=237); groups of pregnant women with spontaneous PB (n=55) and with PB that began with rupture of membranes (n=65); and groups of pregnant women with placenta accreta (n=18) and placenta previa (n=30). The control group was subdivided into subgroups at low and high risk for PE and FGR based on the results of the first expanded combined screening.
Biochemical analyses of PlGF, PAPP-A, and the free β-subunit of human chorionic gonadotropin (β-hCG) levels in maternal serum during the first trimester screening (11–13+6 weeks of pregnancy) were performed using a Delfia Express analyzer (Vallak Oy, Finland). To determine serum levels of PE markers – PlGF and soluble fms-like tyrosine kinase 1 (sFlt-1) – in the third trimester, the Cobas e411 electrochemiluminescent immunoassay (Roche Diagnostics GmbH, Germany) was utilized.
GDM was diagnosed according to the clinical guidelines of the Russian Society of Obstetricians and Gynecologists. Placenta previa and accreta were diagnosed by ultrasound examination and later confirmed by magnetic resonance imaging.
Statistical analysis
Statistical analysis was performed using scripts in the freely available R 4.3.1 language within the RStudio 2024.12.1+563 environment. Comparison of numerical parameters among subgroups at high and low risk of PE/FGR; between the control group and the GDM group; between the control group and the group of pregnancies ending in the birth of a large baby; between the group of pregnancies ending in the birth of a large baby and the group complicated by GDM; between the subgroup of pregnancies with GDM and diet therapy and the subgroup with GDM and insulin therapy; between the control group and the group with spontaneous PB; between the control group and the group with PB due to premature rupture of membranes; and between the control group and the groups with placenta accreta and previa was conducted using the Mann–Whitney test. Data are presented as Me (Q1; Q3), where Me represents the median, and Q1 and Q3 are the 1st and 3rd quartiles, respectively. The Pearson χ2 criterion was used to compare groups based on binary variables, with data presented as n (%), where n is the absolute number of cases in the group, and % represents the proportion of cases. The power of the test (the probability of the absence of type-II error) was calculated. Statistical significance was established at p<0.05, with type II error absent at a power of >0.8.
Results
The outcomes of patients who were identified as having a high risk of PE according to the first-trimester screening results but who did not develop this complication were studied. It emerged that the high-risk group included women who were statistically significantly older (p<0.001) than the low-risk group (median age: 34.3 years vs. 32.5 years). However, it should be noted that the median age in this group was <35 years. As expected, the high-risk group had significantly lower PAPP-A and PlGF hormone levels (0.81 and 0.51 MoM, respectively) than the low-risk group (1.08 and 0.85 MoM, respectively). Notably, the high-risk group had a shorter gestational age at delivery (39 (38.2; 39.5) weeks vs. 39.3 (38.5; 40.1) weeks, p<0.001) and lower neonatal weight (3254 (3027; 3481.5) g vs. 3426 (3190; 3658) g, p<0.001). The high-risk group also had a higher rate of emergency caesarean section (CS) (42/224 (19%) versus the control group (199/1622 (12%)), p=0.01. To identify the sources of these differences, a comparative analysis of the placentas of the patients in the two groups was conducted. This revealed a more frequent decrease in placental weight and delayed chorionic villi maturation in patients at a high risk of developing pregnancy complications (Table 1).
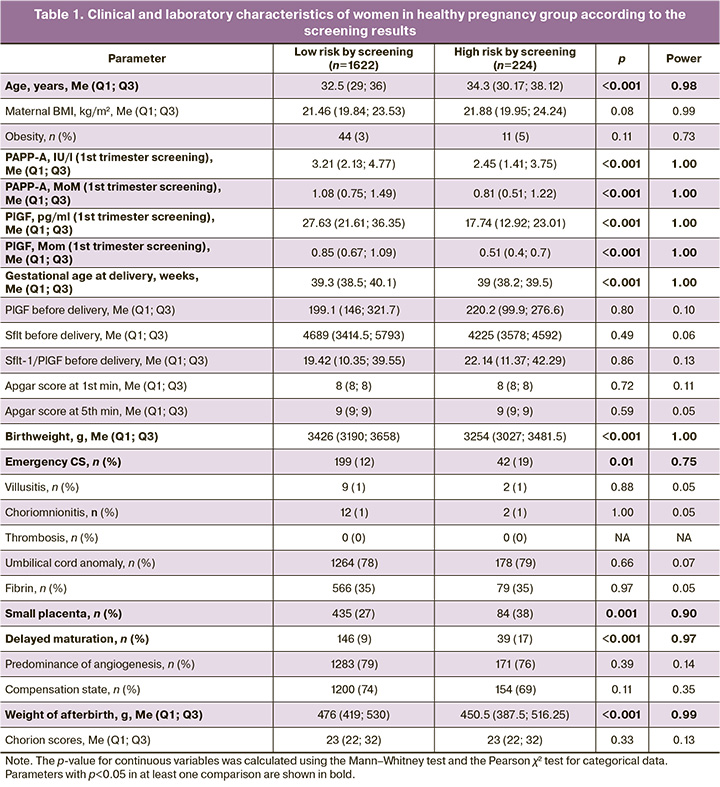
Next, considering the global data on the role of PlGF in identifying pregnant women at risk of developing GDM, we conducted a comparative analysis of pregnancy outcomes in patients with healthy pregnancy and GDM (Table 2). As expected, patients with GDM were significantly (p<0.001) older (34.8 (31.4; 38.3) years), had a higher body mass index (BMI) (23.12 (20.82; 26.75) kg/m2), and a higher incidence of obesity (101/771 (13%)) compared to the control group (32.7 (29.1; 36.2) years, 21.47 (19.82; 23.62) kg/m2, and 60/2098 (3%)). In the group of patients with GDM, a statistically significant (p=0.01) decrease in the PAPP-A level (0.99 (0.71; 1.37) MoM) was observed relative to the control group (1.04 (0.72; 1.46) MoM) during the first trimester screening, with a comparable PlGF concentration (0.82 (0.62; 1.03) MoM for the group with GDM and 0.81 (0.62; 1.04) MoM for the group with healthy pregnancy, p=0.74). However, during the analysis of angiogenic factors one week before delivery, a statistically significant decrease in the level of this molecule was noted (124.25 (87.09; 250.98) pg/ml versus 212.35 (148.55; 295.28) pg/ml, p=0.003) and, accordingly, an increase in the sFlt-1/PlGF ratio (43.39 (15.09; 80.99) versus 19.02 (10.38; 33.71), p=0.002) compared to the control group.
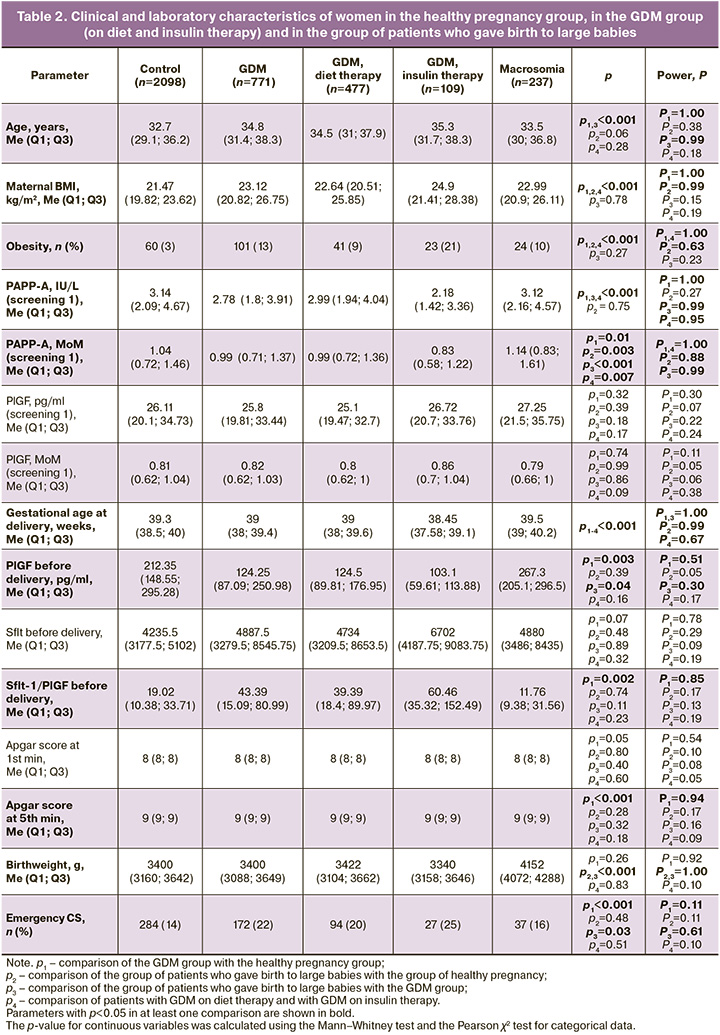
A more detailed analysis of GDM patients examined the differences between pregnant women receiving diet therapy and those receiving insulin therapy (Table 2). It was found that patients receiving insulin had a statistically significant (p<0.001) higher BMI (24.9 (21.41; 28.38) kg/m2) and, accordingly, a more frequent diagnosis of obesity (23/109 (21%)) compared to the group of patients on diet therapy (22.64 (20.51; 25.85) kg/m2, 41/477 (9%)). Moreover, at the first screening, patients who subsequently required insulin therapy showed a significantly (p<0.001) lower concentration of PAPP-A (0.83 (0.58; 1.22) MoM) than those who remained on diet therapy (0.99 (0.72; 1.36) MoM). Also, the concentration of PlGF before delivery in the subgroup with insulin therapy relative to the subgroup with diet therapy was significantly lower (103.1 (59.61; 113.88) pg/ml versus 124.5 (89.81; 176.95) pg/ml, p=0.003), and the sFlt-1/PlGF ratio, respectively, was higher (60.46 (35.32; 152.49) versus 39.39 (18.4; 89.97), p=0.002).
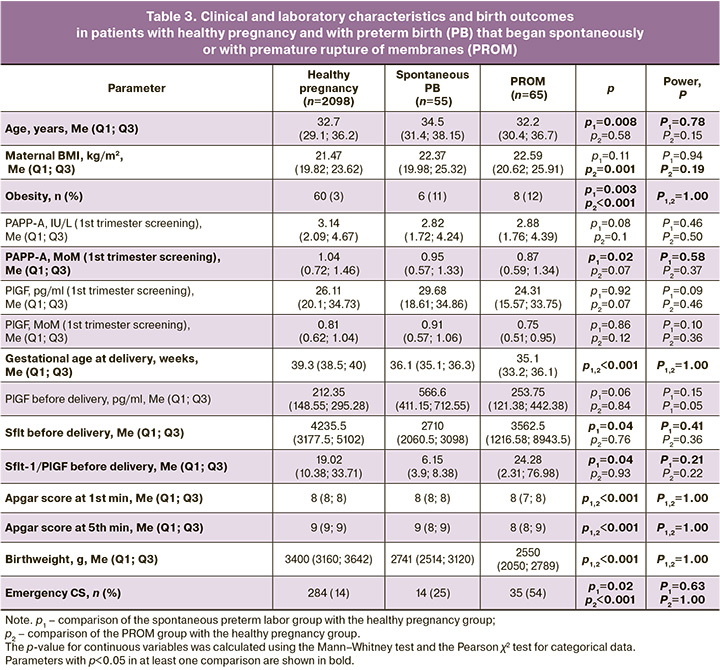
Since patients with GDM, as shown above, gave birth to normal-weight children, we decided to compare the outcomes in patients with GDM and in patients with normal glucose tolerance test results but who gave birth to large-weight newborns. In the group of large-birthweight babies, women were significantly more likely (p<0.001) to be obese (24/237 (10%)) than in the control group (60/2098 (3%)). During the first trimester screening, in women who gave birth to a large-birth-weight baby, the concentration of PAPP-A in the MoM was significantly higher than that in the group of patients who developed GDM (1.14 (0.83; 1.61) vs. 0.99 (0.71; 1.37), p<0.001), with no differences in the concentration of PIGF (0.79 (0.66; 1) vs. 0.81 (0.62; 1.04), p=0.86). However, significant differences in the PlGF level were recorded during the week before delivery: it was significantly higher (p=0.04) in the group with large babies (267.3 (205.1; 296.5) pg/ml) than in the GDM group (124.25 (87.09; 250.98) pg/ml). Delivery in the group of patients with large-weight fetuses (39.5 (39; 40.2) weeks) occurred significantly (p<0.001) later than in the group with healthy pregnancy (39.3 (38.5; 40) weeks) and in the group with GDM (39 (38; 39.4) weeks). The frequency of emergency CS in the group with a large fetus and in the healthy pregnancy group was comparable (284/2098 (14%) and 37/237 (16%), p=0.51) and significantly lower than that in the group of patients with GDM (172/771 (22%)) (Table 2). When studying the features of clinical and laboratory parameters in PB, pregnant women were distributed according to PB phenotypes: PB that began with the development of spontaneous regular labor and PB that began with premature rupture of membranes (PROM). It was found that patients with spontaneous PB were statistically significantly (p=0.03) older than patients in the control group: (34.5 (31.4; 38.15) years versus 32.7 (29.1; 36.2) years), and BMI was statistically significantly higher (p=0.001) in patients with PROM (22.59 (20.62; 25.91) kg/m2) compared to the controls (21.47 (19.82; 23.62) kg/m2); at the same time, the incidence of obesity was statistically significantly higher (p=0.003 and 6/55 (11%) for spontaneous PB; p<0.001 and 8/65 (12%) for PB with PROM) in both subgroups of women with PB compared to healthy pregnancy (60/2098 (3%)) (Table 3). According to the results of the first trimester screening, a significant (p=0.02) decrease in the PAPP-A level, expressed in MoM, was noted in the group of patients with spontaneous PB – 0.95 (0.57; 1.33) relative to the control group (1.04 (0.72; 1.46)). Before delivery, a statistically significant (p=0.04) decrease in the level of the anti-angiogenic factor sFlt-1 was noted in patients with spontaneous PB (2710 (2060.5; 3098) pg/ml) compared to the control group (4235.5 (3177.5; 5102) pg/ml]). The rate of emergency CS was also significantly higher in each PB subgroup: 14/55 (25%) (p=0.02) for the spontaneous PB group and 35/65 (54%) for the PB with PROM group than in the control group (284/2098 (14%) (p<0.001).
Considering that PlGF level reflects the adequacy and depth of placentation, we analyzed the clinical and laboratory characteristics of patients with placentation anomalies, namely placenta accreta and placenta previa (Table 4). In patients with placenta accreta, a statistically significantly (p=0.003 and p<0.001) higher BMI (25 (21.03; 30.2) kg/m2) and a higher incidence of obesity (5/18 (28%)) were recorded than in the control group (21.47 (19.82; 23.62) kg/m2 and 60/2098 (3%)). The PAPP-A level was also significantly elevated (p=0.01) relative to that in the control group: 1.61 (1.16; 1.95) MoM versus 1.04 (0.72; 1.46) MoM. The presence of placenta accreta naturally led to a significantly higher frequency of emergency CS (6/18 (33%)) than that in the control group (284/2098 (14%)) (p=0.04).
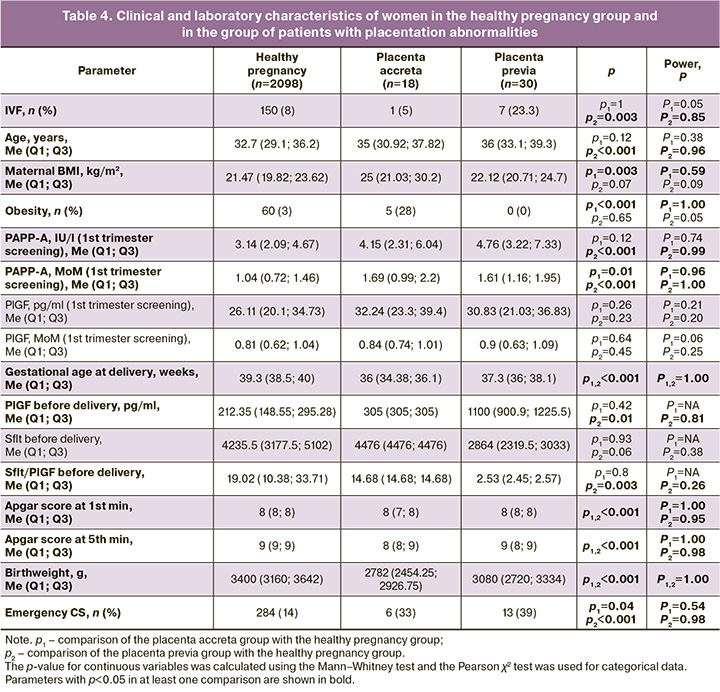
In the group of pregnant women with placenta previa, there was a significantly higher frequency of in vitro fertilization (IVF) (7/30 (23%), p=0.003) and older age (36 (33.1; 39.3) years, p<0.001) than in the control group (150/2098 (8%) and 32.7 (29.1; 36.2) years). They, as well as patients with placenta accreta, showed a statistically significant (p<0.001) increase in PAPP-A levels during the first trimester screening (1.61 (1.16; 1.95) MoM) compared to the control group (1.04 (0.72; 1.46) MoM). Moreover, before delivery, the group with placenta previa had a significantly higher level of PlGF than the control group: 1100 (900.9; 1225.5) pg/ml versus 212.35 (148.55; 295.28) pg/ml (p=0.01). The presence of placenta previa led to a statistically significant (p<0.001) higher frequency of emergency CS (13/30 (39%)) than in the control group (284/2098 (14%)).
Discussion
The results underscore the importance of first-trimester screening and PAPP-A hormone levels in identifying pregnant women at risk for adverse outcomes. Even in healthy pregnancies, patients identified as having a high risk of developing PE based on screening data were statistically more likely to require emergency CS, highlighting the role of the placenta in labor outcomes. Additionally, newborn weights were significantly lower in the high-risk group, further emphasizing the impact of placental factors on the development of a healthy placenta that is capable of meeting the energy and nutritional needs of the growing fetus. This finding was corroborated by the delayed maturation of placental villi observed in the high-risk cohort.
It is important to note that our study did not identify trends in PlGF concentrations as reported by other authors. Among the studied group of pregnant women, comparable PlGF levels were noted; however, a decrease in PAPP-A levels was observed during the first trimester screening in patients who subsequently developed GDM. The most significant decline was found in patients requiring insulin therapy, likely reflecting early disturbances in the bioavailability of insulin-like growth factors (IGF-1 and IGF-2) [20]. However, shortly before delivery, PlGF levels significantly decreased in this group, particularly among those receiving insulin therapy, which is associated with poorer pregnancy outcomes according to global data [21–23]. In contrast, patients who delivered larger newborns exhibited opposite changes in the levels of the studied molecules; they had the highest MoM PAPP-A values during the first trimester screening [24] and the highest PlGF values before delivery. Elevated PAPP-A concentrations may indicate the activation of the insulin-like growth system, promoting increased cellular proliferation in the placenta, which may be linked to accelerated fetal growth. Similarly, elevated PlGF levels in late gestation may suggest adequate placental vascularization, facilitating intensive fetoplacental exchange that contributes to macrosomia.
Our study also indicated no differences in PlGF levels, both in early pregnancy and in the third trimester, between patients with uncomplicated gestations and those experiencing preterm births (PB), which contradicts the existing literature [1, 19, 25]. However, a decrease in PAPP-A at 11–14 weeks of pregnancy is associated with PB [25], a finding supported by our results.
Regarding placentation abnormalities, such as placenta accreta and placenta previa, no differences in PlGF concentrations were observed. However, an increase in PAPP-A levels was noted during the first trimester. Interestingly, early pregnancy PAPP-A increases have been linked to placenta accreta [26], whereas no such association has been observed with placenta previa [27]. The differing PAPP-A levels in these conditions likely arise from variations in pathogenesis: placenta accreta involves abnormal deep trophoblastic invasion into the myometrium with increased PAPP-A production, whereas placenta previa disrupts localization without altering invasion depth, resulting in no change in this marker. Thus, our study did not establish the prognostic significance of PlGF in the first-trimester screening for identifying pregnant women who later develop various complications. However, in the third trimester, statistically significant differences in PlGF levels were observed in patients with GDM (lowest values in those receiving insulin therapy), large fetuses, and placenta previa (highest values), indicating its diagnostic relevance in these complications. This finding aligns with the typical practice of prescribing insulin in the third trimester for GDM, as this protein hormone suppresses VEGF, of which PlGF is a member [28]. Conversely, increased PlGF secretion in macrosomia suggests excessive cytotrophoblastic invasion, which ensures optimal nutrient exchange with the fetus.
The PAPP-A levels in early pregnancy differed significantly between the groups. They were reduced in patients with GDM and PB, whereas they were elevated in those with abnormal placentation and in those who delivered large-weight newborns. PAPP-A is primarily produced by the placenta and plays a vital role in the regulation of growth factors and cytokines that are essential for normal fetal development. Previous studies have demonstrated the role of PAPP-A, measured at 11–14 weeks of pregnancy, in identifying patients at risk of GDM [29, 30]. However, because reduced PAPP-A values remain within the normal range, this biomarker cannot serve as a reliable independent predictor of GDM; instead, its concentration should be evaluated along with maternal characteristics such as age, BMI, and reproductive history. A 2022 study established a threshold value for PAPP-A to identify patients at high risk for developing pregnancy complications such as hypertensive disorders, PB, and FGR [31]. The authors noted that a PAPP-A value of ≤0.40 MoM indicates that 23% of patients may develop one or more of these complications.
In light of these findings, it is essential to consider PAPP-A levels during first-trimester screening to identify women at risk for specific pregnancy complications in a timely manner, thereby optimizing monitoring and initiating preventive measures. Furthermore, assessing PlGF levels in the third trimester presents a promising approach for diagnosing pregnancy complications, potentially enhancing therapeutic strategies, and determining the optimal timing and method of delivery for patients with GDM, large fetuses, or abnormal placentation, to mitigate adverse pregnancy and childbirth outcomes.
Conclusion
These findings suggest the potential clinical significance of dynamically assessing PAPP-A and PlGF levels at various gestational stages for risk stratification of complicated pregnancies. A decrease in PAPP-A hormone concentration during the first trimester was associated with a heightened likelihood of developing GDM and PB, whereas increases were linked to macrosomia and pathological placental invasion. Conversely, PlGF levels did not show significant intergroup differences in early pregnancy, but varied in the third trimester based on the nature of the condition, indicating its diagnostic potential in metabolic and placental disorders. These data provide a foundation for further exploration and discussion regarding the inclusion of these biomarkers in dynamic monitoring algorithms for pregnancy complications with increased risk of adverse outcomes.
References
- Hayes Ryan D., McCarthy F.P., O’Donoghue K., Kenny L.C. Placental growth factor: A review of literature and future applications. Pregnancy Hypertens. 2018; 14: 260-4. https://dx.doi.org/10.1016/j.preghy.2018.03.003
- Yang W., Jiang Y., Wang Y., Zhang T., Liu Q., Wang C. et al. Placental growth factor in beta cells plays an essential role in gestational beta-cell growth. BMJ Open Diabetes Res. Care. 2020; 8(1): e000921. https://dx.doi.org/10.1136/bmjdrc-2019-000921
- Tao J., Xia L.Z., Chen J.J., Zeng J.F., Meng J., Wu S.Y. et al. High glucose condition inhibits trophoblast proliferation, migration and invasion by downregulating placental growth factor expression. J. Obstet. Gynaecol. Res. 2020; 46(9): 1690-701. https://dx.doi.org/10.1111/jog.14341
- Loegl J., Nussbaumer E., Cvitic S., Huppertz B., Desoye G., Hiden U. GDM alters paracrine regulation of feto-placental angiogenesis via the trophoblast. Lab. Investig. 2017; 97(4): 409-18. https://dx.doi.org/10.1038/labinvest.2016.149
- Tao J., Rao Y., Wang J., Tan S., Zhao J., Cao Z. et al. Placental growth factor alleviates hyperglycemia-induced trophoblast pyroptosis by regulating mitophagy. J. Obstet. Gynaecol. Res. 2024; 50(10): 1813-29. https://dx.doi.org/10.1111/jog.16050
- Tenenbaum-Gavish K., Sharabi-Nov A., Binyamin D., Møller H.J., Danon D., Rothman L. et al. First trimester biomarkers for prediction of gestational diabetes mellitus. Placenta. 2020; 101: 80-9. https://dx.doi.org/10.1016/j.placenta.2020.08.020
- Yanachkova V., Staynova R., Naseva E., Kamenov Z. The role of placental growth factor in the prediction of carbohydrate and thyroid disorders during pregnancy. Medicina (Kaunas). 2022; 58(2): 232. https://dx.doi.org/10.3390/medicina58020232
- Lu Y.T., Chen C.P., Sun F.J., Chen Y.Y., Wang L.K., Chen C.Y. Associations between first-trimester screening biomarkers and maternal characteristics with gestational diabetes mellitus in Chinese women. Front. Endocrinol. (Lausanne). 2024; 15: 1383706. https://dx.doi.org/10.3389/fendo.2024.1383706
- Gorkem U., Togrul C., Arslan E. Relationship between elevated serum level of placental growth factor and status of gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2020; 33(24): 4159-63. https://dx.doi.org/10.1080/14767058.2019.1598361
- Alqudah A., Eastwood K.A., Jerotic D., Todd N., Hoch D., McNally R. et al. FKBPL and SIRT-1 are downregulated by diabetes in pregnancy impacting on angiogenesis and endothelial function. Front. Endocrinol. (Lausanne). 2021; 12: 650328. https://dx.doi.org/10.3389/fendo.2021.650328
- Chatzakis C., Papavasiliou D., Mansukhani T., Nicolaides K.H., Charakida M. Maternal vascular-placental axis in the third trimester in women with gestational diabetes mellitus, hypertensive disorders, and unaffected pregnancies. Am. J. Obstet. Gynecol. 2025; 232(5): 489.e1-489.e11. https://dx.doi.org/10.1016/j.ajog.2024.08.045
- James-Todd T., Cohen A., Wenger J., Brown F. Time-specific placental growth factor (PlGF) across pregnancy and infant birth weight in women with preexisting diabetes. Hypertens. Pregnancy. 2016; 35(3): 436-46. https://dx.doi.org/10.3109/10641955.2016.1172085
- Wang F., Zhang L., Zhang F., Wang J., Wang Y., Man D. First trimester serum PIGF is associated with placenta accreta. Placenta. 2020; 101: 39-44. https://dx.doi.org/10.1016/j.placenta.2020.08.023
- Zhang T., Wang S. Potential serum biomarkers in prenatal diagnosis of placenta accreta spectrum. Front. Med. (Lausanne). 2022; 9: 860186. https://dx.doi.org/10.3389/fmed.2022.860186
- Zhang F., Gu M., Chen P., Wan S., Zhou Q., Lu Y. et al. Distinguishing placenta accreta from placenta previa via maternal plasma levels of sFlt-1 and PLGF and the sFlt-1/PLGF ratio. Placenta. 2022; 124: 48-54. https://dx.doi.org/10.1016/j.placenta.2022.05.009
- Jauniaux E., Ayres-de-Campos D., Langhoff-Roos J., Fox K.A., Collins S.; FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int. J. Gynaecol. Obstet. 2019; 146(1): 20-4. https://dx.doi.org/10.1002/ijgo.12761
- Arakaza A., Liu X., Zhu J., Zou L. Assessment of serum levels and placental bed tissue expression of IGF-1, bFGF, and PLGF in patients with placenta previa complicated with placenta accreta spectrum disorders. J. Matern. Fetal Neonatal Med. 2024; 37(1): 2305264. https://dx.doi.org/10.1080/14767058.2024.2305264
- Gao W., Yang L., Shi B. Mapping themes trends and knowledge structure of trophoblastic invasion, a bibliometric analysis from 2012-2021. J. Reprod. Immunol. 2021; 146: 103347. https://dx.doi.org/10.1016/j.jri.2021.103347
- Gladstone R.A., Ahmed S., Huszti E., McLaughlin K., Snelgrove J.W., Taher J. et al. Midpregnancy placental growth factor screening and early preterm birth. JAMA Netw. Open. 2024; 7(11): e2444454. https://dx.doi.org/10.1001/jamanetworkopen.2024.44454
- Conover C.A., Bale L.K., Overgaard M.T., Johnstone E.W., Laursen U.H., Füchtbauer E.M. et al. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004; 131(5): 1187-94. https://dx.doi.org/10.1242/dev.00997
- McLaughlin K., Snelgrove J.W., Audette M.C., Syed A., Hobson S.R., Windrim R.C. et al. PlGF (Placental Growth Factor) testing in clinical practice: evidence from a Canadian Tertiary Maternity Referral Center. Hypertension. 2021; 77(6): 2057-65. https://dx.doi.org/10.1161/HYPERTENSIONAHA.121.17047
- Sherrell H., Dunn L., Clifton V., Kumar S. Systematic review of maternal Placental Growth Factor levels in late pregnancy as a predictor of adverse intrapartum and perinatal outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018; 225: 26-34. https://dx.doi.org/10.1016/j.ejogrb.2018.03.059
- Bowe S., Mitlid-Mork B., Staff A.C., Sugulle M. PlGF and sFlt-1, reduced fetal movements and adverse delivery outcome of a likely placental cause: A real world prospective observational study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025; 307: 34-42. https://dx.doi.org/10.1016/j.ejogrb.2025.01.029
- Monari F., Menichini D., Spano’ Bascio L., Grandi G., Banchelli F., Neri I. et al. A first trimester prediction model for large for gestational age infants: a preliminary study. BMC Pregnancy Childbirth. 2021; 21(1): 654. https://dx.doi.org/10.1186/s12884-021-04127-3
- Chiu C.P.H., Feng Q., Chaemsaithong P., Sahota D.S., Lau Y.Y., Yeung Y.K. et al. Prediction of spontaneous preterm birth and preterm prelabor rupture of membranes using maternal factors, obstetric history and biomarkers of placental function at 11-13 weeks. Ultrasound Obstet. Gynecol. 2022; 60(2): 192-9. https://dx.doi.org/10.1002/uog.24917
- Li Y., Meng Y., Chi Y., Li P., He J. Meta-analysis for the relationship between circulating pregnancy-associated plasma protein A and placenta accreta spectrum. Medicine (Baltimore). 2023; 102(47): e34473. https://dx.doi.org/10.1097/MD.0000000000034473
- Mortaki A., Douligeris A., Panagiotopoulos M., Daskalaki M.A., Pergialiotis V., Antsaklis P. et al. First- and second-trimester aneuploidy screening biomarkers and risk assessment of placenta previa and accreta: a systematic review and meta-analysis. J. Obstet. Gynaecol. Can. 2024; 46(11): 102663. https://dx.doi.org/10.1016/j.jogc.2024.102663
- Kapustin R.V., Kopteeva E.V., Alekseenkova E.N., Tral T.G., Tolibova G.K., Arzhanova O.N. Placental expression of endoglin, placental growth factor, leptin, and hypoxia-inducible factor-1 in diabetic pregnancy and pre-eclampsia. Gynecol. Endocrinol. 2021; 37(sup1): 35-9. https://dx.doi.org/10.1080/09513590.2021.2006513
- Cui J., Li P., Chen X., Li L., Ouyang L., Meng Z. et al. Study on the relationship and predictive value of first-trimester pregnancy-associated plasma protein-A, maternal factors, and biochemical parameters in gestational diabetes mellitus: a large case-control study in Southern China mothers. Diabetes Metab. Syndr. Obes. 2023; 16: 947-57. https://dx.doi.org/10.2147/DMSO.S398530
- Ramezani S., Doulabi M.A., Saqhafi H., Alipoor M. Prediction of gestational diabetes by measuring the levels of pregnancy associated plasma protein-A (PAPP-A) during gestation weeks 11-14. J. Reprod. Infertil. 2020; 21(2): 130-7.
- Kantomaa T., Vääräsmäki M., Gissler M., Sairanen M., Nevalainen J. First trimester low maternal serum pregnancy associated plasma protein-A (PAPP-A) as a screening method for adverse pregnancy outcomes. J. Perinat. Med. 2023; 51(4): 500-9. https://dx.doi.org/10.1515/jpm-2022-0241
Received 07.04.2025
Accepted 05.05.2025
About the Authors
Zulfia S. Khodzhaeva, Dr. Med. Sci., Professor, Deputy Director for Research of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Centerfor Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4, +7(916)407-75-67, zkhodjaeva@mail.ru,
https://orcid.org/0000-0001-8159-3714
Kamilla T. Muminova, PhD, Junior Researcher at the 1st Department of Pregnancy Pathology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4, +7(916)373-77-07, kamika91@mail.ru,
https://orcid.org/0000-0003-2708-4366
Alina A. Poluektova, PhD student, Specialist, Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4, +7(977)636-30-90, a_poluektova@oparina4.ru, https://orcid.org/0009-0003-7892-7017
Anna M. Avdeeva, Student, Faculty of Fundamental Medicine, Moscow Scientific and Educational Institute, Lomonosov Moscow State University, 119991, Russia, Moscow, Leninskie Gory, 1, +7(916)900-06-50, a_avdeeva@oparina4.ru, https://orcid.org/0009-0000-2225-2469
Alisa O. Tokareva, Specialist, Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4, +7(965)128-68-86, a_tokareva@oparina4.ru, https://orcid.org/0000-0001-5918-9045
Evgeny N. Kukaev, PhD in Physics and Mathematics, Senior Researcher at the Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4; Researcher, V.L. Talrose Institute for Energy Problems
of Chemical Physics, +7(916)883-17-85, e_kukaev@oparina4.ru, https://orcid.org/0000-0002-8397-3574
Igor Ivanovich Baranov, Dr. Med. Sci., Professor, Head of the Department of Scientific and Educational Programs, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4; Vice-President of the Russian Society
of Obstetricians and Gynecologists, i_baranov@oparina4.ru, https://orcid.org/0000-0002-9813-2823
Natalia L. Starodubtseva, PhD (Bio), Head of the Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4, +7(916)463-98-67, n_starodubtseva@oparina4.ru,
https://orcid.org/0000-0001-6650-5915
Corresponding author: Zulfia S. Khodzhaeva, zkhodjaeva@mail.ru



