Clinical risk factors for fetal macrosomia
Frankevich N.A., Tokareva A.O., Karapetyan T.E., Kutsenko A.A., Vasilyeva A.G., Chagovets V.V., Frankevich V.E.
Relevance: The prevalence of fetal macrosomia is steadily increasing worldwide and reaches up to 20%. Fetal macrosomia complicates the course of pregnancy and birth, leading to the increase in the number of emergency caesarean sections and perinatal losses by 1.5–3 times. Current prediction strategies are inaccurate, and most patients with fetal macrosomia are sent to labor with the “unknown status”. Current prognostic strategies are inaccurate, and the majority of patients with fetal macrosomia go into labor with the "unknown status".
Objective: To assess the clinical and laboratory risk factors for fetal macrosomia with subsequent development of prognostic mathematical models.
Materials and methods: The case-control study included 110 female patients. Group I (the main group) consisted of 30 patients with gestational diabetes mellitus (GDM). Group II (the control group) consisted of 80 women without GDM. The patients were stratified into four subgroups: Ia and 1b, IIa and IIb) depending on the presence of absence of fetal macrosomia and GDM. The clinical and laboratory risk factors were determined using univariate and multivariate logistic regression.
Results: Risk factors for the development of macrosomia included parity, body mass index before and during pregnancy, macrosomia in history, body weight of the pregnant woman and her partner (baby’s father) at birth, triglyceride and glucose levels at 24–28 weeks of pregnancy, estimated fetal weight during the 3rd ultrasound screening, and baby’s gender. Based on the obtained clinical and laboratory data, mathematical prediction models of macrosomia were constructed. The sensitivity was 100–78%, and specificity was 85–50%, the AUC was 0.76–0.77.
Conclusion: The developed mathematical models can be used to predict the development of fetal macrosomia at or after 24 weeks of pregnancy, both independently of the presence of GDM (also in the group with unknown GDM status) and can be used separately in the group of women with carbohydrate metabolism disorders.
Authors' contributions: Frankevich N.A – the concept and design of the study, clinical data analysis, systematic analysis, manuscript writing; Tokareva A.O. – statistical analysis of the obtained data, manuscript editing; Karapetyan T.E. – clinical data analysis, clinical data management in clinical data analysis, clinical and laboratory data collection and processing; Kutsenko A.A. – clinical and laboratory data collection and processing for subsequent information analysis; Vasilyeva A.G. – clinical and laboratory data collection and processing for subsequent information analysis; Chagovets V.V. – analysis of the obtained data, manuscript editing; Frankevich V.E. – systematic analysis, manuscript editing.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was funded by research funding grant No. 24-64-00006 of the Russian Science Foundation,
https://rscf.ru/project/24-64-00006/
Ethical Approval: The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Protocol No. 4 of April 18, 2024).
Patient Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Frankevich N.A., Tokareva A.O., Karapetyan T.E., Kutsenko A.A., Vasilyeva A.G.,
Chagovets V.V., Frankevich V.E. Clinical risk factors for fetal macrosomia.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2025; 9: 70-81 (in Russian)
https://dx.doi.org/10.18565/aig.2025.150
Keywords
The prevalence of metabolic disorders among pregnant women is steadily increasing worldwide and varies greatly depending on age and ethnicity. Every year in the world about 39 million pregnancies occur against the background of maternal obesity, and in some countries the prevalence exceeds 60% [1]. Excessive maternal weight and obesity are independent factors for the development of gestational diabetes mellitus (GDM). The global standardized prevalence of GDM is 14.0% (95% CI: 13.97–14.04%). It varies in different regions. The regional standardized prevalence of GDM is 7.1% (7.0–7.2%) in North America and Caribbean, 7.8% (7.2–8.4%) in Europe, 10.4% (10.1–10.7%) in South America and Central America, 14.2% (14.0–14.4%) in Africa, 14.7% (14.7–14.8%) in Western Pacific, 20.8% (20.2–21.4%) in South-East Asia, and 27.6% (26.9–28.4%) in Middle East and North Africa [2, 3]. Fetal macrosomia is diagnosed among 15–45% of pregnant women with GDM, that is three times more common than in infants born to mothers with plasma glucose levels. However, a tendency toward excessive fetal growth can also occur in women without carbohydrate metabolism disorders. Known non-modifiable risk factors include ethnicity, late reproductive age (>35 years), multigravidity, multiparity (parity >3), giving birth to a large baby in history or having type 2 diabetes in family history, short interpregnancy interval, and giving birth to a baby boy (p<0.001) [4–7].
Perinatal losses in fetal macrosomia are 1.5–3 times higher than in infants with normal birth weight [6–9]. In pregnancies complicated by diabetes, the fetuses with macrosomia have increased risk (in 5–9% of cases) of shoulder dystocia, Erb's palsy, brachial plexus injuries and tubular bone fractures, and neonatal asphyxia [8–11]. At the same time the parturient women can experience labor and delivery complications (prolonged labor, operative vaginal delivery) or have cesarean delivery. Postpartum complications can be associated with injuries to birth canal, uterine atony, and a high risk of postpartum hemorrhage (3–5 times higher risk) [8]. Neonatal morbidity rate is higher in large for gestational age newborns (birth weight >4000 g). Fetal macrosomia is associated with electrolyte and metabolic disorders in the early neonatal period. For example, the incidence of severe hypoglycemia and neonatal hyperbilirubinemia is 5 and 2 times higher, respectively, compared with babies born to healthy mothers [11]. This cohort of newborns is at high risk for developing Type 2 diabetes, obesity and cardiovascular diseases in adolescence and adulthood [12, 13].
The development of methods for early prediction of fetal macrosomia to reduce the development of this complication both among pregnant women with metabolic disorders (GDM and obesity) and women without these risk factors during pregnancy remains relevant.
The aim of the study was to assess the clinical and laboratory risk factors for fetal macrosomia with subsequent development of prognostic mathematical models.
Material and methods
The case-control study was conducted at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (the Center) from January to September, 2024.
After childbirth at the Center, 110 patients were selected in the study according to inclusion/non-inclusion criteria. Depending on the presence of absence of GDM, 2 groups were formed. Group I (the main group) included 30 patients with GDM. Group II (the comparison group) included 80 women without GDM. The patients were stratified into subgroups Ia and Ib; IIa and IIb) depending on the presence of absence of fetal macrosomia. The subgroup Iа included the patients with GDM who gave birth to babies with birth weight ≥ 4000 g (n=7). The subgroup Ib included women with GDM who gave birth to babies with birth weight from 2501 g to 3999 g (n=23). The subgroup IIа included women without GDM, who gave birth to newborns with birth weight ≥ 4000 g (n=24). The subgroup IIb (the control group) included women without GDM, who gave birth to newborns with birth weight from 2501 g to 3999 g (n=56). All patients signed informed consent to participate in the study. The study was approved by the Ethics Committee of the Center (protocol No. 4 of April 18, 2024).
Inclusion criteria in the main group: European ethnicity, GDM, singleton pregnancy, newborn’s birth weight from 2501 g to 4999 g, patient’s consent to participate in the study.
Inclusion criteria in the comparison group: European ethnicity, absence of GDM, singleton pregnancy, newborn’s birth weight from 2501 g to 4999 g, patient’s consent to participate in the study.
The key feature of inclusion criteria in the study for all patients was undergoing a comprehensive examination including three screening ultrasound examinations and oral glucose tolerance test at 24–28 weeks of pregnancy.
Non-inclusion criteria: type 1 and type 2 diabetes, somatic pathology at the stage of decompensation, oncologic diseases, autoimmune disease, bronchial asthma at the stage of medicated compensation, multiple pregnancy.
All pregnant women at 11–14 weeks, 18–21 weeks, 30–32 weeks of pregnancy underwent ultrasound examination. GDM was diagnosed based on fasting venous plasma glucose level in the first and in the third trimesters, and the results of oral glucose tolerance test (OGTT) in the second trimester (75 g of glucose load after 8–14-hour overnight fast). The procedure included venous blood samples collection after fasting, followed by measurement of 30 min postload plasma glucose levels. When plasma glucose level was >5.1 mmol/L after the first blood sample, the test was terminated (the diagnosis of GDM was made according to the criteria for gestational diabetes mellitus of the Russian Society of Endocrinologists. To continue the test, the patient was asked to drink 75 g of glucose dissolved in 250–300 ml of warm (37–40°С) still water over 5 minutes. The subsequent blood samples were collected to determine venous plasma glucose levels 1 and 2 hours after glucose load.
Statistical analysis
Statistical data processing was performed using the RStudio integrated development environment for R script. The Shapiro-Wilk test was used to test the normality of data distribution. Non-normal distribution of the quantitative data was described using the median (Me) and quartiles (Q1, Q3). The qualitative data are presented as absolute values (%). The comparative analysis of qualitative data was performed used Fisher’s exact test and Persons’ chi-square (c2) test. The comparative analysis of the quantitative data was performed using the Mann–Whitney U test for pairwise сomparisons. The Kruskal–Wallis test was used to compare the differences between more than two groups.
All women were divided in the training cohort and the test cohort according to the 80/20 rule.
In the training cohort, the selected clinical parameters were analyzed by construction of operating curves and calculation of the area under the operating curve, the threshold maximizing the sum of sensitivity and specificity, and the values of sensitivity and specificity.
The potential markers for macrosomia included the parameters with the values in the area under the operating greater than 0.6, and the lower limit of the confidence interval (CI) above 0.5. The markers were converted into factorials: zero (0) represents the values below the threshold, and one (1) represents the values above the threshold determined by the operating curve analysis. The selected potential markers were used to create two types of classification models based on logistic regression: one with and one without the data obtained during screening in the third trimester. The variables were gradually included in the models to minimize the values of the Akaike information criterion (AIC) up to the lower AIC value. In the presence of highly correlated variables, the variable with the lowest AIC was included in the model. Subsequently, the remaining covariates were excluded from the set of variables to be included in the next steps. The variables with the highest probability of regression coefficient of zero and not less than 0.05 were subsequently excluded from the models. Sensitivity, specificity, and the optimal threshold was calculated by 10-fold validation using 10 random splits and constructing operating curve. The model was also tested on the previously separated test set by constructing the operating curve. The quality of model calibration was assessed using the Hosmer–Lemeshow test.
Results
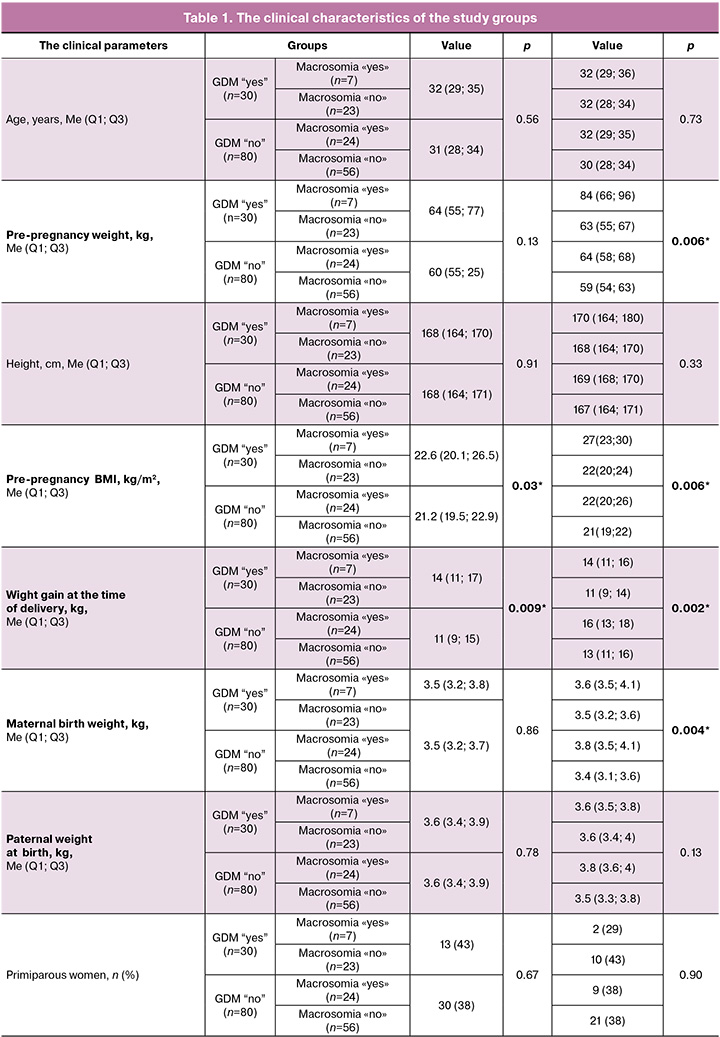
The comparative analysis of the clinical characteristics in the groups was performed. The obtained results in the groups of examined women is presented in Table 1. There was no statistically significant difference between the groups and the subgroups in the median age of patients – 32 years (p=0.56). However, intergroup comparison showed differences between the subgroups depending on fetal birth weight (the presence of absence of macrosomia). Pregnant women with fetal macrosomia were of higher weight – 84 (66; 96) kg, (p=0.006) before the onset of pregnancy among the patients with GDM. There was significant difference between the main group and the comparison group in pre-pregnancy body mass index (BMI) – 22.6 (20.1; 26.5) kg/m2 and 21.2 (19.5; 22.9) kg/м2, respectively, (p=0,03). The patients with GDM and macrosomia entered pregnancy with significantly high BMI compared with the patients in other subgroups (27 (23; 30) kg/m2, p=0.006). Weight gain at the time of delivery differed significantly among the patients with macrosomia, regardless of whether or not they had GDM (14 (11; 16) and 16 (13; 18), p=0.006). At the same time, weight gain was highest at the time of delivery in pregnant women with macrosomia and without GDM. It is interesting to note that birth weight of women in this subgroup was larger (3.8 (3.5; 4.1), p=0.004) compared with other patients. The women in the main group had cesarean delivery significantly more often than women in the comparison group 18/30 (60%), 27/80 (34%), (p=0.02), and up to 71% in cases of fetal macrosomia. In most cases, women with GDM underwent elective operative delivery – 13 (43%), 14 (18%), (p=0,01). In most cases (33%), the patients with fetal macrosomia and without GDM underwent emergency delivery. As it is known today, this is associated with a large number of complications for both mother and fetus. There was significant difference in the length of hospital stay of puerperant women: the patients with GDM stayed in hospital longer – 5 (3; 5) and 4 (3; 5) days, (p=0,02), regardless of fetal birth weight – 5 (3; 5), 5 (4; 5) days, p=0.007). There were no significant differences in neonatal outcomes in newborns depending on GDM (early neonatal complications, Apgar scores at 1t and 5 minutes, and the length of hospital stay, whereas in the subgroups of women with or without fetal macrosomia, macrosomic babies stayed in hospital significantly longer – 4 (4; 5) and 4 (3; 5) days, p=0.009).
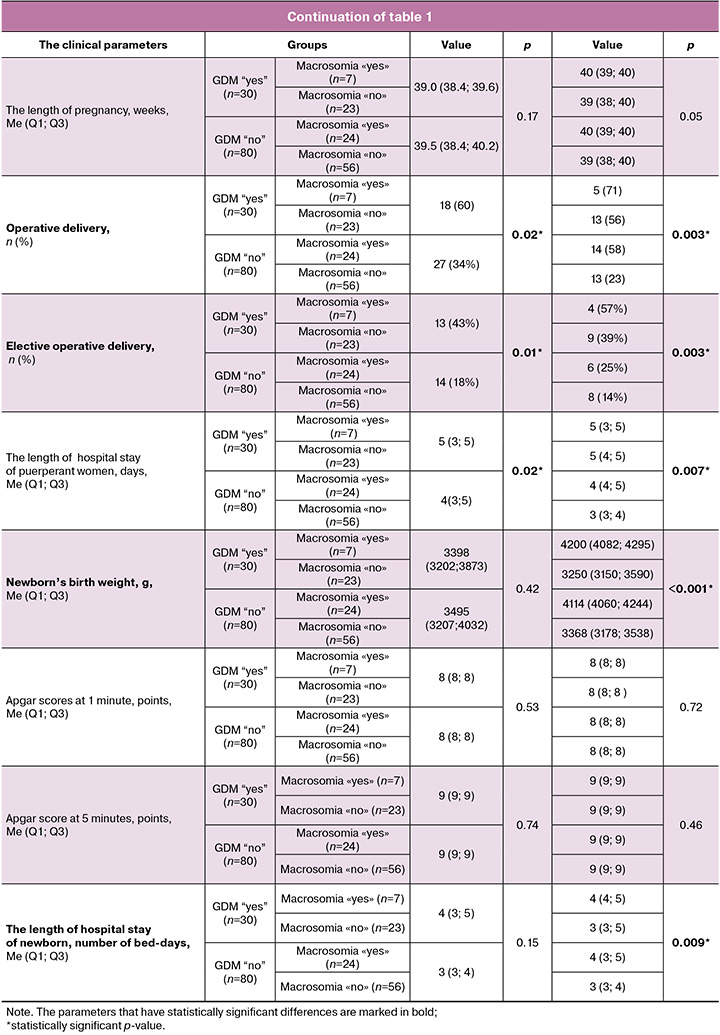
Large for gestational age baby in maternal medical history was significantly more often in patients with macrosomia, regardless of the presence of GDM – 3 (43%) and 6 (25%) versus 0 (0%) and 4 (7%), p=0.002). Also, in these subgroups with fetal macrosomia, Type 1 and Type 2 diabetes mellitus in close family members was more often – in 33% of cases in the subgroup without GDM and in up to 57% of cases with GDM. In the subgroups without macrosomia, family history of diabetes mellitus among close relatives was in 21% of cases without GDM and in 26% of cases with GDM, respectively.
Excessive weight gain during pregnancy was significantly more often among the patients in group I compared with group II (p<0.001), and among women with fetal macrosomia compared with the patients with normal fetal birth weight – 4 (57%) and 18 (75%) versus 5 (22%) and 14 (25%), p<0.001).
According to medical documentation, the diagnosis of carbohydrate metabolism disorders was made in the first and third trimesters of pregnancy according to the results of fasting blood glucose test, and in the second trimester according to the results of oral glucose tolerance test. GDM was diagnosed in women with fetal macrosomia in the first trimester of pregnancy in 10% of cases, in the second trimester in 70% of cases, and in the third trimester in 20% of cases. Dynamic analysis of glycemic index during OGTT showed no significant postprandial glucose levels in women with fetal macrosomia. All patients with fetal macrosomia were diagnosed with GDM based on one fasting venous blood glucose test.
The main therapeutic method for correction of hyperglycemia was diet therapy excluding low-digestible carbohydrates (the patients who required insulin therapy were excluded from further study).
In addition, intragroup and intergroup assessment of laboratory values of blood test that was made as a part of routine examination, and the results were registered in patients' primary medical documentation. There were no significant differences in serum biochemistry parameters found between groups I and II (p>0.05). Comparison of subgroups with fetal macrosomia found significantly increased levels of such serum biochemistry parameters as total cholesterol (TC) and triglyceride (TG) levels in the second trimester (24–28 weeks of pregnancy) (p=0.001).
Prognostic models of fetal macrosomia
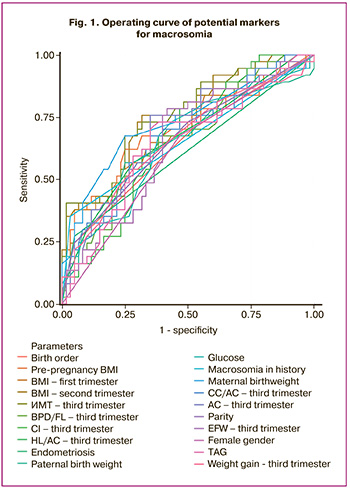
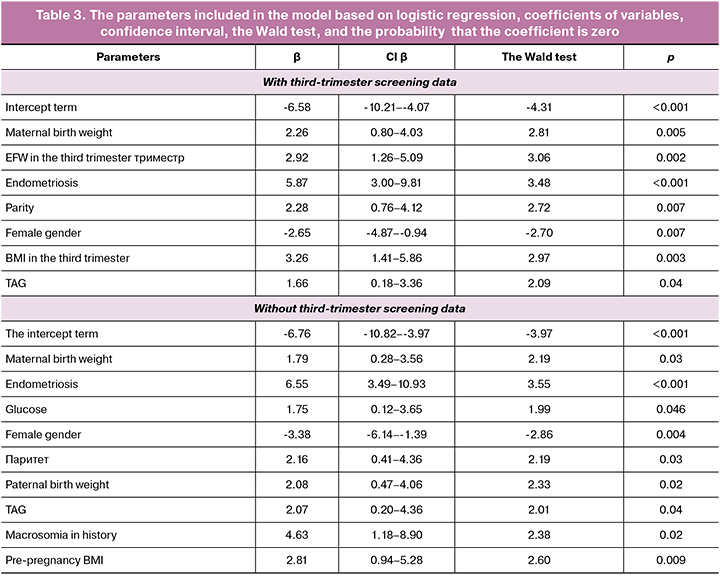
Based on the analysis of operating curves, potential markers for macrosomia were identified: maternal and paternal birth weight (the threshold of 3.58 kg and 3.78 kg, respectively), pre-pregnancy BMI (the threshold of 22.44 kg/m2), endometriosis and cases of macrosomia in previous pregnancies, parity different from zero parity, and birth order (at least the third). Screening in the first trimester identified BMI (22,95 kg/m2) as a potential marker. Screening in the second trimester identified BMI (the threshold value 24.35 kg/m2), blood glucose level (the threshold of 4.75 mmol/L) and triacylglycerol (TAG) level (the threshold of 1.83 mmol/L) as potential markers. Screening in the third trimester identified the following potential markers: weight gain compared with pre-pregnancy weight (the threshold 10.7 kg), BMI (the threshold 26.43 kg/m2), abdominal circumference (AC) (the threshold of 276.85 mm), estimated fetal weight (EFW) (the threshold of 1807 g). Markers include the cephalic index (CI), the ratio of chest circumference to abdominal circumference (CC/AC), the ratio of biparietal diameter to femur length/ (BPD/FL), the ratio of the humeral length to abdominal circumference/(HL/AC) (1.06, 1.34, 0.20, respectively). In addition, baby’s gender is a potential marker (Fig. 1, Table 2).

The final model with third-trimester screening data included the following parameters: maternal birth weight, baby’s gender, parity, triacylglycerol levels during the second screening, endometriosis, estimated fetal weight, and BMI measured in the third trimester. The Hosmer–Lemeshow test was used at p-value of 0.80. With cross-validation of the training cohort, the area under the operating curve was 0.91. The optimal sensitivity and specificity were 85% and 87%, respectively. The threshold value was 0.395. With validation of the test cohort, the area under the operating curve was 0.77. The optimal sensitivity and specificity were 78% and 86%, respectively. The threshold value was 0.57. At the same time, sensitivity, specificity, and accuracy were 78%, 64%, and 70% using the threshold value that was previously determined with cross-validation (Fig. 2a).
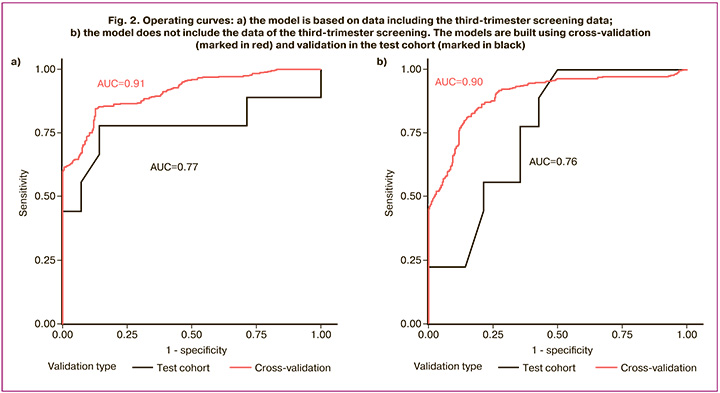
The model without third-trimester screening data, included the following parameters: maternal and paternal birth weight, baby’s gender, endometriosis and macrosomia in medical history, glucose and TG levels in the second-trimester screening, and pre-pregnancy BMI (Table 3). The Hosmer–Lemeshow test was performed at p<0.001. With cross-validation of the training cohort, the area under the operating curve was 0.90. The optimal sensitivity and specificity were 82% and 85%, respectively. The threshold value was 0.42. With validation of the test cohort, the area under the operating curve was 0.76. The optimal sensitivity and specificity were 100% and 50%, respectively. The threshold was 0.57. At the same time, sensitivity, specificity, and accuracy were 78%, 64%, and 70% using the threshold value that was previously determined with cross-validation.
Discussion
Currently, most international groups of researchers have come to an understanding of the main maternal risk factors for fetal macrosomia, although it is still far from the final understanding and solution of this obstetric problem. Thus, the main risk factors for macrosomia include maternal age, pre-pregnancy obesity, excessive weight gain before and during pregnancy, as well as GDM without using insulin [14–19]. In 2023, Journal of the Chinese Medical Association (JCMA) published the data about the identified maternal factors associated with neonatal macrosomia in Taiwanese population (4 262 cases of singleton full-term births). The authors of the study identified significant risk factors such as GDM, 6-month gestational weight gain (6m GWG), and maternal BMI. The odds ratio (OR) of macrosomia was 3.1 in neonates born to mothers with a 6m GWG of ≥15 kg, 6.3 in neonates born to mothers with GDM, and 4.1 in neonates born to mothers with BMI ≥30 kg/m2, respectively. Therefore, the authors suggested the importance of counseling mothers on weight management before and during pregnancy [14]. According to a large meta-analysis in 2023, a clinical marker, such as endometriosis, is important for GDM. The meta-analysis included 18 studies involving 4,600,885 women. The overall risk of GDM in patients with endometriosis was significantly higher than in the control group (OR, 1.23; 95% CI, 1.07–1.51) [20]. Thus, the clinical parameter – endometriosis has a significant impact, both due to high pathogenetic significance and high incidence of both endometriosis and GDM.
The choice of one of the clinical markers of baby’s gender was determined in a number of publications [21–23]. In the study by O’Neill K. et al. [21], 459 known biochemicals in the amniotic fluid in women diagnosed with GDM were assessed using gas chromatography/mass spectrometry. The samples were stratified based on fetal gender. Specific changes were identified based on the offspring’s gender. The authors suggested that sex-specific changes in maternal and fetal metabolism in GDM may explain sex-specific metabolic outcomes that are observed in offspring exposed to GDM in utero. This interesting possibility of sexual dimorphism in metabolic risk is supported in the large population-based study by Ricart W. et al. [22], that included 9,270 women, who gave birth to 4,793 and 4,477 boys and girls, respectively. According to the authors, maternal glucose tolerance consistently influenced the risk of macrosomia in male fetuses, but not in female fetuses, and GDM predicted macrosomia exclusively in male fetuses. Therefore, thorough monitoring of the glycemic status in women carrying male fetuses can be justified. However, confirmation of this sex-specific changes requires further study.
Our analysis of clinical data showed similar results in identifying significant risk factors for fetal macrosomia, namely: greater pre-pregnancy weight (with the highest rate among the patients with GDM, pre-pregnancy BMI, and weight gain until delivery (11–18 kg) regardless of the presence of GDM. Moreover, pregnant women with fetal macrosomia and without GDM exhibited greater weight gain until delivery. Additional interest raised the data indicating significantly greater birth weight in women with macrosomia compared to other patients; history of previous large babies born to multiparous women (regardless of the presence of GDM), and type 1 and type 2 diabetes in close relatives in history (33–57%). It is likely that genetic and behavioral factors (family diet, eating habits) can equally contribute to the development of macrosomia in women without carbohydrate metabolism disorders.
Despite extensive research work is focused on macrosomia, it is impossible to predict the outcomes for women in risk group [19]. Antenatal risk factors are important in predicting macrosomia, but the outcomes for the fetus and mother depend on management of labor [15, 24]. In our study, the rate of cesarean delivery reached 71% in patients with fetal macrosomia. Elective surgical delivery was most often in women with GDM, whereas emergent operative delivery was in most cases (33%) in patients with macrosomia and without GDM, that was associated with a high rate of complications both for the mother and her fetus. The number of bed-days in hospital was significantly higher among the patients with GDM, regardless of fetal birth weight. At the same time, there were no differences in neonatal outcomes (early neonatal complications, Apgar scores at 1and 5 minutes, and the length of hospital stay) with regard to GDM. However, neonatal outcomes differed significantly with regard to birth weight. Thus, the newborns with macrosomia stayed in hospital longer.
The results of the retrospective cohort study by Vitner D. et al., that included 3, 098 mothers and babies with macrosomia, were based on 15 years of observations (2000–2015). The study compared pregnancy management and outcomes between women with correct prediction of fetal macrosomia and women with unknown status based on estimated fetal weight at birth. The primary outcomes included the frequency of cesarean section and postpartum hemorrhage. Secondary outcomes were combined maternal and neonatal outcomes and birth injuries. Fetal macrosomia was predicted in 601 (19.4%) women, and fetal macrosomia was unknown in 2,497 (80.6%) women. The frequency of cesarean section was more than 3.5 times higher in the group with predicted macrosomia (47.2% versus 12.7%, p<0.001), that was consistent with the results of our study (macrosomia was a predictive factor for GDM). Also, the author of the study reported reduced risk of postpartum hemorrhage (OR 0.5, 95% CI 0.2–1.0) in cases of elective cesarean section (the group with predicted macrosomia), and other maternal (OR 0.3, 95% CI 0.2–0.5) and composite neonatal adverse outcomes (OR 0.7, 95% CI 0.6–0.9). Thus, the authors concluded that elective cesarean section is associated with predicted fetal macrosomia and leads to the reduction of the risk of postpartum hemorrhage, improvement of maternal and neonatal outcomes even for infants with the average birth weight of <4500 g [25].
In cases of suspected fetal macrosomia, the patients should be carefully counseled for a birth plan, and if indicated, cesarean delivery should be considered as an option. Methods for fetal weight estimation and prediction of macrosomia include clinical measurements, ultrasound and magnetic resonance imaging. However, current prediction strategies, including clinical examination and ultrasound, are inaccurate. Therefore, a search for new methods of prediction and early diagnosis of fetal macrosomia is extremely relevant today.
In our study, an attempt was made to predict fetal macrosomia, regardless of the presence or absence of GDM, starting from the first screening (11–13 weeks of pregnancy). In the study by Yuan Y. et al. (2023), an attempt was made to create a prognostic model of fetal macrosomia based on clinical and laboratory biomarkers in maternal blood that differed in women with GDM and macrosomia (GDM-M) and women with GDM and normal birth weight (GDM-N). The model included the parameters, such as pre-pregnancy BMI, weight gain at 24 weeks of pregnancy, parity, plasma glucose level 2 hours after a 75-g glucose load (glucose tolerance test at 24 weeks of pregnancy), high-density and low-density lipoproteins at 24 weeks, and plasma CLUL1, VCAN, and RNASE3 expression at 24 weeks. The obtained prognostic model demonstrated its effectiveness in predicting macrosomia in women with GDM [26]. TG levels have been currently identified as predictors of fetal macrosomia in women with GDM, regardless of BMI and glucose levels [27]. TG levels in the second and third trimesters of pregnancy positively correlated with newborn’s birth weight in the absense of diabetes mellitus [28]. The study by Wang X. et al. (2018) reported that each 1 mmol/L increase in TG in the third trimester resulted in a 27% increase in macrosomia risk in women without diabetes [29]. In pregnant women with GDM, positive correlation was found between TG levels, free fatty acids and fetal macrosomia, and fetal fat mass, regardless of glycemic control, BMI and weight gain during pregnancy.
The results of our study showed statistically significant increase in the levels of TC and TG (p=0.001) at 24–28 weeks of pregnancy in the group with fetal macrosomia. The prognostic model that can be used for verification of women at high risk of fetal macrosomia, regardless of GDM in the first trimester, included the following parameters: paternal and maternal birth weight, baby’s gender, endometriosis and macrosomia in maternal medical history, glucose level and TG level during the screening in the second trimester, and pre-pregnancy BMI. With cross-validation, the area under the operating curve was 0.90. Sensitivity and specificity were 82% and 85%, respectively.
It is important to note that comparative analysis of currently existing data and the findings in our study indicate significant inconsistency. It can be due to the sample size, ethnic characteristics, and a number of other environmental factors that significantly influence the selection of markers of GDM. It is unlikely that a meta-analysis of the data currently accumulated in world literature can fully address this issue. A large, multicenter studies that will take into account regional differences are likely needed to validate data on GDM.
Despite the limitations of the study, the obtained data can be useful in routine duties of practicing obstetrician-gynecologist and contribute to early diagnosis of GDM and macrosomia, and timely of personalized correction of metabolic disorders in pregnant women with GDM.
Conclusion
Early identification of risk groups for fetal macrosomia may allow to take preventive measures by changing lifestyle and dietary habits among this cohort of women in the first trimester of pregnancy, personalization of obstetric management strategies, selection of the optimal time for delivery to reduce adverse maternal and fetal outcomes.
The study identified clinical prognostic markers for fetal macrosomia and developed pilot prognostic mathematical models with good sensitivity and specificity (100–78% and 85–50%, AUC = 0.76–0.77, respectively). These models can be used to predict the development of fetal macrosomia at or after 24 weeks of pregnancy, both regardless of the presence of GDM (also in the group with unknown GDM status) and can be used separately in the group of women with carbohydrate metabolism disorders.
References
- Langley-Evans S.C., Pearce J., Ellis S. Overweight, obesity and excessive weight gain in pregnancy as risk factors for adverse pregnancy outcomes: a narrative review. J. Hum. Nutr. Diet. 2022; 35(2): 250-64. https://dx.doi.org/10.1111/jhn.12999
- Wang H., Li N., Chivese T., Werfalli M., Sun H., Yuen L. et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International association of diabetes in pregnancy study group’s criteria. Diabetes Res. Clin. Pract. 2022; 183: 109050. https://dx.doi.org/10.1016/j.diabres.2021.109050
- Российская ассоциация эндокринологов. Российское общество акушеров-гинекологов. Клинические рекомендации. Гестационный сахарный диабет. Диагностика, лечение, акушерская тактика, послеродовое наблюдение. 2020. [Russian Association of Endocrinologists. Russian Society of obstetricians and gynecologists. Clinical Guidelines. Gestational diabetes mellitus. Diagnosis, treatment, obstetric tactics, postpartum monitoring. 2020 (in Russian)]. https://opc33.ru/wp-content/uploads/2021/07/kr_gsd_2020.pdf
- Beta J., Khan N., Khalil A., Fiolna M., Ramadan G., Akolekar R. Maternal and neonatal complications of fetal macrosomia: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2019; 54(3): 319-25. https://dx.doi.org/10.1002/uog.20278.
- Frick A.P., Syngelaki A., Zheng M., Poon L.C., Nicolaides K.H. Prediction of large-for-gestational-age neonates: screening by maternal factors and biomarkers in the three trimesters of pregnancy. Ultrasound Obstet. Gynecol. 2016; 47(3): 332-9. https://dx.doi.org/10.1002/uog.15780
- Goldstein R.F., Abell S.K., Ranasinha S., Misso M., Boyle J.A., Black M.H. et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017; 317(21): 2207-25. https://dx.doi.org/10.1001/jama.2017.3635
- Isaku M., Vrapi E., Bimbashi T., Cala I., Perdja K., Hoxhallari R. et al. Perinatal outcomes among cases of predicted and unpredicted macrosomia. Gynecol. Obstet. Reprod. Med. 2023; 29(2): 93-8. https://dx.doi.org/10.21613/gorm.2022.1379
- Boulvain M., Thornton J.G. Induction of labour at or near term for suspected fetal macrosomia. Cochrane Database Syst. Rev. 2023; 3. https://dx.doi.org/10.1002/14651858.CD000938.pub3
- Mehlman C.T. Neonatal brachial plexus palsy. The Pediatric Upper Extremity. 2015; 123(4): 589-605. https://dx.doi.org/10.1007/978-1-4614-8515-5_27
- Кравченко Е.Н., Серов В.Н., Баев О.Р. Факторы риска родовой травмы. Акушерство и гинекология. 2022; 9: 5-10. [Kravchenko E.N., Serov V.N., Baev O.R. Risk factors for birth injury. Obstetrics and Gynecology. 2022; (9): 5-10 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.9.5-10
- Kamana K., Sumisti S., Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann. Nutr. Metab. 2015; 66: 14-20. https://dx.doi.org/10.1159/000371628
- Whincup P.H., Kaye S.J., Owen C.G., Huxley R., Cook D.G., Anazawa S. et al. Birth weight and risk of type 2 diabetes a systematic review. JAMA. 2008; 300(24): 2886-97. https://dx.doi.org/10.1001/jama.2008.886
- Szabo A.J. Transferred maternal fatty acids stimulate fetal adipogenesis and lead to neonatal and adult obesity. Med. Hypotheses. 2019; 122: 82-8. https://dx.doi.org/10.1016/j.mehy.2018.10.022
- Liu C.H., Yang S.T., Wang P.H. Maternal factors associated with fetal macrosomia. Journal of the Chinese Medical Association. 2023; 86(5): 455-6. https://dx.doi.org/10.1097/JCMA.0000000000000894
- Moodley T., Moodley J. A retrospective identification of risk factors associated with fetal macrosomia. Afr. J. Reprod. Health. 2022; 26(7): 127-34. https://dx.doi.org/10.29063/ajrh2022/v26i7.13
- Romero R., Kingdom J., Deter R., Lee W., Vintzileos A. Fetal growth: evaluation and management. Am. J. Obstet. Gynecol. 2018; 218(2S): S608. https://dx.doi.org/10.1016/j.ajog.2018.01.010
- Rizzo G., Patrizi L., Mappa I. Can we improve the diagnosis of fetal macrosomia? J. Clin. Ultrasound. 2022; 50(7): 974-5. https://dx.doi.org/10.1002/jcu.23238
- Jenabi E., Salehi A.M., Farashi S., Salimi Z. The environmental risk factors associated with fetal macrosomia: an umbrella review. Pediatr. Neonatol. 2024; 65(3): 217-21. https://dx.doi.org/10.1016/j.pedneo.2023.09.007
- Ewington L., Black N., Leeson C., Al Wattar B.H., Quenby S. Multivariable prediction models for fetal macrosomia and large for gestational age: a systematic review. BJOG. 2024; 131(12): 1591-602. https://dx.doi.org/10.1111/1471-0528.17802
- Salmeri N., Li Piani L., Cavoretto P.I., Somigliana E., Viganò P., Candiani M. Endometriosis increases the risk of gestational diabetes: a meta-analysis stratified by mode of conception, disease localization and severity. Sci. Rep. 2023; 13(1): 8099. https://dx.doi.org/10.1038/s41598-023-35236-y
- O’Neill K., Alexander J., Azuma R., Xiao R., Snyder N.W., Mesaros C.A. et al. Gestational diabetes alters the metabolomic profile in 2nd trimester amniotic fluid in a sex-specific manner. Int. J. Mol. Sci. 2018; 19(9): 2696. https://dx.doi.org/10.3390/ijms19092696
- Ricart W., López J., Mozas J., Pericot A., Sancho M.A., González N. et al. Maternal glucose tolerance status influences the risk of macrosomia in male but not in female fetuses. J. Epidemiol. Community Health. 2009; 63(1): 64-8. https://dx.doi.org/10.1136/jech.2008.074542
- Боташева Т.Л., Андреева В.О., Рымашевский А.Н., Тезиков Ю.В., Липатов И.С., Фабрикант А.Д., Лебеденко Е.Ю., Железнякова Е.В. Роль половой принадлежности плода в патогенезе гестационного сахарного диабета и акушерских осложнений. Акушерство и гинекология. 2022; 9: 33-41. [Botasheva T.L., Andreeva V.O., Rymashevskiy A.N., Tezikov Yu.V., Lipatov I.S., Fabrikant A.D., Lebedenko E.Yu., Zheleznyakova E.V. The role of fetal gender in the pathogenesis of gestational diabetes mellitus and obstetric complications. Obstetrics and Gynecology. 2022; (9): 33-41 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.9.33-41
- Одинокова В.А., Шмаков Р.Г. Современные аспекты акушерской тактики при фетальной макросомии. Акушерство и гинекология. 2022; 7: 21-7. [Odinokova V.A., Shmakov R.G. Modern aspects of obstetric tactics for fetal macrosomia. Obstetrics and gynecology. 2022; (7): 21-7 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.7.21-27
- Vitner D., Bleicher I., Kadour-Peero E., Lipworth H., Sagi S., Gonen R. Does prenatal identification of fetal macrosomia change management and outcome? Arch. Gynecol. Obstet. 2019; 299(3): 635-44. https://dx.doi.org/10.1007/s00404-018-5003-2
- Yuan Y., Zhu Q., Yao X., Shi Z., Wen J. Maternal circulating metabolic biomarkers and their prediction performance for gestational diabetes mellitus related macrosomia. BMC Pregnancy and Childbirth. 2023; 23: 113. https://dx.doi.org/10.1186/s12884-023-05440-9
- Zhang Y., Zhang H.H., Lu J.H., Zheng S.Y., Long T., Li Y.T. et al. Changes in serum adipocyte fatty acid-binding protein in women with gestational diabetes mellitus and normal pregnant women during mid- and late pregnancy. Journal of Diabetes Investigation. 2016; 7(5): 797-804. https://dx.doi.org/10.1111/jdi.12484
- Ning H., Tao H., Weng Z., Zhao X. Plasma fatty acid-binding protein 4 (FABP4) as a novel biomarker to predict gestational diabetes mellitus. Acta Diabetol. 2016; 53(6): 891-8. https://dx.doi.org/10.1007/s00592-016-0867-8
- Wang X., Guan Q., Zhao J., Yang F., Yuan Z., Yin Y. et al. Association of maternal serum lipids at late gestation with the risk of neonatal macrosomia in women without diabetes mellitus. Lipids Health Dis. 2018; 17(1): 78. https://dx.doi.org/10.1186/s12944-018-0707-7
Received 11.06.2025
Accepted 15.08.2025
About the Authors
Natalia A. Frankevich, Dr. Med. Sci., Senior Researcher at the Department of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Moscow, Russia, Ac. Oparina str., 4, natasha-lomova@yandex.ru, https://orcid.org/0000-0002-6090-586XAlisa O. Tokareva, PhD (in Physics and Mathematics), specialist at the Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, alisa.tokareva@phystech.edu,
https://orcid.org/0000-0001-5918-9045
Tamara E. Karapetyan, Dr. Med. Sci., Senior Researcher at the Department of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Moscow, Russia, Ac. Oparina str., 4, tomamed02@mail.ru, https://orcid.org/0000-0003-0025-3182
Anastasia A. Kutsenko, PhD student, Department of Obstetrics and Gynecology, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moskovsky tract, 2, maori.nastya@yandex.ru, https://orcid.org/0009-0007-6146-561X
Angela G. Vasilyeva, applicant at the Department of Obstetrics and Gynecology, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moskovsky tract, 2, angela.grigorjevna@yandex.ru, https://orcid.org/0009-0006-7975-1115
Vitaly V. Chagovets, PhD (in Physics and Mathematics), Head of the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, v_chagovets@oparina4.ru,
https://orcid.org/0000-0002-5120-376X
Vladimir E. Frankevich, Dr. Sci. (in Physics and Mathematics), Director for Science – Head of the Department of Systems Biology in Reproduction, Institute of Translational Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparina str., 4, v_frankevich@oparina4.ru, https://orcid.org/0000-0002-9780-4579
Corresponding author: Natalia A. Frankevich, natasha-lomova@yandex.ru



