Study the modifying effect of obesity on uterine fibroid’s clinical characteristics in Syrian women
Alali O.M., Churnosov M.I.
Objective: To evaluate the modifying effect of obesity on clinical manifestations of uterine fibroids (UF) in Syrian women.
Materials and methods: 1,000 Syrian women, ages 22–83, from different cities participated in the study, which ran from June 2023 to August 2024. They were divided based on obesity and disease into two groups: with obesity BMI ≥30 kg/m2 (160 patients and 146 controls), and without obesity BMI <30 kg/m2 (340 patients and 354 controls). The study employed a questionnaire consisting of 65 items that provided information on the clinical features and risk factors for the development of UF. Version 30 of SPSS was used to analyze data.
Results: Revealed the features of the clinical characteristics of the non-obese group that were statistically significant in patients compared to controls: an older age, a shorter menstrual cycle length and a lower rate of endometrial ablation, and didn't reveal specific features in the obese group. In both studied groups, obese and non-obese, in patients compared to controls, the age of menopause was earlier, and by the time elapsed since the last birth, the percentage was lower in the categories: ≤5 years and 11–15 years, while within the categories: 6–10 years, 16–19 years, and ≥20 years, it was higher.
Conclusion: This study demonstrated the modifying effect of obesity on clinical manifestations of UF in Syrian women. That will provide additional clarification of the impact of obesity on the risk/prevalence of UF, which will be beneficial for both its prevention and diagnosis.
Authors' contributions: Alali O.M., Churnosov M.I. – conception and design of the study; Alali O.M. – data collection and analysis, manuscript drafting; Churnosov M.I. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Belgorod State National Research University.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Alali O.M., Churnosov M.I. Study the modifying effect of obesity on
uterine fibroid’s clinical characteristics in Syrian women.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (11): 120-128 (in Russian)
https://dx.doi.org/10.18565/aig.2025.193
Keywords
Uterine fibroids (UF) (also known as leiomyomas) are benign tumours that are dependent on sex steroid hormones, arising from the smooth muscle layer of the uterus (myometrium) [1]. They are the most common neoplasms affecting 20–40% of women in their reproductive age [2], with a lifetime prevalence of 30–70% [3]. UF are the primary reason for hysterectomy in the United States, incurring annual healthcare expenses in the billions [4].
Obesity and being overweight are important factors that affect women’s reproductive health [5, 6], and are considered the most prominent illness predisposing factors [5–7]. Prior researches have indicated that obesity is a risk factor for the development of UF by several biological mechanisms [5–7], and increases the probability of endometrial polyps and symptomatic uterine fibroids in women's reproductive systems[8].
The relationship between obesity and the female reproductive system is said to be influenced by a number of mechanisms. One mechanism explains the endocrine response that occurs when obesity enhances the conversion of circulating androgens to oestrogens and creates extra adipose tissue. According to a different mechanism, obesity may raise peripheral unbound oestrogen levels by decreasing the liver's synthesis of sex hormone binding globulin (SHBG) [4, 5].
According to other research, gaining more weight after the age of 18 is also linked to a higher chance of developing UF. But for one study, women who are severe overweight had a lower risk of developing UF [9]. A greater frequency of multiple and larger uterine fibroids was observed to be related to raised waist-to-hip ratio (WHR) and body mass index (BMI) among women who had their fibroids surgically removed [10], and that keeping a healthy weight can help prevent UF [7, 11]. According to WHR, the obese body type had a greater incidence of irregular vaginal bleeding, numerous tumours, tumour degeneration, and lesion diameters of 40 mm than the normal body type group. The data indicated that, regardless of the type of obesity, an increase in peripheral adipose tissue is related with a higher risk of UF [11]. BMI has been shown to have a major impact on fibroids' development. Women who weigh 70 kg have three times the risk of developing fibroids compared to those who weigh 50 kg [12]. Furthermore, according to a prior study, visceral fat raises the risk of fibroids by stimulating the synthesis of inflammatory mediators such tumour necrosis factor-α (TNF-α) [6].
Several epidemiological studies have been conducted to investigate the relationship between obesity and the risk of UF in premenopausal women, with contradictory results [13–15]. Some research found a positive correlation between obesity and the risk of UF [5, 15, 16]. While others revealed no significant correlation [13, 14].
Not all ethnic groups have a positive correlation between a higher BMI and an increased chance of developing fibroids [9, 17, 18]. Case-control studies undertaken in China, Korea, and Japan discovered that higher BMI levels were related with an increased incidence of UF. One Japanese case-control study, however, discovered that higher risks of UF were linked to body fat percentage rather than BMI [13].
The traits of Asian women who may be at a higher risk of developing UF are not well understood. There was only one study conducted on Saudi women, where discovered that obesity is a risk factor for Saudi women, as it had a positive role in promoting the growth of UF [16]. Furthermore, to present, the effect of obesity on the initiation and development of UF is still mostly unknown. Thus, in this study, we aimed to evaluate the modifying effect of obesity on clinical manifestations of uterine fibroids (UF) in Syrian women.
Materials and methods
Between June 2023 and August 2024, a survey was carried out in Syria at both public and private hospitals located in cities such as Damascus, Hama, Tartus, Latakia, and Homs. These include of Tartus Maternity and Children's Hospital, Latakia University Hospital, Homs University Hospital, Al-Ahli Private Hospital, and Latakia Maternity and Children's Hospital.
The study involved 1,000 Syrian women, ages 22–83, divided based on obesity and disease into: the obese group (BMI≥30) (146 controls and 160 patients), and the non-obese group (BMI<30) (354 controls and 340 patients). Women with UF were the patients, while women without any symptoms of benign disorders of the reproductive organs were the controls. Women under the age of twenty, those of non-Syrian ancestry, and anyone born outside of Syria were all excluded. Following an explanation of the study's goal, participants gave their informed permission. A 65-question questionnaire that assessed clinical features and risk factors was created, consisting of yes/no categorical items and multiple-choice questions (MCQ) that were translated into Arabic for the participants' convenience. The design of the questionnaire was modified from previously published research.
Statistical analysis
We estimated that a sample size (1000) was divided based on their body mass index (BMI) into the obese group BMI≥30 (146 controls and 160 patients) and the non-obese group BMI<30 (354 controls and 340 patients). All statistical analysis for this study was conducted using IBM Corporation's Statistical Packages for Software Sciences (SPSS) version 30.0.0.0 (172). The normality of the assessed quantitative variables was examined using the Shapiro-Wilk test. P Shapiro–Wilk was less than 0.001 for all indices, indicating that their distribution was not normal. Thus, the median and interquartile range Me (Q1; Q3) were utilized to characterize these quantitative variables, and the Mann–Whitney test was employed for comparison analysis. Binary categorical variables were compared between the case and control groups using the chi-squared test for qualitative variables. The groups were compared using the Mann–Whitney test for variables with three or more categories. For all qualitative characteristics, the results were shown as numbers or percentages of all participants. It was deemed statistically significant when the P value was <0.05.
Results
The study conducted on 1,000 Syrian women, who ranged in age from 22 to 83, with the majority in the 36–50 age group, divided them based on obesity and disease into the obese group (BMI≥30) (146 controls and 160 UF cases) and the non-obese group (BMI<30) (354 controls and 340 UF cases).
In investigating the clinical characteristics and risk factors of UF in Syrian women within the non-obese group (BMI <30), the results found that patients, compared to controls, had an older age (45 (35; 56) years and 43 (28; 57.7) years, respectively, P=0.002), a shorter menstrual cycle length (28 (25; 30) days and 28 (26; 30) days, respectively, P=0.027), and an earlier age at menopause (45 (40; 49) years and 47 (45; 49) years, respectively, P<0.001). Also, the time elapsed since the first birth showed that the percentage of patients compared to controls, was lower in both categories: ≤5 years (16.80 (44) and 27.76 (78), respectively) and 11–15 years (24.04 (63) and 39.86 (112), respectively) and higher in the following categories: 6-10 years (15.65 (41) and 11.39 (32), respectively), 16–19 years (18.32 (48) and 2.85 (8), respectively), and ≥20 years (25.19 (66) and 18.15 (51), respectively, P<0.001)); the rate of endometrial ablation in patients was also lower 0.88(3) compared to controls 3.12(11), P=0.036, as shown in the table.
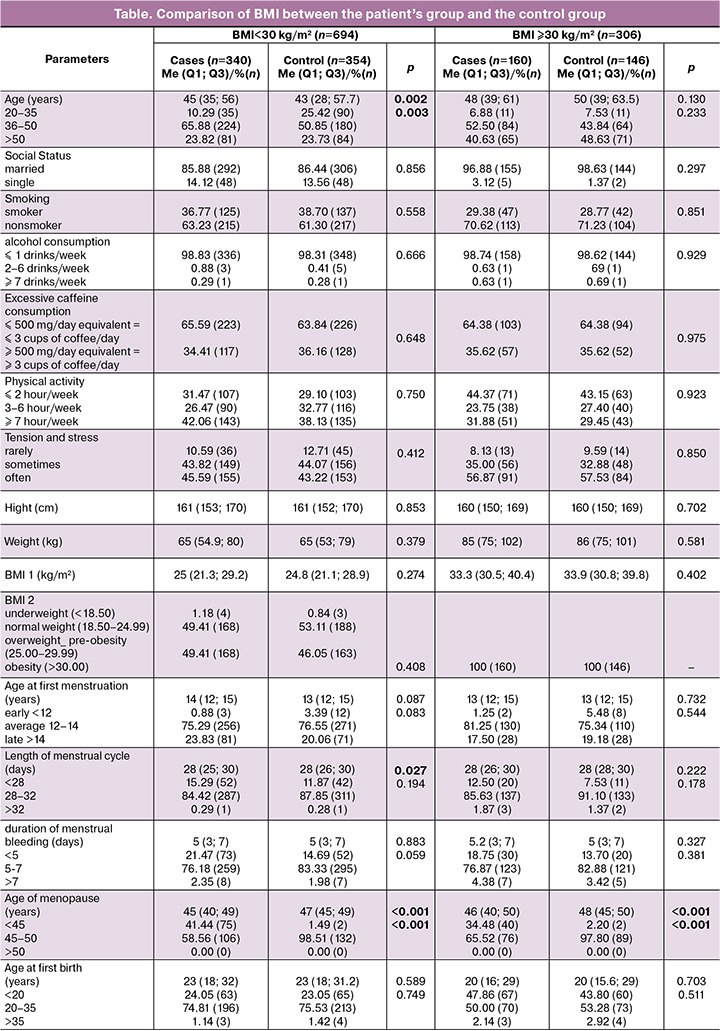


In investigating the clinical characteristics and risk factors of UFs in Syrian women within the obese group (BMI≥30), the results showed that patients, compared to controls, had an earlier age at menopause (46 (40; 50) years and 48 (45; 50) years, respectively, P<0.001). Also, the time elapsed since the first birth showed that the percentage of patients compared to controls was lower in both categories: ≤5 years (10.80 (15) and 12.50 (17), respectively) and 11–15 years (21.86 (29) and 49.26 (67), respectively), and higher in the following categories: 6–10 years (11.51 (16) and 9.56 (13), respectively), 16–19 years (20.14 (28) and 2.21 (3), respectively), and ≥20 years (36.69 (51) and 26.47 (36), respectively, P=0.013), as shown in the table.
By comparing the two studied groups (BMI<30 and BMI≥30), it was concluded that the age of menopause was earlier in both groups in the patients compared to the controls. As for the time elapsed since the last birth, it was similar within the obese and non-obese groups. The percentage of patients was lower than in the controls in the categories ≤5 years and 11–15 years, while within the categories 6–10 years, 16–19 years, and ≥20 years, it was higher. Whereas it showed that only within the non-obese group (BMI<30), patients compared to controls, had an older age, a shorter menstrual cycle length, and a lower rate of endometrial ablation.
Discussion
Based on the results of this study, it was concluded that there was statistical significance for a number of parameters specific to the non-obese group (BMI<30), which found that patients, compared to controls, had an older age (45 (35; 56) years and 43 (28; 57.7) years, respectively, P=0.002), where the greatest percentage was within the age group 36-50 years (65.88(224) and 50.85(180), respectively P=0.003). These results are consistent with the results of a study conducted by Turner B.M. et al., who suggested that age linked to an increase in the prevalence of leiomyomas, with a rapid increase noted in leiomyomas diagnosed among women in their 40s [19].
It also agreed with the findings of another study conducted on Saudi women by Moawad R. et al.[16], as well as with the outcomes of other studies conducted in the United States and Belgium, which have suggested that 60–70% of women have one or more UFs by the age of 50 [20], owing to the accumulation of hormonal alterations that occur over the reproductive lifetime in women. Their reliance on the ovarian steroids progesterone and estrogen is a noteworthy characteristic of UFs [21]. It has been suggested by experimental and clinical research that progesterone and estrogen promote the development of UFs during the reproductive years [16, 21].
Menstrual cycle length showed significant differences, shorter in patients compared to controls (28 (25; 30) days and 28 (26; 30) days, respectively, P=0.027). The relationship between menstrual cycle patterns and the risk of UFs is still unclear. However, a study conducted on Korean women revealed results consistent with our study, as women with long menstrual cycles were less susceptible to developing UF. These results are biologically reasonable due to the decrease in the length of the menstrual cycle with the arrival of menopause, which is accompanied by a shortened length of the follicular phase [22]. In addition, research on female nurses was conducted in the US and Japan. In the NHS II, longer and/or irregular cycles were substantially linked to a decreased incidence of UL [23], and the Japan Nurses' Health Study found that the risks of UL were negatively correlated with lengthy cycle duration at ages 18–22 [24].
While in the obese group (BMI≥30), no statistical significance was observed for any specific parameters.
By comparing the two studied groups, obese (BMI≥30) and non-obese (BMI<30), our findings demonstrated a statistically significant link between menopause age and UFs in both groups, with menopause occurring later in those aged 45–50 years than those under 45, where the percentage of women with UFs who had menopause between 45 and 50 years old was higher compared to those under the age of 45. The main reason for menopause was the fact that these women had either a myomectomy, where the percentage reached 95/160 (59.37%) among the obese group compared to the non-obese group 145/340 (42.65%), or a hysterectomy, where the percentage reached 63/160 (39.37%) among the obese group compared to the non-obese group 87/340 (25.59%), which is logical, as menopause occurs after that. Because female gonadal steroid hormones play a part in promoting UF growth, the premenopausal state was linked to a noticeably greater risk of UF than the postmenopausal state. However, because they do not have menstruation-related symptoms, postmenopausal women may underreport UFs [25]. Another study by Ponomarenko M.S. et al. [26] suggested that late menopause prolongs lifelong exposure to estrogens, an effect similar to that of early menarche, which results in stimulation of UF growth and promote EH [27, 28].
In terms of reproductive health, the time elapsed since the last birth was statistically significant, with most UF patients having intervals of more than 5 years, especially ≥20 years. This is in line with the results of the Stuart E.A. et al. study, which showed that in both the black (the Black Women’s Health Study [100% black women]) and white (the Nurses’ Health Study II [1% black women]) populations, the risk of developing UFs was roughly 2–3 times higher for women who had given birth five years or more ago than for those who had given birth more recently [25]. Furthermore, Nwonuma C. et al. revealed that women who have had pregnancies more recently are at a lower risk of developing fibroids compared to those who have had pregnancies in the distant past [29], and a 20–50% lower UF risk in women with previous live births than nulliparous women [30].
Conclusion
This study demonstrated the modifying effect of obesity on clinical manifestations of UF in Syrian women. That will provide additional clarification of the impact of obesity on the risk/prevalence of UF, which will be beneficial for both its prevention and diagnosis.
References
- Алали О.М., Чурносов М.И. Полногеномные исследования миомы матки. Акушерство и гинекология. 2023; 7: 28-38. [Alali O.M., Churnosov M.I. Genome-wide studies of uterine leiomyomas. Obstetrics and Gynecology. 2023; (7): 28-38 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.156
- Ponomarenko I., Reshetnikov E., Polonikov A., Verzilina I., Sorokina I., Yermachenko A. et al. Candidate genes for age at menarche are associated with uterine leiomyoma. Front. Genet. 2021; 11: 512940. https://dx.doi.org/10.3389/fgene.2020.512940
- Alsudairi H.N., Alrasheed A.T., Dvornyk V. Estrogens and uterine fibroids: an integrated view. Research results in biomedicine. 2021; 7(2): 156-63. https://dx.doi.org/10.18413/2658-6533-2021-7-2-0-6
- Harmon Q.E., Patchel S., Denslow S., Wegienka G., Baird D.D. Body mass index and uterine fibroid development: a prospective study. J. Clin. Endocrinol. Metab. 2024; 109(11): e2016-23. https://dx.doi.org/10.1210/clinem/dgae036
- Qin H., Lin Z., Vásquez E., Luan X., Guo F., Xu L. Association between obesity and the risk of uterine fibroids: a systematic review and meta-analysis. J. Epidemiol. Community Health. 2021; 75(2): 197-204. https://dx.doi.org/10.1136/jech-2019-213364
- Maghraby N., El Noweihi A.M., El-Melegy N.T., Mostafa N.A., Abbas A.M., El-Deek H.E. et al. Increased expression of fibroblast activation protein is associated with autophagy dysregulation and oxidative stress in obese women with uterine fibroids. Reprod. Sci. 2022; 29(2): 448-59. https://dx.doi.org/10.1007/s43032-021-00810-0
- Алали О.М., Чурносов М.И. Этиопатогенез миомы матки (обзор). Гинекология. 2023; 25(1): 22-30. [Alali O.M., Churnosov M.I. The etiopathogenesis of uterine leiomyomas: a review. Gynecology. 2023; 25(1): 22-30. (in English)]. https://dx.doi.org/10.26442/20795696.2023.1.201827
- Sparic R., Mirkovic L., Malvasi A., Tinelli A. Epidemiology of uterine myomas: a review. Int. J. Fertil. Steril. 2016; 9(4): 424-35. https://dx.doi.org/10.22074/ijfs.2015.4599
- Sharami S.H., Fallah Arzpeyma S., Shakiba M., Montazeri S., Milani F., Kazemi S. et al. Relationship of uterine fibroids with lipid profile, anthropometric characteristics, subcutaneous and preperitoneal fat thickness. Arch. Iran. Med. 2019; 22(12): 716-21.
- Pan H., Qin F., Deng F. Clinical value of body mass index and waist-hip ratio in clinicopathological characteristics and prognosis of uterine leiomyomata. Evid. Based Complemen. Alternat. Med. 2021; 2021: 8156288. https://dx.doi.org/10.1155/2021/8156288
- Manta L., Suciu N., Toader O., Purcărea R.M., Constantin A., Popa F. The etiopathogenesis of uterine fibromatosis. J. Med. Life. 2016; 9(1): 39-45.
- Gürbüz T., Yardımcı O., Udum S., Günay T. The relationship between body mass index and clinical complications among patients undergoing myomectomy. Journal of Surgery and Medicine. 2020; 4(11): 1027-30. https://dx.doi.org/10.28982/josam.805122
- Lee J.E., Song S., Cho E., Jang H.J., Jung H., Lee H.Y. et al. Weight change and risk of uterine leiomyomas: Korea Nurses’ Health Study. Curr. Med. Res. Opin. 2018; 34(11): 1913-9. https://dx.doi.org/10.1080/03007995.2018.1462783
- Haan Y.C., Diemer F.S., Van der Woude L., Van Montfrans G.A., Oehlers G.P., Brewster L.M. The risk of hypertension and cardiovascular disease in women with uterine fibroids. J. Clin. Hypertens (Greenwich). 2018; 20(4): 718-26. https://dx.doi.org/10.1111/jch.13253
- Sun K., Xie Y., Zhao N., Li Z. A case‑control study of the relationship between visceral fat and development of uterine fibroids. Exp. Ther. Med. 2019; 18(1): 404-10. https://dx.doi.org/10.3892/etm.2019.7575
- Muawad R., Dabbagh R., Sabr Y. Association of health and lifestyle factors with uterine fibroids among Saudi women: a case–control study. J. Taibah Univ. Med. Sci. 2022; 17(6): 1039-46. https://dx.doi.org/10.1016/j.jtumed.2022.06.005
- Ciebiera M., Włodarczyk M., Słabuszewska-Jóźwiak A., Nowicka G., Jakiel G. Influence of vitamin D and transforming growth factor β3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil. Steril. 2016; 106(7): 1787-92. https://dx.doi.org/10.1016/j.fertnstert.2016.09.007
- Keizer A.L., Semmler A., Kok H.S., van Kesteren P.J.M., Huirne J.A.F., Hehenkamp W.J.K. Modifiable prognostic factors in uterine fibroid development: a systematic review of literature. J. Obstet. Gynaecol. 2024; 44(1): 2288225. https://dx.doi.org/10.1080/01443615.2023.2288225
- Turner B.M., Cramer S.F., Heller D.S., Tavassoli F.A. Apoplectic leiomyomas: does ethnicity make a difference? a clinicopathologic study. Virchows Arch. 2022; 480(3): 645-54. https://dx.doi.org/10.1007/s00428-021-03225-z
- Donnez J., Dolmans M.M. Uterine fibroid management: from the present to the future. Hum. Reprod. Update. 2016; 22(6): 665-86. https://dx.doi.org/10.1093/humupd/dmw023
- Wise L.A., Laughlin-Tommaso S.K. Epidemiology of uterine fibroids: from menarche to menopause. Clin. Obstet. Gynecol. 2016; 59(1): 2-24. https://dx.doi.org/10.1097/GRF.0000000000000164
- Song S., Park S., Song B.M., Lee J.E., Cha C., Park H.Y. Risk of uterine leiomyomata with menstrual and reproductive factors in premenopausal women: Korea nurses’ health study. BMC Women's Health. 2023; 23(1): 305. https://dx.doi.org/10.1186/s12905-023-02447-4
- Terry K.L., De Vivo I., Hankinson S.E., Missmer S.A. Reproductive characteristics and risk of uterine leiomyomata. Fertil. Steril. 2010; 94(7): 2703-7. https://dx.doi.org/10.1016/j.fertnstert.2010.04.065
- Yasui T., Hayashi K., Okano H., Kamio M., Mizunuma H., Kubota T. et al. Uterine leiomyomata: a retrospective study of correlations with hypertension and diabetes mellitus from the Japan Nurses’ Health Study. J. Obstet. Gynaecol. 2018; 38(8): 1128-34. https://dx.doi.org/10.1080/01443615.2018.1451987
- Stewart E.A., Cookson C.L., Gandolfo R.A., Schulze‐Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017; 124(10): 1501-12. https://dx.doi.org/10.1111/1471-0528.14640
- Ponomarenko M.S., Reshetnikov E.A., Churnosova M.M., Reshetnikova Y.N., Churnosov V.I., Ponomarenko I.V. Comorbidity and syntropy of benign proliferative diseases of the female reproductive system: non-genetic, genetic, and epigenetic factors (review). Research Results in Biomedicine. 2023; 9(4): 544-56. https://dx.doi.org/10.18413/2658- 6533-2023-9-4-0-9
- Пономарева Т.А. Генетические варианты глобулина, связывающего половые гормоны, и гормональный профиль больных генитальным эндометриозом. Научные результаты биомедицинских исследований. 2025; 11(1): 75-90. [Ponomareva T.A. Genetic variants of sex hormone-binding globulin and hormonal profile in patients with genital endometriosis. Research Results in Biomedicine. 2025; 11(1): 75-90 (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2025- 11-1-0-4
- Чурносов В.И. Ассоциации полиморфных локусов генов-кандидатов с уровнем половых гормонов у больных гиперплазией эндометрия. Научные результаты биомедицинских исследований. 2025; 11(2): 243-62. [Churnosov V.I. Associations of polymorphic loci of candidate genes with the level of sex hormones in patients with endometrial hyperplasia. Research Results in Biomedicine. 2025; 11(2): 243-62 (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2025-11-2-0-3
- Nwonuma C., Irokanulo E., Bamgboye F., Akinduko A., Okeniyi F., Eigbe C. Uterine fibroid: risk factors and therapeutic interventions. In: 2024 International Conference on science, engineering and business for driving sustainable development goals (SEB4SDG), 2024 Apr 2. Omu-Aran, Nigeria: IEEE, 2024: 1-10. https://dx.doi.org/10.1109/SEB4SDG60871.2024.10630388
- Ahmed A., Sharif M.A. Prevalence and clinical characteristics of uterine fibroids in women of reproductive age in district hyderabad. The Research of Medical Science Review. 2025; 3(1): 635-47.
Received 15.07.2025
Accepted 15.10.2025
About the Authors
Ola Mohamad Alali, PhD student, Homs, Syria, Belgorod State National Research University, Belgorod, Russia, alali@bsuedu.ru, https://orcid.org/0000-0003-4370-6719Mikhail I. Churnosov, Dr. Med. Sci., Professor, Belgorod State National Research University, Belgorod, Russia, churnosov@bsuedu.ru, https://orcid.org/0000-0003-1254-6134



