Experience of myo-inositol use in patients with oligomenorrhea and insulin resistance
Spiridonova N.V., Popova E.I., Levin V.A., Deviatov I.M.
Oligomenorrhea (OM) is a menstrual disorder that can be associated with various causes including disorders of carbohydrate and fat metabolism. One of the manifestations of impaired carbohydrate metabolism is insulin resistance (IR) which leads to hyperinsulinemia. Excess insulin stimulates the adrenal glands and results in hyperandrogenism, which in turn reduces the sensitivity of estrogen-dependent ovarian receptors to insulin. This condition is further complicated by polycystic ovary syndrome.
Objective: To study the effect of administration of myo-inositol (MI) in combination with alpha-lactalbumin (α-LA) on the length of the menstrual cycle and the rate of carbohydrate metabolism in women with OM and IR.
Materials and methods: An observational study included 30 reproductive-aged patients of with OM and IR, who were administered a three-month course of treatment with the Inofert Forte complex containing MI 600 mg + α-LA + folic acid 200 mcg. The patients were evaluated for the clinical, anamnestic and anthropometric data, bioimpedance analysis of body composition, and the results of an oral glucose tolerance test (OGTT) with insulin before and after the course of therapy.
Results: After the course of treatment with a combination of MI + α-LA, the length of the menstrual cycle became normal in 13/22 (59.1%) patients and was 34.2 (1.0) days in these women. The average length of the menstrual cycle was 38.4 (1.9) days (p<0.001). A decrease in fasting glucose levels was found in 16/30 (53.3%) patients, in 20/30 (66.7%) patients after one hour and in 30/30 (100%) patients after two hours (Δ0.66±0.35, p<0.001). A decrease in fasting insulin levels was noted in 19/30 (63.3%) of the examined patients two hours after OGTT (p<0.04).
Conclusion: The addition of the complex containing MI 600 mg + α-LA + folic acid 200 mcg (Inofert Forte) to the therapy of patients with OM and IR may have a positive effect on the menstrual cycle and may improve metabolic parameters.
Authors' contributions: Spiridonova N.V. – developing the concept and design of the study; Spiridonova N.V., Popova E.I. – collecting and processing the material; Popova E.I., Levin V.A. – statistical processing of the data; Popova E.I., Deviatov I.M. – writing the text; Spiridonova N.V., Levin V.A. – editing the article.
Conflicts of interest: The authors declare no possible conflicts of interest.
Funding: The study was conducted without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of the Samara State Medical University, Ministry of Health of Russia.
Patient Consent for Publication: The patients signed informed consent for the publication of their anonymized data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Spiridonova N.V., Popova E.I., Levin V.A., Deviatov I.M.
Experience of myo-inositol use in patients with oligomenorrhea and insulin resistance.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (8): 179-186 (in Russian)
https://dx.doi.org/10.18565/aig.2025.139
Keywords
Menstrual dysfunction remains a topical issue in modern gynecology. Oligomenorrhea in women of reproductive age is characterized by an increase in cycle length to more than 38 days or a decrease in the number of menstrual periods to less than 10 per year and can be associated with a variety of causes [1]. One of them is a violation of carbohydrate metabolism. In particular, it is associated with a change in the susceptibility of cells to the effects of insulin, leading to a state of insulin resistance and hyperinsulinemia [2].
The relationship between hyperinsulinemia and increased menstrual cycle length is regulated at two levels – central and peripheral. The central influence is based on increased secretion of luteinizing hormone, coupled with a relative decrease in follicle-stimulating hormone (FSH) production. Peripheral mechanisms are more extensive: a decrease in the level of sex hormone-binding globulin and insulin-like growth factor-1, which act as transport molecules, as well as stimulation of androgen secretion by the adrenal glands under the influence of insulin. Together, these mechanisms lead to the production of excess androgens and subsequent follicular atresia [3]. According to literature data, healthy women with sufficient estrogen levels are less susceptible to insulin resistance, as estrogens activate a large number of estrogen-dependent insulin receptors in all body tissues [4]. When a hormonal imbalance occurs towards the androgen increase, the following chain of reactions is observed: the relative predominance of the amount of androgens causes sensitivity decrease of estrogen-dependent receptors to insulin in tissue cells, provoking the development of pathological hyperinsulinemia, leading to the accumulation of visceral fat and excessive production of androgen. Estrogen deficiency triggers increased insulin levels, perpetuating the vicious cycle of insulin resistance. Weight gain and increased body fat can also contribute to disruptions not only in the duration of the menstrual cycle but also in reproductive function in general [5]. Obesity can become the cause of changes in the function of not only the sex glands, but also the functioning of the diencephalic brain structures, resulting in hormonal imbalance and hyperandrogenic anovulation, which are difficult to reverse. The predominance of visceral fat is of high importance, since it is more relevant to consider the presence of visceral fat and the proportion of adipose body tissue as a marker of metabolic changes, rather than the body mass index (BMI). There is evidence that women with visceral obesity, even with normal BMI, showed a significant increase in low-density lipoproteins (LDL) and a decrease in high-density lipoproteins (HDL), which in turn indicates an imbalance in fat metabolism [6]. Polycystic ovary syndrome (PCOS) is a risk factor for the development of infertility, androgen-dependent dermatopathy (acne, hirsutism, alopecia), carbohydrate metabolism disorders (impaired glucose tolerance, diabetes mellitus (DM) type 2), dyslipidemia, hyperandrogenism, cardiovascular diseases, endometrial hyperplasia, mood disorders, and cancer. As a result of PCOS in the mother during the period of intrauterine fetus development, infancy and adolescence, excess in androgens lead to the formation of obesity of the abdominal and visceral type, and at a later age - to the insulin resistance and hyperinsulinemia, which subsequently completes the vicious circle of insulin resistance ("hyperinsulinemia – hyperandrogenism – PCOS") at the epigenetic level.
For the diagnosis of disorders and the formation of risk groups for the development of metabolic syndrome, one of the relevant methods is bioimpedance analysis (BIA) of body composition, which is based on the calculation of the difference in electrical resistance of body tissues. BIA is an informative and minimally invasive method that can be used as a criterion for the drug therapy effectiveness [7]. BIA provides an accurate value for the amount of fat mass, total body fluid, mineral content, and other parameters that can serve as markers of chronic and acute diseases [8]. According to some authors, determining the parameters of women's body composition, and especially the proportion of fat mass, can be used to predict the risk of cardiovascular diseases: a correlation has been noted between an increase in BMI and the development of metabolic syndrome [9].
One approach to treating menstrual irregularities is the use of medications containing inositols. Inositols are hexahydric sugar alcohols with several stereoisomers that act as second messengers for hormones such as insulin and FSH. Inositols play a significant role in carbohydrate metabolism and follicle maturation. Possessing insulinomimetic properties, myo-inositol (MI) enhances glucose uptake into cells by stimulating the translocation of the GLUT4 transporter protein to the cell membrane, causing a postprandial decrease in glucose levels, which may reduce the risk of developing type 2 diabetes [10, 11]. In addition, MI has a stimulating effect on the expression of the ovarian aromatase gene, preventing the synthesis of androgens from cholesterol in theca cells [12]. Despite the positive effect of MI on insulin resistance, there is a phenomenon of resistance of the body to the molecules of MI itself, caused by absorption defects in the digestive tract. To overcome this problem, alpha-lactalbumin (α-LA) is used, which has a beneficial effect on absorption in the intestinal tract, preventing the development of inflammatory reactions [13,14]. Currently, a promising therapy for the correction of menstrual cycle disorders in patients with PCOS and carbohydrate and lipid metabolism disorders is the administration of MI in combination with α-LA – Inofert Forte.
Aim of study: to study the effect of the use of MI in combination with α-LA on the duration of the menstrual cycle and the state of carbohydrate metabolism in women with oligomenorrhea and insulin resistance.
Materials and methods
30 patients were included into the study. Inclusion criteria: aged 18–45 years and the presence of oligomenorrhea, insulin resistance, and PCOS. Exclusion criteria: use of combined hormonal contraceptives, metformin therapy, insulin therapy, and the presence of severe somatic diseases.
The study was conducted at the Department of Obstetrics and Gynecology of the Samara State Medical University, Ministry of Health of Russia (Head of the Department – Dr. Med. Sci., Prof. N.V. Spiridonova) in the antenatal clinic of Samara City Hospital No. 1 during the period from January 01, 2024 to December 31, 2024. The study was conducted on an outpatient basis; patients were to visit the doctor at least three times. At the first visit, patients underwent a general examination, including calculation of BMI and waist-to-hip ratio (WHR). Laboratory tests included an extended oral glucose tolerance test (OGTT) measuring fasting glucose and insulin levels, 1 hour after exercise, and 2 hours after exercise. All patients underwent a baseline body composition analysis using the BIA MEDASS device (STC MEDASS, Russia) based on a standardized panel of two sensors with computerized data processing.
After the examination, the patients were prescribed a complex of MI 600 mg + α-LA + folic acid 200 mcg (Inofert Forte), 1 capsule 2 times a day, orally, for a course of 3 months. Recommendations to modify the lifestyle were also given: eating according to the Harvard’s Healthy Eating Plate principle, keeping food diaries, increasing physical activity to 8,000–10,000 steps per day. After 1 and 2 months of treatment, doctors from the women's clinic of the Samara City Hospital No. 1 provided telemedicine consultations to support patients' motivation regarding lifestyle modification and compliance monitoring. 3 months later, the patients came for a follow-up visit, where they underwent a repeat examination with biometric measurements, BIA, an extended OGTT with insulin, and a survey on therapy tolerability. 10 patients refused a repeat OGTT.
Statistical analysis
Mathematical processing of the results was carried out using the statistical package SPSS21, license number 20130626-3 (An IBM Company; USA) and Microsoft Excel (Microsoft; USA). The normality of the distribution was calculated using the Shapiro–Wilk test. If p<0.05, the distribution is abnormal (since the differences between the studied distribution and the normal distribution are statistically significant); if p>0.05, the distribution is normal (since the differences between the studied distribution and the normal distribution are insignificant). Normally distributed quantitative parameters were described using the arithmetic mean (M) and standard deviation (SD). Categorical data were presented using absolute values (n) and percentages (%). Comparison of related populations (before-after analysis) in a two-stage study and the mean difference were calculated using the paired Student's t-test (a parametric statistical criterion). The obtained values were considered statistically significant at p<0.05.
Results
The average age of the examined women patients was 28.52 (7.78) years. The age of menarche was 13.13 (1.42) years, respectively. 8 out of 30 (26.7%) patients had a regular menstrual cycle, and 22 out of 30 (73.3%) had amenorrhea. The average duration of amenorrhea before treatment was 87.5 (6.9) days. Infertility (primary and secondary in equal proportions) was diagnosed in 2/30 (6.7%) patients, and 4/30 (13.3%) were examined by an endocrinologist for hyperprolactinemia. All patients also had clinical manifestations of insulin resistance in the form of Acanthosis nigricans.
Following a course of treatment with the combination of MI+α-LA (Inofert Forte), the duration of the menstrual cycle normalized in 13/22 (59.1%) women and accounted for 34.2 (1.0) days. The average duration of the menstrual cycle was 38.4 (1.9) days (p<0.001) (Table 1).
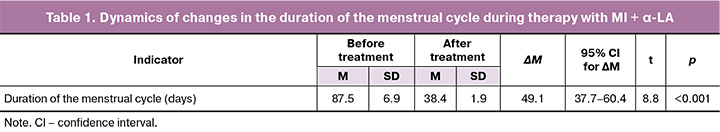
Anthropometric parameters indicated the presence of metabolic disorders (BMI=30.57 (6.38) kg/m2), with 3/30 (10.0%) patients having grade 3 obesity. The average waist circumference (WC) value at the beginning of treatment was 93.25 (13.68) cm, hip circumference (HC) – 113.66 (9.65) cm, WHC ratio comprised 0.81 (0.07).
According to the results of the extended insulin OGTT after treatment, the following dynamics were observed: in 16/30 (53.3%) patients, a decrease in fasting glucose levels was noted (Δ0.03 (0.21)), in 20/30 (66.7%) there was a decrease in glucose levels 1 hour after load (Δ0.41 (1.19)). In all patients glucose levels significantly decreased 2 hours after load (Δ0.66 (0.35), p<0.001) (Table 2).
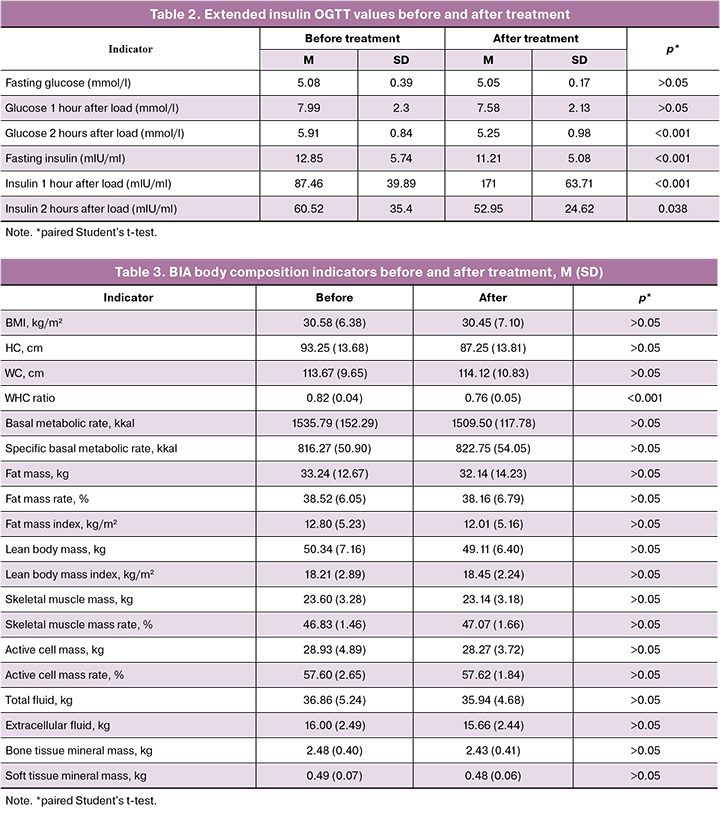
In 19 (63.3%) of the study patients, a decrease in fasting insulin levels and 2 hours after the OGTT was observed (Δ1.64 (1.42) and Δ7.57 (19.06), respectively). One hour after the test all patients showcased an increase in the postprandial hyperinsulinemia (Δ83.54 (23.82), p<0.001) (Figure), which reflects the nature of the insulin resistance phenomenon and proves its presence in the study participants.
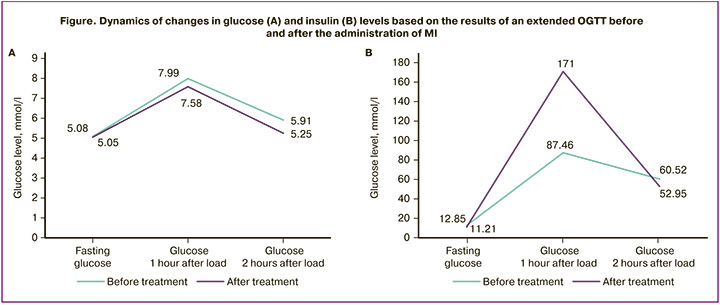
A decrease in BMI was observed during body composition analysis, likely indicating a positive effect of the MI+α-LA (Inofert Forte) complex on both carbohydrate and fat metabolism. Some of the BMI reduction may be due to visceral fat loss rather than fluid and/or mineral losses, which presumes the impact of MI catabolic nature. However, despite the recommendations given to each patient on the need to increase physical activity, the results of the BIA clearly demonstrate that the indicators correlating with the level of physical activity after 3 months did not change. It means that the patients did not enhanced their physical activity (Table 3).
At the third follow-up visit, all patients reported no side effects concerning the treatment.
Discussion
The results of our study showed that 13/22 (59.1%) patients experienced normalization of the menstrual cycle, which is consistent with the results of the studies by Greff D. et al. (2023) and Efendieva R.M. et al. (2025) [15, 16].
The data obtained by Gudović A. et al. (2024) and Kamenov Z. et al. (2023) prove the effectiveness of using a therapy complex containing MI and α-LA (Inofert Forte) in the treatment of patients with PCOS, oligomenorrhea and insulin resistance [17, 18]. Changes in carbohydrate metabolism after the course of treatment most likely indicate enhanced compensatory responses. The most significant decrease in glucose levels was observed 2 hours after exercise. The true decline in glucose levels demonstrated after the course of MI treatment coincides with the findings of Pintaudi B. et al. (2018) [19]. Considering the fact that the study participants initially did not have severe carbohydrate metabolism disorders, the results of our study confirm that the MI+α-LA complex can be used for prevention of type 2 diabetes, as well as gestational diabetes, which is confirmed by Chulkov V.S. et al. (2018) [20].
The effect of increasing postprandial hyperinsulinemia can be regarded as a sign of improved tissue sensitivity to insulin. Thus, Ametov A.S. (2007), when analyzing insulin curves during OGTT, compared it in insulin resistance and type 2 diabetes and noted that an important indicator of the body's ability to compensate is the duration of peak insulin values over time [21]. We obsserved a similar situation, when after the treatment the level of postprandial insulin became higher than the initial one, but the rate of the level decline also increased, which indicates a positive effect in the combination of MI and α-LA and the need to extend the duration of the course of treatment with repeated measurements of insulin levels.
In the study by Muraca E. et al. (2020), which included patients with infertility and hypothyroidism, BIA served as a criterion for the effectiveness of hypothyroidism therapy. The author concluded that BIA can be a criterion for assessing disorders in women with gynecological and endocrine diseases, and can also be used to decide on changing hormonal therapy [22]. The results of the study by Cheng K. et al. (2021) demonstrated that obese female patients had an increase in the amount of total and extracellular fluid in the body, along with the initial signs of sarcopenia in BIA [23]. Kurmaev D.P. et al. (2022) conducted BIA in 129 patients with type 2 diabetes. Based on the study results, the authors concluded that BIA can be a safe and accurate method to assess the reduction in the proportion of skeletal muscle mass and obesity [24]. The BIA data obtained in our study indicates a positive effect of the MI+α-LA complex (Inofert Forte) on body parameters with a significant decrease in the WHC ratio and with the absence of an increase in physical activity. Dobrokhotova Yu.E. et al. (2020) concluded that the simultaneous use of combined hormonal contraceptives and drugs containing MI led to a decrease in total cholesterol, triglycerides, LDL, and an increase in HDL, which demonstrates a positive effect [25]. The decrease in the WHC ratio in our study indicates a change in the nature of adipose tissue deposits from predominantly visceral to gynoid, which, according to studies by Dolgikh Yu.A. et al. (2024) and Khamoshina M.B. et al. (2020), can catalyze fat metabolism [26, 27].
Conclusion
Given the positive effect on the menstrual cycle and metabolic parameters in patients with oligomenorrhea and insulin resistance, the administration of a combination of MI + α-LA (Inofert Forte) can be considered as one of the promising components of the complex treatment of menstrual dysfunction and metabolic syndrome.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Аменорея и олигоменорея. М.; 2024. 62 с. [Ministry of Health of the Russian Federation. Clinical guidelines. Amenorrhea and oligomenorrhea. Moscow; 2024. 62 p. (in Russian)].
- Dupont J., Scaramuzzi R.J. Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle. Biochem. J. 2016; 473(11): 1483-501. https://dx.doi.org/10.1042/BCJ20160124
- Титова М.С., Колодина М.И., Ляшенко А.С., Ляшенко Е.Н. Применение мио-инозитола у женщин с синдромом поликистозных яичников при вспомогательных репродуктивных технологиях. Медицинский совет. 2022; 16(16): 50-6. [Titova M.S., Kolodina M.I., Lyashenko A.S., Lyashenko E.N. Use of myo-inositol in women with polycystic ovary syndrome in the application of auxiliary reproductive technologies. Meditsinskiy Sovet. 2022; 16(16): 50-6 (in Russian)]. https://dx.doi.org/10.21518/2079-701X-2022-16-16-50-56
- Li M., Chi X., Wang Y., Setrerrahmane S., Xie W., Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022; 7(1): 216. https://dx.doi.org/10.1038/s41392-022-01073-0
- Аганезова Н.В., Аганезов С.С. Ожирение и репродуктивное здоровье женщины. Акушерство и гинекология. 2016; 6: 18-25. [Aganezova N.V., Aganezov S.S. Obesity and women's reproductive health. Obstetrics and Gynecology. 2016; (6): 18-25 (in Russian)]. https://dx.doi.org/10.18565/aig.2016.6.18-25
- Anagnostis P., Tarlatzis B.C., Kauffman R.P. Polycystic ovarian syndrome (PCOS): long-term metabolic consequences. Metabolism. 2018; 86: 33-43. https://dx.doi.org/10.1016/j.metabol.2017.09.016
- Оксюта В.Н. Исследование биоимпедансных показателей у женщин с бесплодием при гипотиреозе. Репродуктивное здоровье. Восточная Европа. 2013; 6(30): 81-7. [Oksyuta V.N. Study of bioimpedance parameters in women with infertility and hypothyroidism. Reproductive health. Eastern Europe. 2013; 6(30): 81-7 (in Russian)].
- Николаев Д.В., Щелыкалина С.П. Биоимпедансный анализ состава тела человека: медицинское применение, терминология. Клиническое питание и метаболизм. 2021; 2(2): 80-91. [Nikolaev D.V., Shchelykalina S.P. Bioimpedance analysis of human body composition: medical applications, terminology. Clinical nutrition and metabolism. 2021; 2(2): 80-91 (in Russian)]. https://dx.doi.org/10.17816/clinutr72132
- Дадаева В.А., Еганян Р.А., Розанов В.Б., Елиашевич С.О., Громова А.В., Котова М.Б., Иванова Е.И., Драпкина О.М. Особенности компонентного состава тела, физического и психического здоровья женщин с избыточным весом. Профилактическая медицина. 2022; 25(9): 60-9. [Dadaeva V.A., Eganyan R.A., Rozanov V.B., Eliashevich S.O., Gromova A.V., Kotova M.B., Ivanova E.I., Drapkina O.M. Body composition, physical and mental health of overweight females. Russian Journal of Preventive Medicine. 2022; 25(9): 60-9 (in Russian)]. https://dx.doi.org/10.17116/profmed20222509160
- Турчинец А.И., Уварова Е.В., Хащенко Е.П., Кумыкова З.Х. Таргетная терапия синдрома поликистозных яичников. Медицинский совет. 2023; 17(5): 7-13. [Turchinets A.I., Uvarova E.V., Khashchenko E.P., Kumykova Z.Kh. Target therapy of polycystic ovary syndrome. Meditsinskiy Sovet. 2023; 17(5): 7-13 (in Russian)]. https://dx.doi.org/10.21518/ms2023-060
- Пустотина О.А. Инозитол и липоевая кислота в лечении инсулинорезистентности у женщин с синдромом поликистозных яичников. Акушерство и гинекология. 2020; 12: 209-16. [Pustotina O.A. Inositol and lipoic acid in the treatment of insulin resistance in women with polycystic ovary syndrome. Obstetrics and Gynecology. 2020; (12): 209-16 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.209-216
- Чернуха Г.Е., Пронина В.А. Коррекция метаболической дисфункции как метод восстановления функции репродуктивной системы у женщин. Медицинский совет. 2023; 17(5): 90-7. [Chernukha G.E., Pronina V.A. Metabolic dysfunction correction as a method of restoring the function of the reproductive system in women. Meditsinskiy Sovet. 2023; 17(5): 90-7 (in Russian)]. https://dx.doi.org/10.21518/ms2023-087
- Monastra G., Sambuy Y., Ferruzza S., Ferrari D., Ranaldi G. Alpha-lactalbumin effect on myo-inositol intestinal absorption: in vivo and in vitro. Curr. Drug Deliv. 2018; 15(9): 1305-11. https://dx.doi.org/10.2174/1567201815666180509102641
- Montanino O.M., Buonomo G., Calcagno M., Unfer V. Effects of myo-inositol plus alpha-lactalbumin in myo-inositol-resistant PCOS women. J. Ovarian Res. 2018; 11(1): 38. https://dx.doi.org/10.1186/s13048-018-0411-2
- Greff D., Juhász A.E., Váncsa S., Váradi A., Sipos Z., Szinte J. et al. Inositol is an effective and safe treatment in polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2023; 21(1): 10. https://dx.doi.org/10.1186/s12958-023-01055-z
- Эфендиева Р.М., Дикке Г.Б., Абусуева З.А., Шилова Н.В. Опыт восстановления менструального цикла у пациенток с олиго-/аменореей и ожирением с помощью комплекса, содержащего миоинозитол и D-хироинозитол в соотношении 5:1, фолиевую кислоту и марганец. Акушерство и гинекология. 2025; 2: 110-20. [Efendieva R.M., Dikke G.B., Abusueva Z.A., Shilova N.V. Experience in restoring the menstrual cycle in patients with oligo-/amenorrhea and obesity using a complex containing myo-inositol and D-chiro-inositol in a 5:1 ratio, folic acid, and manganese. Obstetrics and Gynecology. 2025; (2): 110-20 (in Russian)]. https://dx.doi.org/10.18565/aig.2025.31
- Gudović A., Bukumirić Z., Milincic M., Pupovac M., Andjić M., Ivanovic K. et al. The comparative effects of myo-inositol and metformin therapy on the clinical and biochemical parameters of women of normal weight suffering from polycystic ovary syndrome. Biomedicines. 2024; 12(2): 349. https://dx.doi.org/10.3390/biomedicines12020349
- Kamenov Z., Gateva A., Dinicola S., Unfer V. Comparing the efficacy of myo-inositol plus α-lactalbumin vs. myo-inositol alone on reproductive and metabolic disturbances of polycystic ovary syndrome. Metabolites. 2023; 13(6): 717. https://dx.doi.org/10.3390/metabo13060717
- Рintaudi B., Di Vieste G., Corrado F., Lucisano G., Giunta L., D'Anna R. et al. Effects of myo-inositol on glucose variability in women with gestational diabetes. Eur. Rev. Med. Pharmacol. Sci. 2018; 22(19): 6567-72. https://dx.doi.org/10.26355/eurrev_201810_16073
- Чулков В.С., Гаврилова Е.С., Чулков В.С., Минина Е.Е. Репродуктивное здоровье и кардиометаболический риск. Сибирское медицинское обозрение. 2018; 4(112): 13-21. [Chulkov V.S., Gavrilova E.S., Chulkov V.S., Minina E.E. Reproductive health and cardiometabolic risk. Siberian Medical Review. 2018; 4(112): 13-21 (in Russian)]. https://dx.doi.org/10.20333/2500136-2018-4-13-21
- Аметов А.С. Секреция инсулина в норме и при сахарном диабете 2 типа. Сахарный диабет. 2007: 10(4): 11-6. [Ametov A.S. Insulin secretion in normal conditions and in type 2 diabetes. Diabetes mellitus. 2007; 10(4): 11-6 (in Russian)]. https://dx.doi.org/10.14341/2072-0351-5860
- Muraca E., Ciardullo S., Oltolini A., Zerbini F., Bianconi E., Perra S. et al. Resting energy expenditure in obese women with primary hypothyroidism and appropriate levothyroxine replacement therapy. J. Clin. Endocrinol. Metab. 2020; 105(4): dgaa097. https://dx.doi.org/10.1210/clinem/dgaa097
- Cheng K.Y., Chow S.K., Hung V.W., Wong C.H., Wong R.M., Tsang C.S. et al. Diagnosis of sarcopenia by evaluating skeletal muscle mass by adjusted bioimpedance analysis validated with dual-energy X-ray absorptiometry. J. Cachexia Sarcopenia Muscle. 2021; 12(6): 2163-73. https://dx.doi.org/10.1002/jcsm.12825
- Курмаев Д.П., Булгакова С.В., Тренева Е.В., Четверикова И.С., Косарева О.В., Шаронова Л.А., Долгих Ю.А., Мусиенко С.К. Биоимпедансный анализ состава тела женщин пожилого и старческого возраста с сахарным диабетом 2 типа. Современные проблемы здравоохранения и медицинской статистики. 2022; 5: 219-36. [Kurmaev D.P., Bulgakova S.V., Treneva E.V., Chetverikova I.S., Kosareva O.V., Sharonova L.A., Dolgikh Yu.A., Musienko S.K. Bioimpedance analysis of the body composition in elderly and old women with type 2 diabetes mellitus. Current problems of health care and medical statistics. 2022; (5): 219-36 (in Russian)]. https://dx.doi.org/10.24412/2312-2935-2022-5-219-236
- Доброхотова Ю.Э., Лапина И.А., Чирвон Т.Г., Таранов В.В. Новые возможности интегративной терапии пациенток с синдромом поликистозных яичников и нарушениями углеводного и липидного обмена. Результаты сравнительного исследования. РМЖ. Мать и дитя. 2020; 3(3): 169-73. [Dobrokhotova Yu.E., Lapina I.A., Chirvon T.G., Taranov V.V. New prospects of the integrative therapy for polycystic ovary syndrome in women with the disorders of carbohydrate and lipid metabolism: a comparative study. Russian Journal of Woman and Child Health. 2020; 3(3): 169-73 (in Russian)]. https://dx.doi.org/10.32364/2618-8430-2020-3-3-169-173
- Долгих Ю.А., Булгакова С.В., Шаронова Л.А., Тренева Е.В., Косарева О.В., Курмаев Д.П. Синдром поликистозных яичников и метаболический синдром: возможные пути коррекции метаболических нарушений. Экспериментальная и клиническая гастроэнтерология. 2024; (2): 5-14. [Dolgikh Yu.A., Bulgakova S.V., Sharonova L.A., Treneva E.V., Kosareva O.V., Kurmaev D.P. Polycystic ovary syndrome and metabolic syndrome: possible ways to correct metabolic disorders. Experimental and Clinical Gastroenterology. 2024; (2): 5-14 (in Russian)]. https://dx.doi.org/10.31146/1682-8658-ecg-222-2-5-14
- Хамошина М.Б., Епишкина-Минина А.А., Рябова В.А., Елагин И.Б. В петле метаболического риска. StatusPraesens. Гинекология, акушерство, бесплодный брак. 2020; 4(69): 35-41. [Khamoshina M.B., Epishkina-Minina A.A., Ryabova V.A., Elagin I.B. In the metabolic risk loop. StatusPraesens. Gynecology, obstetrics, infertile marriage. 2020; 4(69): 35-41 (in Russian)].
Received 27.05.2025
Accepted 25.07.2025
About the Authors
Nataliya V. Spiridonova, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, https://orcid.org/0000-0003-3928-3784Evgeniya I. Popova, applicant at the Department of Obstetrics and Gynecology, Samara State Medical University, Ministry of Health of Russia,
443099, Russia, Samara, Chapaevskaya str., 89, evgeniya1popova@gmail.com, https://orcid.org/0000-0002-7249-1721
Vitaly A. Levin, endocrinologist, Ornament Health AG, Lucerne, Switzerland, https://orcid.org/0009-0000-8758-0842
Ilia M. Deviatov, student at the Medical Institute, Patrice Lumumba Peoples’ Friendship University of Russia, 117198, Russia, Moscow, Miklukho-Maklaya str., 6,
https://orcid.org/0000-0002-6025-8148
Corresponding author: Evgeniya I. Popova, evgeniya1popova@gmail.com



