The BAIAP2L1 gene variant as a risk factor for the development of genital endometriosis in combination with endometrial hyperplasia
Ponomareva T.A., Altukhova O.B., Ponomarenko I.V., Churnosov M.I.
Objective: To investigate the association between single nucleotide variants (SNVs) and the occurrence of genital endometriosis combined with endometrial hyperplasia in the Russian population in the following genes: NC_000017.11 (SHBG):g.7618597G>T (rs12150660), NC_000001.11 (PRMT6):g.107003753A>G (rs17496332), NC_000007.14 (BAIAP2L1):g.98364050T>A (rs3779195), NC_000012.12 (SLCO1B1):g.21178615T>C (rs4149056), and NC_000015.10 (NR2F2):g.96165062T>C (rs8023580).
Materials and methods: GWAS-significant single nucleotide variants (SNVs) associated with sex hormone-binding globulin (SHBG) levels were genotyped in 286 patients with genital endometriosis, including 183 patients with both genital endometriosis and endometrial hyperplasia and 103 patients with isolated genital endometriosis (control group). Logistic regression was used to assess the association between GWAS-significant SNVs linked to SHBG levels and the development of genital endometriosis in combination with endometrial hyperplasia. Allelic, additive, recessive, and dominant models were applied using the gPLINK software.
Results: The A allele of the BAIAP2L1 (rs3779195) gene was identified as a risk factor for the development of endometriosis in combination with endometrial hyperplasia (OR=2.85; 95% CI 1.36–5.97; pperm=0.006 for the additive model; OR=1.91; 95% CI 1.15–3.17; pperm=0.014 for the allelic model; OR=2.72; 95% CI 1.26–5.88; pperm=0.012 for the dominant model). The rs3779195 locus is located in the intronic region of the BAIAP2L1 gene, characterizes DNA interaction with the Foxp1 transcription factor, and is in linkage disequilibrium with 20 SNV, four of which (rs6950023, rs6967728, rs77032872, and rs3779196) exhibit significant regulatory potential.
Conclusion: The A allele of BAIAP2L1 (rs3779195) is associated with an increased risk of genital endometriosis in combination with endometrial hyperplasia.
Authors' contributions: Ponomareva T.A. – conducting the study, summarizing the data, drafting of the manuscript; Ponomarenko I.V. – editing of the manuscript; Altukhova O.B. – conducting the study, conception and plan of the article; Churnosov M.I. – reviewing, final editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the St. Ioasaph Belgorod Regional Clinical Hospital.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Ponomareva T.A., Altukhova O.B., Ponomarenko I.V., Churnosov M.I.
The BAIAP2L1 gene variant as a risk factor for the development of genital endometriosis
in combination with endometrial hyperplasia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (7): 84-91 (in Russian)
https://dx.doi.org/10.18565/aig.2025.71
Keywords
Endometriosis is a chronic hormone-dependent neuroinflammatory gynecological disease characterized by the presence of tissue resembling the endometrium, located outside the uterine cavity [1, 2]. This condition affects approximately 10–15% of women of reproductive age [3], with the highest incidence occurring between the ages of 25 and 35 [4]. Notably, cases of endometriosis have been reported both before menarche and after menopause [3]. As a chronic disease, endometriosis poses not only medical challenges but also economic and social issues, often being associated with other uterine disorders [5, 6].
Hyperestrogenism is known to trigger various proliferative and invasive processes that underlie the development of conditions such as endometriosis, endometrial cancer, uterine fibroids, and endometrial hyperplasia [7–9]. The shared pathogenetic mechanisms and risk factors for these diseases increase the likelihood of their co-occurrence [10]. Literature indicates a high prevalence of endometrial hyperplasia (over 70%) among patients with endometriosis [11]. The development of both endometriosis and endometrial hyperplasia is multifactorial [12], influenced by anatomical, hormonal, immunological, environmental, epigenetic, and genetic factors [12–14]. Given the significant role of sex hormones in the etiopathogenesis of proliferative uterine diseases, the protein responsible for their transport and binding is of great importance [15]. Sex hormone-binding globulin (SHBG) is a plasma glycoprotein that regulates the levels of bioavailable sex hormones, thereby controlling their biological activity [16]. Genome-wide association studies (GWAS) have identified significant single-nucleotide variants (SNVs) associated with SHBG levels [17–19]. However, there is a lack of studies examining the genetic determinants of SHBG that contribute to the combination of genital endometriosis and endometrial hyperplasia, highlighting the relevance of this research.
This study aimed to investigate the association between single nucleotide variants (SNVs) and the occurrence of genital endometriosis combined with endometrial hyperplasia in the Russian population in the following genes: NC_000017.11 (SHBG):g.7618597G>T (rs12150660), NC_000001.11 (PRMT6):g.107003753A>G (rs17496332), NC_000007.14 (BAIAP2L1):g.98364050T>A (rs3779195), NC_000012.12 (SLCO1B1):g.21178615T>C (rs4149056), and NC_000015.10 (NR2F2):g.96165062T>C (rs8023580).
Materials and methods
This study included 183 patients with genital endometriosis combined with endometrial hyperplasia, and a control group of 103 patients with isolated genital endometriosis was formed. Samples were collected at the Perinatal Center of St. Ioasaph Belgorod Regional Clinical Hospital. All women in the study were Russian residents of the Central Black Earth region of Russia. The study groups included women with confirmed diagnoses of genital endometriosis and endometrial hyperplasia (confirmed by morphological examination after surgery).
DNA samples extracted from venous blood using the phenol-chloroform extraction method were used for the genetic analysis. The selection of SNVs for this study was carried out based on the following criteria: 1) associations between SNVs and SHBG levels established in genome-wide studies (at a statistical significance level of p≤5×10-8) [20]; 2) significant regulatory potential (regSNP) and association with gene expression (eSNP); 3) minor allele frequency in the European population of at least 5%. The regulatory potential of SNP was assessed in silico using the bioinformatics database HaploReg [21]. A total of 5 SNVs were examined in the study: NC_000017.11 (SHBG): g.7618597G>T (rs12150660), NC_000001.11 (PRMT6): g.107003753A>G (rs17496332), NC_000007.14 (BAIAP2L1): g.98364050T>A (rs3779195), NC_000012.12 (SLCO1B1): g.21178615T>C (rs4149056), NC_000015.10 (NR2F2): g.96165062T>C (rs8023580). The polymerase chain reaction (PCR) method (real-time PCR technology using TagMan probes and variant-specific reagent kits (synthesized by Test-Gen, Ulyanovsk, Russia)) was used for genotyping, performed on a CFX96 amplifier (BioRad, USA). The functional effects of the BAIAP2L1 gene variant (rs3779195), which showed significant associations with the development of genital endometriosis in combination with endometrial hyperplasia, and SNVs strongly linked to it (r2≥0.8) were analyzed in detail in silico using the bioinformatics resources HaploReg [21] and Gtex Portal [22]. The relationship of SNVs (reference and alternative alleles) with a change in the regulatory motif of DNA (the affinity of the motif to transcription factors) was determined by the difference between the LOD scores of the alternative (alt) and reference (ref) alleles: LOD (alt) − LOD (ref). A negative value of this indicator indicates an increase in the affinity of this motif for the reference allele, and conversely, a positive value demonstrates the association of the alternative allele with an increase in the affinity of the analyzed DNA motif.
Statistical analysis
Statistical analysis was performed using Statistica software. The assumption of normality was assessed using the Shapiro–Wilk test. Numerical variables were not normally distributed and were reported as the median (Me) and interquartile range (Q1; Q3). Categorical variables were reported as frequencies and percentages (%). The statistical significance of between-group differences for continuous variables was tested using the Mann–Whitney U test. The Pearson χ2 test was used to compare groups using categorical variables. The critical level of significance when testing statistical hypotheses was taken to be 0.05 (p<0.05).
Statistical analysis of the results of genotyping of the SNVs under consideration among patients with genital endometriosis in combination with endometrial hyperplasia and the control group included an assessment of the correspondence of the established distribution of genotypes to that expected in accordance with Hardy–Weinberg equilibrium (HWE) and the calculation of the observed (H1) and expected (H2) heterozygosity indicators. Pearson’s χ2 test was used to assess the correspondence of genotype frequencies to the state of Hardy–Weinberg equilibrium. The relationship between the studied SNVs and the formation of genital endometriosis in combination with endometrial hyperplasia was assessed using logistic regression. Four main genetic models were tested (allelic model 1, additive model 2, dominant model 3, recessive model 4) with corrections for covariates (age and body mass index (BMI)) and multiple comparisons (an adaptive permutation test was performed with the determination of the pperm indicator). Odds ratios (OR) with 95% confidence interval (95% CI) were used to assess the strength and direction of associative relationships. Statistical significance was set at pperm≤0.05. We used PLINK v1.07 software [23] to assess the correspondence between the established distribution of genotypes and the distribution expected under Hardy–Weinberg equilibrium, as well as to analyze the association between alleles and genotypes and the risk of developing genital endometriosis in combination with endometrial hyperplasia.
Results and discussion
The median age of patients with genital endometriosis and endometrial hyperplasia was 42 (36; 48) years, which was 11 years higher than that of patients with isolated genital endometriosis – 31 (27; 37.5) years (p<0.001). The median BMI among patients with endometrial hyperplasia and genital endometriosis was 27.3 (22.8; 30.5) kg/m2, the comparison group – 23.8 (20.9; 27.8) kg/m2 (p<0.001) (Table 1). Among patients with genital endometriosis and endometrial hyperplasia, the proportion of women with obesity was higher (p=0.039) and the proportion of women with normal body weight was lower (p=0.034) than in the comparison group (patients with isolated genital endometriosis).
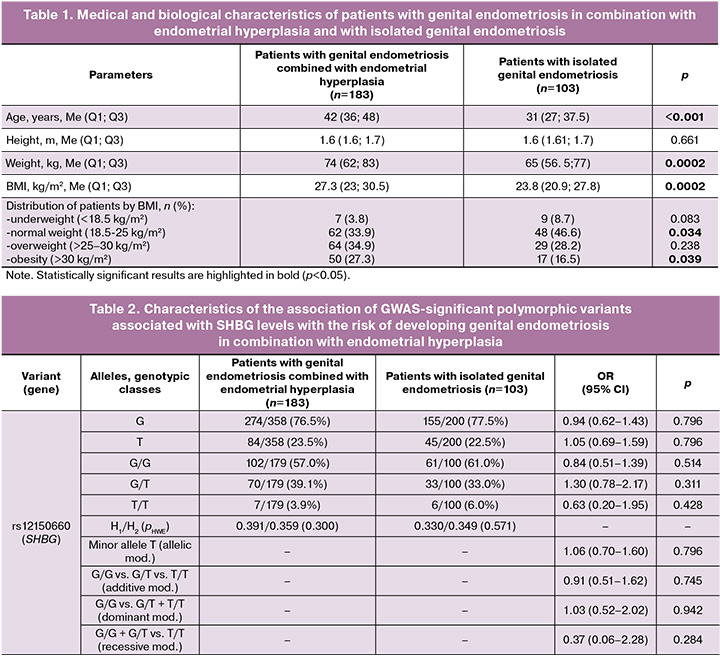
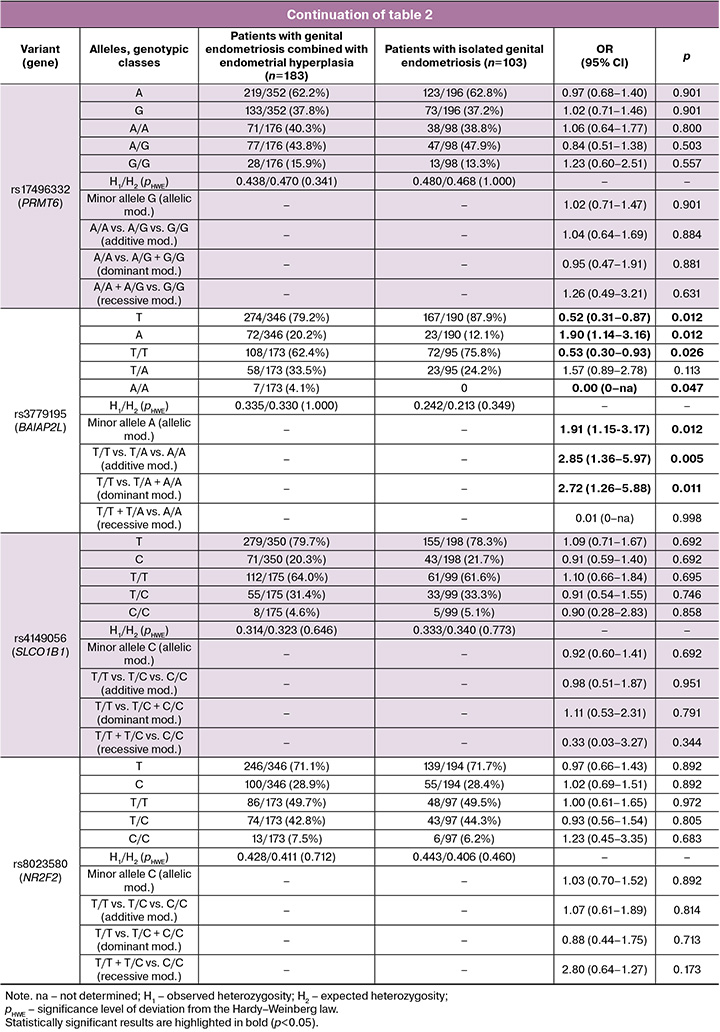
Analysis of the observed distribution of genotypes for the five SNVs studied (SHBG (rs12150660), PRMT6 (rs17496332), BAIAP2L1 (rs3779195), SLCO1B1 (rs4149056), and NR2F2 (rs8023580)) showed correspondence with the expected distribution according to the Hardy–Weinberg law.
Analysis of the involvement of SHBG gene variants in the development of genital endometriosis in combination with endometrial hyperplasia showed a statistically significant association between the A allele variant of the BAIAP2L1 gene (rs3779195) and the risk of developing genital endometriosis in combination with endometrial hyperplasia within the framework of allelic, additive, and dominant genetic models (Table 2). The genetic variant A BAIAP2L1 (rs3779195) served as a risk factor for the formation of genital endometriosis in combination with endometrial hyperplasia (OR=2.85; 95% CI 1.36–5.97; p=0.005; pperm=0.006 for the additive model; OR=1.91; 95% CI 1.15–3.17; p=0.012; pperm=0.014 for the allelic model; OR=2.72; 95% CI 1.26–5.88; p=0.011; pperm=0.012 for the dominant model).
Thus, within the framework of this study, a genetic risk factor for the development of genital endometriosis in conjunction with hyperplasia was identified as the A allele of the BAIAP2L1 gene (rs3779195).
Analysis of the regulatory effects of BAIAP2L1 SNV (rs3779195) revealed its significant functional role in the body. Using materials from the HaploReg (v4.2) online resource, it was determined that the rs3779195 SNV is located in the intronic region of the BAIAP2L1 gene, which determines the interaction between DNA and the transcription factor Foxp1. The A allele variant (rs3779195) increases the regulatory region's affinity to Foxp1 (difference in LOD scores of alleles A and T: 0.9).
According to the data presented in the bioinformatics database GTEx portal (https://www.gtexportal.org/home/), the BAIAP2L1 gene variant (rs3779195) affects the transcription levels of five genes (BAIAP2L1, BRI3, LMTK2, RP11-307C18.1, and TECPR1) in more than 25 different organs and tissues, which are important for the pathogenesis of endometriosis. Thus, the A allele variant (OR=1.83–2.85) is associated with reduced expression of the RP11-307C18.1 gene in adipose tissue (NES (Normalized Enrichment Score)=-0.88; p=3.1e-57), thyroid gland (NES=-0.69; p=7.6e-36), adrenal glands (NES=-0.67; p=6.4e-12), pituitary gland (NES=-0.65; p=8.2e-13), blood (NES=-0.62; p=7.2e-38), as well as with low transcription of the BRI3 gene in adipose tissue (NES=-0.19; p=5.9e-12). In addition, this allelic variant was associated with increased expression of TECPR1 (NES=0.17; p=0.0000045), LMTK2 (NES=0.17; p=0.000038), and BAIAP2L1 (NES=0.27; p=1.1e-10) in the thyroid gland and TECPR1 (NES=0.11; p=0.0000058) in the blood.
Thus, SNVs BAIAP2L1 (rs3779195), which influences the interaction of DNA with the transcription factor Foxp1, is able to regulate the increase/decrease in the expression of five genes (RP11-307C18.1, BRI3, TECPR1, LMTK2, and BAIAP2L1), indicating its important functional significance in the body.
The BAIAP2L1 gene variant (rs3779195) is in linkage disequilibrium with 20 SNVs, at least four of which (rs6950023, rs6967728, rs77032872 and rs3779196) are characterized by pronounced regulatory potential. Thus, SNVs rs6967728 and rs6950023 are localized in the region of histones marking enhancers (in 24 different organs and tissues) and promoters (in blood cells and the thyroid gland), DNase-hypersensitive sites (in 32 organs and tissues), the region of the regulatory motif for the transcription factor Nkx3, and binding sites with 10 and 11 regulatory proteins, respectively. The SNVs rs3779196 and rs77032872 are located in the region of histones marking enhancers (in 20 and 21 organs and tissues, respectively) and promoters (in two and nine organs and tissues), DNase-1 hypersensitivity regions (in 13 and 26 organs and tissues), regions of DNA regulatory motifs (in six and four organs and tissues), and binding sites with regulatory proteins (8 proteins for rs3779196). Notably, 17 of the 20 SNVs strongly linked to BAIAP2L1 (rs3779195) affected the expression of seven genes (PRMT6, RP11-307C18.1, BAIAP2L1, BRI3, TECPR1, LMTK2, ASNS, and AC004967.7) in various organs and tissues (adipose tissue, liver, thyroid gland, hypothalamus, ovaries), which are sites of SHBG synthesis and are important in the pathogenesis of endometriosis.
Thus, the BAIAP2L1 gene variant (rs3779195) under consideration and the 20 SNVs strongly linked to it are functionally significant in relation to seven genes (PRMT6, RP11-307C18.1, BAIAP2L1, BRI3, TECPR1, LMTK2, ASNS, and AC004967.7) in a large number of organs and tissues, which may underlie the association of this gene variant and the SNVs strongly linked to SHBG levels and the development of endometriosis.
GWAS materials (https://www.ebi.ac.uk/gwas/) indicate associations of several variants linked (r2≥0.8) with SNVs BAIAP2L1 (rs3779195) with SHBG levels in the body: BRI3 (rs6950023), p=2×10-101, r2=0.9, D’=-0.96; BRI3/BAIAP2L1 (rs7015), p=2×10-195, r2=0.85, D’=-0.97; BRI3/BAIAP2L1 (rs13232861), p=6×10-28, r2=0.81, D’=-0.96; BAIAP2L1 (rs1688606), p=2×10-24, r2=0.98, D’=-0.99; BAIAP2L1 (rs112758337), p=2×10-37, r2=0.98, D’=0.99; BAIAP2L1 (rs4268041), p=1×10-34, r2=0.91, D’=-0.98 [18, 24]. Thus, the region of the genome containing the significant BAIAP2L1 SHBG gene variant (rs3779195) plays a significant role in regulating SHBG concentration in the body.
According to the literature, a genome-wide study conducted by Coviello A.D. et al. involving a sample of 21,791 individuals of European descent established that the BAIAP2L1 gene variant (rs3779195) is associated with SHBG levels in the body. Specifically, its A allele variant was linked to lower levels of this protein (β=-0.028, p=3.0×10-8) [17]. A subsequent GWAS study by Harrison S. et al., which included 306,248 Europeans, corroborated these findings, showing that the A allele of the BAIAP2L1 gene (rs3779195) is associated with low SHBG levels (β=-2.41, p=9×10-9) [18]. Thus, based on GWAS data [17, 18], allele A serves as a marker of low SHBG levels and correlates with an increased risk of developing genital endometriosis when combined with endometrial hyperplasia.
SHBG, primarily responsible for the transport and binding of steroids in the blood plasma, regulates the levels of free (biologically active) testosterone and estrogens in the body [15]. Concurrently, low SHBG levels are associated with elevated steroid levels [16, 25–27]. Therefore, the SNVs BAIAP2L1 (rs3779195), which our data indicate, is associated with an increased risk of developing genital endometriosis in conjunction with endometrial hyperplasia, is linked to low SHBG levels and, consequently, high levels of testosterone and estrogen. The literature suggests that endometriosis is a hormone-dependent disease in which increased estrogen synthesis and progesterone resistance play significant roles in its etiopathogenesis [28, 29]. Notably, hyperestrogenism is a recognized risk factor for both endometriosis and endometrial hyperplasia [29].
Conclusion
This study established an association between the GWAS-significant variant of BAIAP2L1 (rs3779195) and the development of genital endometriosis in combination with endometrial hyperplasia. The A allele of the BAIAP2L1 gene (rs3779195) was found to be a risk factor for the development of genital endometriosis in combination with endometrial hyperplasia (OR=1.91–2.85). The rs3779195 variant is located in the intronic region of BAIAP2L1, influencing the interaction of DNA with the Foxp1 transcription factor and affecting the expression of five genes (RP11-307C18.1, BRI3, TECPR1, LMTK2, and BAIAP2L1). This variant is in linkage disequilibrium with 20 SNVs, which are also characterized by significant regulatory potential.
References
- Crump J., Suker A., White L. Endometriosis: a review of recent evidence and guidelines. Aust. J. Gen. Pract. 2024; 53(1-2): 11-8. https://dx.doi.org/10.31128/AJGP/04-23-6805
- Wang P.H., Yang S.T., Chang W.H., Liu C.H., Lee F.K., Lee W.L. Endometriosis: part I. Basic concept. Taiwan. J. Obstet. Gynecol. 2022; 61(6): 927-34. https://dx.doi.org/10.1016/j.tjog.2022.08.002
- Smolarz B., Szyłło K., Romanowicz H. Endometriosis: epidemiology, classification, pathogenesis, treatment and genetics (review of Literature). Int. J. Mol. Sci. 2021; 22(19): 10554. https://dx.doi.org/10.3390/ijms221910554
- Koninckx P.R., Fernandes R., Ussia A., Schindler L., Wattiez A., Al-Suwaidi S. et al. Pathogenesis based diagnosis and treatment of endometriosis. Front. Endocrinol. (Lausanne). 2021; 12: 745548. https://dx.doi.org/10.3389/fendo.2021.745548
- Zondervan K.T., Becker C.M., Missmer S.A. Endometriosis. N. Engl. J. Med. 2020; 382(13): 1244-56. https://dx.doi.org/10.1056/NEJMra1810764
- Ponomarenko M.S., Reshetnikov E.A., Churnosova M.M., Reshetnikova Y.N., Churnosov V.I., Ponomarenko I.V. Comorbidity and syntropy of benign proliferative diseases of the female reproductive system: non-genetic, genetic, and epigenetic factors (review). Research Results in Biomedicine. 2023; 9(4): 544-56. https://dx.doi.org/10.18413/2658-6533-2023-9-4-0-9
- Пономарева Т.А., Алтухова О.Б., Пономаренко И.В., Чурносов М.И. Современные представления о механизмах развития и факторах риска эндометриоза. Акушерство и гинекология. 2024; 7: 12-20. [Ponomareva T.A., Altukhova O.B., Ponomarenko I.V., Churnosov M.I. Novel concepts in the pathogenesis and risk factors of endometriosis. Obstetrics and Gynecology. 2024; (7): 12-20 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.110
- Пономаренко И.В., Полоников А.В., Верзилина И.Н., Чурносов М.И. Молекулярно-генетические детерминанты развития эндометриоза. Вопросы гинекологии, акушерства и перинатологии. 2019; 18(1): 82-6 [Ponomarenko I.V., Polonikov A.V., Verzilina I.N., Churnosov M.I. Molecular-genetic determinants of the development of endometriosis. Gynecology, Obstetrics, and Perinatology. 2019; 18(1): 82-6 (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2019-1-82-86
- Signorile P.G., Baldi A., Viceconte R., Ronchi A., Montella M. Pathogenesis of endometriosis: focus on adenogenesis-related factors. In Vivo. 2023; 37(5): 1922-30. https://dx.doi.org/10.21873/invivo.13288
- Adilbayeva A., Kunz J. Pathogenesis of endometriosis and endometriosis-associated cancers. Int. J. Mol. Sci. 2024; 25(14): 7624. https://dx.doi.org/10.3390/ijms25147624
- Kim H., Kim H.J., Ahn H.S. Does endometriosis increase the risks of endometrial hyperplasia and endometrial cancer? Gynecol. Oncol. 2023; 169: 147-53. https://dx.doi.org/10.1016/j.ygyno.2022.06.021
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Молекулярные механизмы и факторы риска развития эндометриоза. Акушерство и гинекология. 2019; 3: 26-31. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Molecular mechanisms of and risk factors for endometriosis. Obstetrics and Gynecology. 2019; (3): 26-31 (in Russian)]. https://dx.doi.org/10.18565/aig.2019.3.26-31
- Радзинский В.Е., Алтухова О.Б. Молекулярно-генетические детерминанты бесплодия при генитальном эндометриозе. Научные результаты биомедицинских исследований. 2018; 4(3): 28-37. [Radzinsky V.E., Altukhova O.B. Molecular-genetic determinants of infertility in genital endometriosis. Research Results in Biomedicine. 2018; 4(3): 28-37 (in Russian)]. https://dx.doi.org/10.18413/2313-8955-2018-4-3-0-3
- Андреев А.Е., Клейменова Т.С., Дробинцева А.О., Полякова В.О., Кветной И.М. Сигнальные молекулы, вовлеченные в образование новых нервных окончаний при эндометриозе (обзор). Научные результаты биомедицинских исследований. 2019; 5(1): 94-107. [Andreev A.E., Kleimenova T.S., Drobintseva A.O., Polyakova V.O., Kvetnoy I.M. Signal molecules involved in formation of new nerve endings in endometriosis (review). Research Results in Biomedicine. 2019; 5(1): 94-107 (in Russian)]. https://dx.doi.org/10.18413/2313-8955-2019-5-1-0-7
- Narinx N., David K., Walravens J., Vermeersch P., Claessens F., Fiers T. et al. Role of sex hormone-binding globulin in the free hormone hypothesis and the relevance of free testosterone in androgen physiology. Cell. Mol. Life Sci. 2022; 79(11): 543. https://dx.doi.org/10.1007/s00018-022-04562-1
- Simons P.I.H.G., Valkenburg O., Stehouwer C.D.A., Brouwers M.C.G.J. Sex hormone-binding globulin: biomarker and hepatokine? Trends Endocrinol. Metab. 2021; 32(8): 544-53. https://dx.doi.org/10.1016/j.tem.2021.05.002
- Coviello A.D., Haring R., Wellons M., Vaidya D., Lehtimäki T., Keildson S. et al. A genome-wide association meta-analysis of circulating sex hormone-binding globulin reveals multiple loci implicated in sex steroid hormone regulation. PLOS Genet. 2012; 8(7): e1002805. https://dx.doi.org/10.1371/journal.pgen.1002805
- Harrison S., Davies N. M., Howe L. D., Hughes A. Testosterone and socioeconomic position: mendelian randomization in 306,248 men and women in UK Biobank. Sci. Adv. 2021; 7(31): eabf8257. https://dx.doi.org/10.1126/sciadv.abf8257
- Пономарева Т.А., Алтухова О.Б., Пономаренко И.В., Чурносов М.И. Роль генетических факторов в формировании эндометриоидных поражений. Акушерство, гинекология и репродукция. 2023; 17(4): 443-54 [Ponomareva T.A., Altukhova O.B., Ponomarenko I.V., Churnosov M.I. The role of genetic factors in developing endometrioid lesions. Obstetrics, Gynecology and Reproduction. 2023; 17(4): 443-54 (in Russian)]. https://dx.doi.org/110.17749/2313-7347/ob.gyn.rep.2023.434
- Golovchenko I., Aizikovich B., Golovchenko O., Reshetnikov E., Churnosova M., Aristova I. et al. Sex hormone candidate gene polymorphisms are associated with endometriosis. Int. J. Mol. Sci. 2022; 23(22): 13691. https://dx.doi.org/10.3390/ijms232213691
- HaploReg v4.2. https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php (accessed 02.04.2025).
- GTExPortal. https://gtexportal.org/home (accessed 24.06.2025).
- PLINK v1.07. https://zzz.bwh.harvard.edu/plink/ (accessed 02.04.2025).
- Ruth K.S., Day F.R., Tyrrell J., Thompson D.J., Wood A.R., Mahajan A. et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 2020; 26(2): 252-8. https://dx.doi.org/10.1038/s41591-020-0751-5
- Пасенов К.Н. Особенности ассоциаций SHBG-связанных генов с раком молочной железы у женщин в зависимости от наличия наследственной отягощенности и мутаций в генах BRCA1/CHEK2. Научные результаты биомедицинских исследований. 2024; 10(1): 69-88. [Pasenov K.N. Features of associations of SHBG-related genes with breast cancer in women, depending on hereditary burden and mutations in BRCA1/CHEK2 genes. Research Results in Biomedicine. 2024; 10(1): 69-88 (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2024-10-1-0-4
- Головченко И.О. Генетические детерминанты уровня половых гормонов у больных эндометриозом. Научные результаты биомедицинских исследований. 2023; 9(1): 5-21. [Golovchenko I.O. Genetic determinants of sex hormone levels in endometriosis patients. Research Results in Biomedicine. 2023; 9(1): 5-21 (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2023-9-1-0-1
- Yuan S., Wang L., Sun J., Yu L., Zhou X., Yang J. et al. Genetically predicted sex hormone levels and health outcomes: phenome-wide Mendelian randomization investigation. Int. J. Epidemiol. 2022; 51(6): 1931-42. https://dx.doi.org/10.1093/ije/dyac036
- Пономарева Т.А. Генетические варианты глобулина, связывающего половые гормоны, и гормональный профиль больных генитальным эндометриозом. Научные результаты биомедицинских исследований. 2025; 11(1): 75-90. [Ponomareva T.A. Genetic variants of sex hormone-binding globulin and hormonal profile in patients with genital endometriosis. Research Results in Biomedicine. 2025; 11(1): 75-90 (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2025-11-1-0-4
- Nees L.K., Heublein S., Steinmacher S., Juhasz-Böss I., Brucker S., Tempfer C.B. et al. Endometrial hyperplasia as a risk factor of endometrial cancer. Arch. Gynecol. Obstet. 2022; 306(2): 407-21. https://dx.doi.org/10.1007/s00404-021-06380-5
Received 13.03.2025
Accepted 01.07.2025
About the Authors
Tatyana A. Ponomareva, PhD student, Department of Medical and Biological Disciplines, Belgorod National Research University, 85 Pobedy str., 308015, Belgorod, Russia; Obstetrician-Gynecologist, Belgorod Regional Clinical Hospital of St. Joasaph, 8/9 Nekrasov str., 308007, Belgorod, Russia, rybaarbusova@icloud.com,https://orcid.org/0009-0007-8533-9319
Oksana B. Altukhova, Dr. Med. Sci., Associate Professor, Head of the Department of Obstetrics and Gynecology, Belgorod National Research University, 85 Pobedy str., 308015, Belgorod, Russia; Head of Gynecological Department, Belgorod Regional Clinical Hospital of St. Joasaph, 8/9 Nekrasov str., 308007, Belgorod, Russia,
altuhova_o@bsuedu.ru, https://orcid.org/0000-0003-4674-8797
Irina V. Ponomarenko, Dr. Med. Sci., Associate Professor, Department of Medical and Biological Disciplines, Belgorod National Research University, 85 Pobedy str.,
308015, Belgorod, Russia, ponomarenko_i@bsuedu.ru, https://orcid.org/0000-0002-5652-0166
Mikhail I. Churnosov, Dr. Med. Sci., Professor, Head of the Department of Medical and Biological Disciplines, Belgorod National Research University, 85 Pobedy str.,
308015, Belgorod, Russia, churnosov@bsuedu.ru, https://orcid.org/ 0000-0003-1254-6134
Corresponding author: Tatyana A. Ponomareva, rybaarbusova@icloud.com



