Association of polymorphic variants rs699517 and rs2790 of the maternal TYMS gene with newborn birthweight
Reshetnikova Y.N., Ponomarenko I.V., Churnosov M.I., Reshetnikov E.A.
Objective: To investigate the associations between maternal folate cycle gene polymorphisms and newborn birthweight, as well as evaluate their functional effects.
Materials and methods: A molecular genetic study was conducted on five polymorphic gene loci involved in folic acid and methionine metabolism (rs699517 TYMS, rs2790 TYMS, rs1979277 SHMT1, rs1805087 MTR, and rs1801394 MTRR) using the genomic DNA of 317 pregnant women.
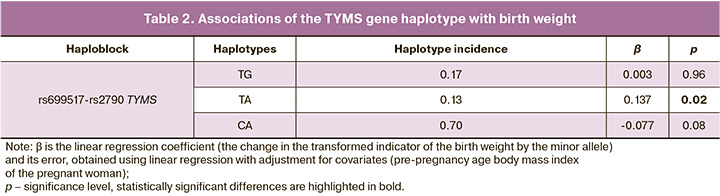
Results: The birthweight of newborns with different maternal genotypes for the loci studied showed slight changes, but no significant differences were observed (p>0.05): rs699517 TYMS (3449.63 vs. 3551.44 g), rs2790 TYMS (3441.82 vs. 3511.29 g), rs1979277 SHMT1 (3484.05 vs. 3527.66 g), rs1805087 MTR (3477.50 vs. 3522.08 g), rs1801394 MTRR (3464.74 vs. 3550.98 g). Among the haplotypes, the TA haplotype (rs699517-rs2790 TYMS) occurred at a frequency of 0.13 and showed a significant association with birthweight (β=0.14, p=0.02, pperm=0.03). However, the TG haplotype (frequency 0.17) and the CA haplotype (frequency 0.70) were not significantly associated with birthweight.
Conclusion: The TA haplotype rs699517–rs2790 of TYMS may be associated with higher birthweight.
Authors' contributions: Churnosov M.I., Reshetnikov E.A. – conception and design of the study; Reshetnikova Yu.N. – material collection and processing, drafting of the manuscript; Ponomarenko I.V., Reshetnikova Yu.N. – statistical analysis; Churnosov M.I., Reshetnikov E.A. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Medical Institute of Belgorod State National Research University (Ref. No: 2/13.02.2008).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Reshetnikova Y.N., Ponomarenko I.V., Churnosov M.I., Reshetnikov E.A. Association of polymorphic variants rs699517 and rs2790 of the maternal TYMS gene with newborn birthweight.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (3): 73-78 (in Russian)
https://dx.doi.org/10.18565/aig.2023.294
Keywords
Fetal anthropometric characteristics are critical indicators of pregnancy outcomes and depend directly on the normal placentation process [1]. Impaired placental development can lead to placental insufficiency, resulting in pregnancy complications, such as fetal growth restriction (FGR) and pre-eclampsia [2–4].
Neonates with higher or lower birth length and weight are at an increased risk of developing lifelong health problems, including mortality, compared to neonates with average levels of these parameters [5, 6].
Folic acid metabolism plays an essential role in fetal and placental development. It is necessary for the methylation processes associated with the biosynthesis of DNA and RNA and the regulation of gene expression to ensure cell growth, proliferation, and differentiation [7]. Impaired function of enzymes critical to the folate and methionine cycles can result in folic acid deficiency and hyperhomocysteinemia [8]. Elevated levels of homocysteine can lead to increased apoptosis in the placenta and reduced secretion of human chorionic gonadotropin, contributing to placental insufficiency and FGR [8–11].
Recent molecular genetic studies have shown that gene polymorphisms within the folate and methionine cycle enzymes may be linked to FGR and/or low birth weight [12–15]. For example, polymorphisms C677T and A1298C of methylenetetrahydrofolate reductase (MTHFR) have been associated with lower birth weight in the children of smoking women in the Japanese population [12]. In Indian populations, the C677T MTHFR polymorphic locus was also shown to correlate with lower birth weight [13, 15]. However, a meta-analysis by Wu H. et al. [16] found no association between C677T MTHFR polymorphism and birth weight. Similarly, a recent study in a Chinese female population that examined the relationship between five folate metabolism gene polymorphisms (C677T and A1298C MTHFR, A66G MTRR, A2756G MTR, rs3819102 TYMS) and low birth weight yielded no significant results [17].
The inconsistency in molecular genetic study outcomes regarding the role of folate and methionine cycle gene polymorphisms in determining newborn weight underscores the need for further research. This will help to identify maternal genetic factors linked to newborn weight and, upon gathering substantive evidence of these connections and their implications, potentially recommend them for application in practical medicine.
Our study aimed to explore the association between polymorphisms in genes involved in folic acid/methionine metabolism and newborn weight in pregnant women in the Central Black Earth region of Russia.
Materials and methods
This study included 317 pregnant women who were examined at the Perinatal Center of the Belgorod Regional Clinical Hospital from 2008 to 2015. All the women provided informed consent for inclusion in the study. This study was reviewed and approved by the Research Ethics Committee of the Medical Institute of Belgorod State National Research University (Ref. No: 2/13.02.2008). The inclusion criteria were singleton pregnancy resulting in a live birth, Russian nationality, the and Central Black Earth region of Russia as the place of birth and residence. The exclusion criteria were multiple pregnancies, congenital malformations of the fetus or newborn, and congenital uterine anomalies.
For molecular genetic studies, five polymorphic loci of the folate and methionine cycle genes were selected: rs699517 TYMS, rs2790 TYMS, rs1979277 SHMT1, rs1805087 MTR, rs1801394 MTRR. These loci are functionally significant because they are associated with epigenetic changes and transcription of the corresponding genes (data obtained in silico using the online resource Haploreg v.4.2, https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php).
DNA was extracted from the venous blood using the standard phenol-chloroform extraction method. Genotyping of samples was performed by PCR DNA synthesis on a CFX96 amplifier (Bio-Rad) using standard oligonucleotide primers and probes, followed by the analysis of polymorphic loci using the allele discrimination method [18].
Statistical analysis
Associations between maternal SNPs and birth weight were assessed by log-linear regression analysis using gPLink software [19]. For this analysis, transformed birth weight values were used because their distribution in the study sample (assessed by the Shapiro–Wilk test) did not meet the normality assumption. To assess the direction of the association, the regression coefficient (β) and its error (SE) were used (describing the change in the transformed birth weight variable to the minor allele). Individual SNP effects were assessed using four genetic models (allelic, additive, dominant, and recessive) with adjustment for covariates (age and maternal pre-pregnancy body mass index) and multiple comparisons (adaptive permutation procedures were used to calculate the pperm index).
Linkage disequilibrium between SNP pairs was assessed using the D' and Pearson’s r2 correlation coefficients. Haploblocks were constructed based on the "confidence interval " algorithm (with D'>0.8) implemented in the gPLINK program. Analysis of the associations between haplotypes and birth weight was performed using log-linear regression with adjustment for covariates (maternal pre-pregnancy age and body mass index) and permutation procedures (1000 permutations were performed). Pperm<0.05 was considered statistically significant.
Using modern online bioinformatics resources, we studied the relationships between SNPs that showed significant associations with birth weight, non-synonymous substitutions (PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/), epigenetic effects (HaploReg (v4.2), https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php), expression, and alternative splicing of genes (GTEx portal, https://www.gtexportal.org/home/).
Results and discussion
There were no statistically significant differences in the analysis of the associations of folate cycle gene polymorphisms with birth weight (Table 1).
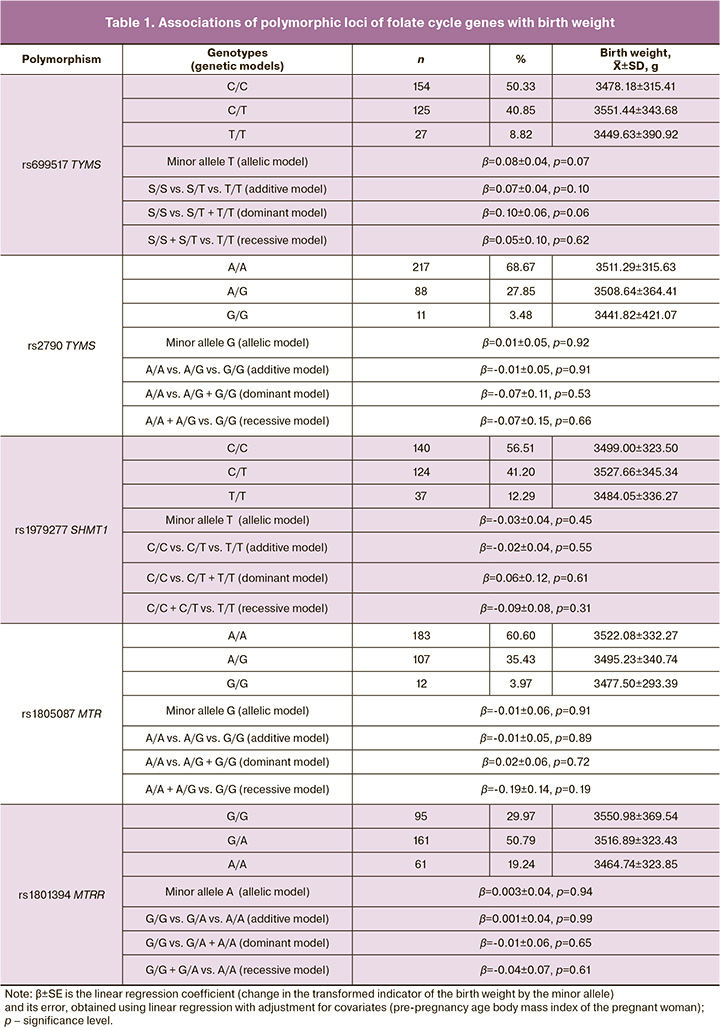
Linkage disequilibrium was studied for polymorphic loci located on the same chromosome in the study group of women. Using the “Confidence intervals” algorithm with a linkage coefficient level D´>0.8, haploblocks were determined based on the studied SNPs. As a result, one haploblock was established, including polymorphic loci rs699517 and rs2790 of the TYMS gene (chromosome 18). Haplotype associations within the identified haploblock revealed that the TA rs699517–rs2790 TYMS haplotype was associated with birth weight (β=0.14, p=0.0195) (Table 2).
According to the online resource HaploReg (v4.2), rs699517 and rs2790 TYMS have important regulatory potentials. These polymorphic markers are located at the site of DNase hypersensitivity in whole blood, and rs699517 TYMS is localized in the regulatory pattern region, which is a binding site for the transcription factor NRSF. For this factor, the difference between the logarithm of the odds (LOD) scores of allelic variants T and C was ΔLOD scores = 2.4. Thus, the T allele of rs699517 TYMS, which is associated with higher birth weight, increases the affinity for this transcription factor.
Data from the online resource GTEx Portal indicated an association of rs699517 and rs2790 TYMS with the level of gene expression in various organs and tissues associated with birth weight. Allele C of the rs699517 TYMS polymorphic locus is associated with the expression of the ENOSF1 gene in skeletal muscle (β=-0.40, p=1.1*10-25, pFDR≤0.05), fibroblast cell culture (β=0.24, p =1.6*10-15, pFDR≤0.05), adrenal glands (β=0.38, p=1.6*10-9, pFDR≤0.05), subcutaneous adipose tissue (β=-0, 19, p=1.1*10-8, pFDR≤0.05); gene RP11-806L2.6 in the thyroid gland (β=0.38, p=1.8*10-13, pFDR≤0.05), subcutaneous adipose tissue (β=0.38, p=1.8*10-13, pFDR≤0.05); adrenal glands (β=0.51, p=1.2*10-8, pFDR≤0.05), fibroblast cell culture (β=0.23, p=7.7*10-5, pFDR≤0.05 ).
Allele A of the rs2790 TYMS polymorphic locus is associated with the expression of the ENOSF1 gene in skeletal muscle (β=-0.49, p=2.3*10-29, pFDR≤0.05), fibroblast cell culture (β=0.29, p=1.7*10-15, pFDR≤0.05), adrenal glands (β=0.43, p=5.5*10-9, pFDR≤0.05), subcutaneous adipose tissue (β=-0, 26, p=8.1*10-11, pFDR≤0.05); gene RP11-806L2.6 in the thyroid gland (β=0.40, p=4.0*10-11, pFDR≤0.05), subcutaneous adipose tissue (β=0.35, p=1.7*10-8, pFDR≤0.05), adrenal glands (β=0.51, p=1.6*10-6, pFDR≤0.05).
It was established that the C allele of rs699517 TYMS and the A allele of rs2790 TYMS are associated with the level of alternative splicing of the ENOSF1 gene in the thyroid gland (β=-0.54, p=1.1*10-21, pFDR≤0.05 and β=-0.90, p=4.5*10-47, pFDR≤0.05, respectively), subcutaneous adipose tissue (β=0.46, p=2.1*10-15, pFDR≤0.05 and β=0.41, p=3.2*10-9, pFDR≤0.05, respectively), visceral adipose tissue (β=-0.41, p=1.4*10-15, pFDR≤0.05 and β=-0.56, p=2.8*10-23, pFDR≤0.05, respectively), fibroblast cell culture (β=-0.23, p=2.7*10-7, pFDR≤0.05 and β=-0.40, p=3.6*10-7, pFDR≤0.05, respectively). In addition, the C allele of rs699517 TYMS affects the level of alternative splicing of the ENOSF1 gene in the adrenal glands (β=0.60, p=2.2*10-15, pFDR≤0.05).
As a result, rs699517 and rs2790 TYMS, associated with birth weight, have important functional effects and are localized in the region of DNase hypersensitivity in whole blood, associated with the level of mRNA expression of the ENOSF1 gene and the RP11-806L2.6 gene in skeletal muscle, adipose tissue, adrenal glands, thyroid gland, and fibroblast cell culture. In addition, these SNPs affect the level of alternative splicing of the ENOSF1 gene in adipose tissue, adrenal glands, thyroid glands, and fibroblast cell cultures. In addition, rs699517 TYMS determines DNA sensitivity for the transcription factor NRSF.
According to information from the GeneCards database (https://www.genecards.org), TYMS encodes the enzyme thymidylate synthase, which is involved in the folate cycle. The folate cycle is associated with a cascade of transformations of folic acid into tetrahydrofolates, which are methyl group donors used in purine biosynthesis [7]. Thymidylate synthase catalyzes the conversion of deoxyuridine monophosphate (dUMP) to deoxythymine monophosphate (dTMP), which is necessary for the biosynthesis of deoxynucleotides and is critical for DNA replication [7]. To carry out this reaction, 10-methylenetetrahydrofolate (methylene-THF) was used as the cofactor. Inhibition of thymidylate synthase under the influence of various factors leads to a decrease in cellular dTMP and accumulation of dUMP. Ultimately, the absence of dTMP leads to inhibition of DNA synthesis, incorrect incorporation of dUMP into DNA, and damage (double- and single-strand breaks) with subsequent cell apoptosis, potentially leading to disorders in placental vascular endothelial cells and the development of placental insufficiency [11, 20]. Therefore, it is logical to identify genetic markers of TYMS associated with the course of pregnancy and the disorders that arise during this process. However, the role of TYMS gene polymorphisms in birth weight remains largely unexplored. In molecular genetic studies on different populations, the association of the rs699517 and rs2790 TYMS polymorphic loci with the risk of developing a number of diseases has been studied, including ischemic stroke [21, 22], ventricular septal defect [23], lymphoblastic leukemia [24], and colorectal cancer [25]. Simultaneously, attention has been drawn to the ambiguity and inconsistency of the results of such studies.
The multidirectional nature of the identified associations of rs699517 and rs2790 TYMS with the formation of various diseases may be due to pronounced differences in the allele frequencies of these polymorphic loci in different ethnic groups worldwide [24, 26].
Conclusion
The TA haplotype rs699517-rs2790 TYMS in pregnant women may be associated with higher birth weight. Thus, these polymorphic loci have significant functional implications. They are located in areas of DNase hypersensitivity in whole blood and correlate with the mRNA expression levels of the ENOSF1 and RP11-806L2.6 gene in various tissues, including skeletal muscle, adipose tissue, adrenal gland, thyroid gland, and fibroblast cell cultures. They also affect the alternative splicing of the ENOSF1 gene in these tissues. The rs699517 TYMS polymorphism determines the sensitivity of DNA to transcription factor NRSF.
References
- Society for Maternal-Fetal Medicine (SMFM). Electronic address: pubs@smfm.org; Martins J.G., Biggio J.R., Abuhamad A. Society for Maternal-Fetal Medicine Consult Series #52: diagnosis and management of fetal growth restriction: (replaces Clinical Guideline Number 3, April 2012). Am. J. Obstet. Gynecol. 2020; 223(4): B2-B17. https://dx.doi.org/10.1016/j.ajog.2020.05.010.
- Головченко О.В. Молекулярно-генетические детерминанты преэклампсии. Научные результаты биомедицинских исследований. 2019; 5(4): 139-49. [Golovchenko O.V. Molecular genetic determinants of preeclampsia. Research Results in Biomedicine. 2019; 5(4): 139-49. (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2019-5-4-0-11.
- Решетников Е.А. Поиск ассоциаций генов-кандидатов, дифференциально экспрессирующихся в плаценте, с риском развития плацентарной недостаточности с синдромом задержки роста плода. Научные результаты биомедицинских исследований. 2020; 6(3): 338-49. [Reshetnikov E.A. Search for associations of candidate genes differentially expressed in the placenta with the risk of developing placental insufficiency with fetal growth retardation. Research Results in Biomedicine. 2020; 6(3): 338-49. (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2020-6-3-0-5.
- Баев Т.О., Панова И.А., Кузьменко Г.Н., Клычева М.М., Назаров С.Б. Состояние микроциркуляции у беременных женщин с гипертензивными расстройствами в III триместре беременности. Научные результаты биомедицинских исследований. 2023; 9(1): 113-28. [Baev T.O., Panova I.A., Kuz’menko G.N., Klycheva M.M., Nazarov S.B. The state of microcirculation in pregnant women with hypertensive disorders in the third trimester of pregnancy. Research Results in Biomedicine. 2023; 9(1): 113-28. (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2023-9-1-0-8.
- Pels A., Beune I.M., van Wassenaer-Leemhuis A.G., Limpens J., Ganzevoort W. Early-onset fetal growth restriction: a systematic review on mortality and morbidity. Acta Obstet. Gynecol. Scand. 2020; 99(2): 153-66. https://dx.doi.org/10.1111/aogs.13702.
- D'Agostin M., Di Sipio Morgia C., Vento G., Nobile S. Long-term implications of fetal growth restriction. World J. Clin. Cases. 2023; 11(3): 2855-63. https://dx.doi.org/10.12998/wjcc.v11.i13.2855.
- Ducker G.S., Rabinowitz J.D. One-carbon metabolism in health and disease. Cell Metab. 2017; 25(1): 27-42. https://dx.doi.org/10.1016/j.cmet.2016.08.009.
- Yeter A., Topcu H.O., Guzel A.I., Ozgu E., Danisman N. Maternal plasma homocysteine levels in intrauterine growth retardation. J. Matern. Fetal Neonatal Med. 2015; 28(6): 709-12. https://dx.doi.org/10.3109/14767058.2014.929110.
- Jiang H.L., Cao L.Q., Chen H.Y. Blood folic acid, vitamin B12, and homocysteine levels in pregnant women with fetal growth restriction. Genet. Mol. Res. 2016; 15(4): gmr15048890. https://dx.doi.org/10.4238/gmr15048890.
- Liu C., Luo D., Wang Q., Ma Y., Ping L., Wu T. et al. Serum homocysteine and folate concentrations in early pregnancy and subsequent events of adverse pregnancy outcome: the Sichuan Homocysteine study. BMC Pregnancy Childbirth. 2020; 20(1): 176. https://dx.doi.org/10.1186/s12884-020-02860-9.
- Gaiday A., Balash L., Tussupkaliyev A. The role of high concentrations of homocysteine for the development of fetal growth restriction. Rev. Bras. Ginecol. Obstet. 2022; 44(4): 352-9. https://dx.doi.org/10.1055/s-0042-1743093.
- Yila T.A., Sasaki S., Miyashita C., Braimoh T.S., Kashino I., Kobayashi S. et al. Effects of maternal 5,10-methylenetetrahydrofolate reductase C677T and A1298C Polymorphisms and tobacco smoking on infant birth weight in a Japanese population. J. Epidemiol. 2012; 22(2): 91-102. https://dx.doi.org/10.2188/jea.JE20110039.
- Sukla K.K., Tiwari P.K., Kumar A., Raman R. Low birthweight (LBW) and neonatal hyperbilirubinemia (NNH) in an Indian cohort: association of homocysteine, its metabolic pathway genes and micronutrients as risk factors. PLoS One. 2013; 8(8): e71587. https://dx.doi.org/10.1371/journal.pone.0071587.
- Liew S.C., Gupta E.D. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. Eur. J. Med. Genet. 2015; 58(1): 1-10. https://dx.doi.org/10.1016/j.ejmg.2014.10.004.
- Tiwari D., Bose P.D., Das S., Das C.R., Datta R., Bose S. MTHFR (C677T) polymorphism and PR (PROGINS) mutation as genetic factors for preterm delivery, fetal death and low birth weight: a Northeast Indian population based study. Meta Gene. 2015; 3: 31-42. https://dx.doi.org/10.1016/j.mgene.2014.12.002.
- Wu H., Zhu P., Geng X., Liu Z., Cui L., Gao Z. et al. Genetic polymorphism of MTHFR C677T with preterm birth and low birth weight susceptibility: a meta-analysis. Arch. Gynecol. Obstet. 2017; 295(5): 1105-18. https://dx.doi.org/10.1007/s00404-017-4322-z.
- Wang S., Duan Y., Jiang S., Bi Y., Pang X., Liu C. et al. Relationships between maternal gene polymorphisms in one carbon metabolism and adverse pregnancy outcomes: a prospective mother and child cohort study in China. Nutrients. 2022; 14(10): 2108. https://dx.doi.org/10.3390/nu14102108.
- Иванова Т.А. Пол-специфические особенности межлокусных взаимодействий, определяющих подверженность к гипертонической болезни. Научные результаты биомедицинских исследований. 2024; 10(1): 53-68. [Ivanova T.A. Sex-specific features of interlocus interactions determining susceptibility to hypertension. Research Results in Biomedicine. 2024; 10(1): 53-68. (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2024-10-1-0-3.
- Пономаренко И.В., Решетников Е.А., Полоников А.В., Чурносов М.И. Полиморфный локус rs314276 гена LIN28B ассоциирован с возрастом менархе у женщин Центрального Черноземья России. Акушерство и гинекология. 2019; 2: 98-104. [Ponomarenko I.V., Reshetnikov E.A., Polonikov A.V., Churnosov M.I. The polymorphic locus rs314276 of the LIN28A gene is associated with the age at menarche in women of the Central Black Earth Region of Russia. Obstetrics and Gynecology. 2019; (2): 98-104. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.2.98-104.
- Thomas F., Motsinger-Reif A.A., Hoskins J.M., Dvorak A., Roy S., Alyasiri A. et al. Methylenetetrahydrofolate reductase genetic polymorphisms and toxicity to 5-FU-based chemoradiation in rectal cancer. Br. J. Cancer. 2011; 105(11): 1654-62. https://dx.doi.org/10.1038/bjc.2011.442.
- Kim J.O., Park H.S., Ko E.J., Sung J.H., Kim J., Oh S.H. et al. The 3'-UTR polymorphisms in the thymidylate synthase (TS) gene associated with the risk of ischemic stroke and silent brain infarction. J. Pers. Med. 2021; 11(3): 200. https://dx.doi.org/10.3390/jpm11030200.
- Yu F., Shi L., Wang Q., Xing X., Li Z., Hou L. et al. The association between thymidylate synthase gene polymorphisms and the risk of ischemic stroke in Chinese Han population. Biochem. Genet. 2024; 62(1): 468-84. https://dx.doi.org/10.1007/s10528-023-10431-8.
- Zhao J.Y., Sun J.W., Gu Z.Y., Wang J., Wang E.L., Yang X.Y. et al. Genetic polymorphisms of the TYMS gene are not associated with congenital cardiac septal defects in a Han Chinese population. PLoS One. 2012; 7(2): e31644. https://dx.doi.org/10.1371/journal.pone.0031644.
- Wang S.M., Zeng W.X., Wu W.S., Sun L.L., Yan D. Genotype and allele frequencies of TYMS rs2790 A>G polymorphism in a Chinese paediatric population with acute lymphoblastic leukaemia. J. Clin. Pharm. Ther. 2018; 43(4): 507-12. https://dx.doi.org/10.1111/jcpt.12678.
- Dong S.Q., Wang T.M., Zhang J.B., He Y.Q., Xue W.Q., Wu Z.Y. et al. Polymorphisms in TYMS for prediction of capecitabine-induced hand-foot syndrome in Chinese patients with colorectal cancer. Cancer Res. Treat. 2021; 53(3): 724-32. https://dx.doi.org/10.4143/crt.2020.457.
- Shen R., Liu H., Wen J., Liu Z., Wang L.E., Wang Q. et al. Genetic polymorphisms in the microRNA binding-sites of the thymidylate synthase gene predict risk and survival in gastric cancer. Mol. Carcinog. 2015; 54(9): 880-8. https://dx.doi.org/10.1002/mc.22160.
Received 18.12.2023
Accepted 13.03.2024
About the Authors
Yuliya N. Reshetnikova, PhD Student at the Department of Biomedical Disciplines, Belgorod State National Research University, 308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, resh_yul@mail.ru, https://orcid.org/0009-0004-6123-4086Irina V. Ponomarenko, Dr. Med. Sci., Professor at the Department of Biomedical Disciplines, Belgorod State National Research University, 308015, Russia, Belgorod,
Pobedy str., 85, +7(4722)30-13-83, ponomarenko_i@bsu.edu.ru, https://orcid.org/0000-0002-5652-0166
Evgeny A. Reshetnikov, Dr. Bio. Sci., Professor at the Department of Biomedical Disciplines, Belgorod State National Research University, 308015, Russia, Belgorod,
Pobedy str., 85, +7(4722)30-13-83, reshetnikov@bsu.edu.ru, https://orcid.org/0000-0002-5429-6666
Mikhail I. Churnosov, Dr. Med. Sci., Professor, Head of the Department of Biomedical Disciplines, Belgorod State National Research University, 308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, churnosov@bsu.edu.ru, https://orcid.org/0000-0003-1254-6134
Corresponding author: Evgeny A. Reshetnikov, reshetnikov@bsu.edu.ru



