Potential of angiogenesis-related serum markers for predicting placenta accrete spectrum in pregnant women with placenta previa
Objective: To investigate the predictive value of angiogenesis-related serum markers in the third trimester of pregnancy to assess the likelihood of placenta accreta spectrum (PAS) and the prognosis of emergency delivery in patients with placenta previa. Materials and methods: This case-control study included women in the third trimester of pregnancy (26¬38 weeks) with (group I, n=46) and without PAS (group 2, n=42) managed at the Perinatal Center of Krasnodar Regional Clinical Hospital No. 2. All participants were tested for serum vascular endothelial growth factor (VEGF), placental growth factor (PlGF), soluble fms-like tyrosine kinase-1 (sFlt-1), determined by enzyme- linked immunosorbent assay. PAS was verified intraoperatively during delivery and by histological examination of the surgical specimens. The correlation between biomarker levels of and the depth of placental implantation was analyzed. The predictive value of biomarkers was assessed using multivariate logistic regression and ROC analysis. Results: With an increase in gestational age, patients with PAS group had significantly higher levels PlGF and lower sFlt-1/PlGF ratio than patients without PAS with a difference between groups at >37+0 weeks gestation [Me (IQR) : 778 (751-1057) versus 190 (171-369)pg/ml, p=0.005 and 1.1 (0.8-1.4) versus 8.0 (2.8-20.3), p=0.003, respectively]. The ratio of sFlt-1/PlGF <2.5 predicts PAS with sensitivity (Se) of 65.2% (49.8-78.6%), specificity (Sp) of 59.5%, (43.3-74, 4%), positive predictive value (PPV) of 63.8% (53.6-72.9%), negative predictive value (NPV) of 61.0% (45.9-71.4%), area under ROC-curve (AUC) of0.624(0.514-0.725), p=0.018. The inclusion of maternal age and a history of caesarean section in the predictive model increases the predictive accuracy: Se = 87.0% (73.7-95.1%), Sp = 78.6% (63.2-89.7%), PPV = 81.6% (71.1-89.9%), NPV = 84.6% (72.0-92.1%), AUC=0.886 (0.800-0.944), p<0.001. The PIGFlevel (pg/ml) statistically significantly correlates with the PAS depth (Spearman's rho=0.292; p<0.01). The sFlt-1/PlGF ratio <2.5, combined with maternal age and a history of cesarean section predicts the likelihood of emergency delivery with placenta previa with Se=40.0% (21.1-61.3%), Sp=92, 1% (82.4-97.4%), PPV=66.7% (38.4-88.2%), NPV=79.5% (68.4¬80.0%), AUC=0.789(0.689-0.869), p<0.001. Conclusion: Increased levels of angiogenesis-related serum markers in the third trimester can be used to predict PAS and emergency delivery in pregnant women with placenta previa.Makukhina T.B., Penzhoyan G.A., Amirkhanyan A.M.
Keywords
The placenta accreta spectrum (PAS) is a severe pregnancy complication with a potential risk of adverse maternal and fetal outcomes. The incidence of PAS has been steadily growing in the last ten years, reaching 9:1000 in specialized centers [1]. The strategy for the prevention of adverse PAS outcomes includes early antenatal hospitalization of pregnant women in level 3 centers, early planned surgical delivery with skilled multidisciplinary teams for PAS management and high-tech equipment, taking into account the high risk of massive blood loss, trauma to adjacent organs, hysterectomy and neonatal complications [2–9].
Antenatal diagnosis of PAS is based on risk factors assessment and diagnostic imaging [3, 5, 10]. At the same time, currently available research evidence suggests that the accuracy of antenatal diagnostic imaging, including ultrasound examination and magnetic resonance imaging (MRI)) was lower than previously thought, and false-positive results lead to invasive interventions and early delivery of patients without PAS [5, 11]. Thus, it is essential to study the feasibility of using biological markers to diagnose abnormal placental invasion [12–20].
Among potential serum PAS predictors, markers of angiogenesis are of particular interest [12–14, 15, 19]. On the one hand, the local imbalance of these proteins as a factor of uncontrolled trophoblast invasion has been confirmed by several morphological studies [12, 15, 21]. On the other hand, the disproportion in serum levels of soluble fms-like tyrosine kinase-1 (sFlt-1) and placental growth factor (PlGF) have been proven to have a significant role in the genesis of preeclampsia. This fact suggests the feasibility of early prediction of these severe pregnancy complications [22–24] and the prospects for using this group of analytes in routine clinical practice as a multimodal biomarker. This highlights the need for research investigating the patterns of changes in their blood concentration in pregnant women with various obstetric complications.
The presents study aimed to investigate the predictive value of angiogenesis-related serum markers, including vascular endothelial growth factor (VEGF), PlGF, and sFlt-1 in the third trimester of pregnancy, to assess the depth of placental invasion, the likelihood of PAS, and emergency delivery in patients with placenta previa (PP).
Materials and methods
This case-control study was conducted at the Perinatal Center of Krasnodar Regional Clinical Hospital No. 2. from 01.12.2019 to 01.03.2021. The study was approved by the Ethics Committee of the Krasnodar Regional Clinical Hospital No. 2 (Minutes No. 90 dated 11/13/2019). The study was conducted following the principles of the WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human. The study analyzed serum markers of angiogenesis (PlGF, sFlt-1, VEGF) and the standard examination following Appendix 1 to the Order of the Ministry of Health of the Russian Federation No. 572Н 01.11.2012. The study included women with PP in the third trimester hospitalized in the Obstetric Department of Pathology of Pregnancy of the Perinatal Center. The sampling was carried out after obtaining the patients' written informed consent before the administration of steroids and blood transfusion (if necessary).
Inclusion criteria were antenatal diagnosis O44.0–O44.1 with/without O43.2 (ICD X), documented by ultrasound and /without MRI, gestational age 26–38 weeks, informed written consent of the patient to participate in the study.
Exclusion criteria were multifetal pregnancy, preeclampsia, antenatal fetal death, congenital fetal malformations, which required early termination of pregnancy.
Consent to participate in the study was obtained in 93 patients who met the inclusion criteria. Subsequently, five patients were excluded from the study, two due to loss to follow-up, two due to the exclusion of PP diagnosis, one woman who developed postpartum preeclampsia. A total of 88 patients were included in the study.
Clinical groups
The patients were divided into groups according to the clinical diagnosis, documented intraoperatively, and histological examination of the surgical specimens (if any). The PAS depth was determined following the FIGO recommendations [25]. The specialists who performed the enzyme immunoassay were unaware of the clinical diagnosis of the patients.
Methodology
Peripheral venous blood samples were collected from the study subjects into vacuum tubes with a coagulation activator. After exposure at room temperature for 30 minutes, the samples were centrifuged for 15 minutes at 2500 rpm with centrifugal acceleration RCF 1100 G (Liston C2202 centrifuge (www.liston.ru). Further, the serum samples were immediately frozen and stored at a temperature of -80°C until the analysis. The enzyme immunoassay results were measured and processed using an automatic photometer for microplates INVITROLOGIC (LLC Medico-biological Union, Russia). Quantification of serum markers of angiogenesis was performed using an enzyme immunoassay kit for the quantitative determination of human VEGF receptor 1 (Bender MedSystems Austria, BMS268/3 h VEGF-R1), cat # BMS268/3; cultures, serum, plasma, and urine (R&D Systems, USA, Inc., Quantikine Human PlGF Immunoassay) Cat. No. DPG00 and ELISA kit for the quantitative determination of human VEGF-A (Bender MedSystems Austria, BMS277/2 h VEGF-A), cat. No. BMS277/2.
The range of measurements on the INVITROLOGIC photometer was for PlGF: 7–1000 pg/ml; for VEGF-A: 7.9–1000 pg/ml; for sFlt-1: 0.03–10.0 ng/ml. The coefficient of variation of reproducibility for sFlt-1 within one batch was 5.5%, between batches – 5.1%; for PlGF 5.4 and 11.2%; for VEGF-A 6.2 and 4.3%, respectively.
Groups of PP patients with and without PAS were compared in terms of clinical and demographic characteristics, including race, age, number of pregnancies, parity, number of cesarean sections (CS), body mass index (BMI), somatic comorbidity (chronic arterial hypertension, obesity), obstetric comorbidity (gestational diabetes mellitus), nicotine addiction, gestational age at the time of sampling of biomaterial for analysis. We also compared the outcomes of pregnancies between groups, including gestational age at the time of delivery, emergency delivery, intraoperative blood loss volume, massive blood loss, need for blood transfusion, hysterectomy rate, low birth weight, and neonatal asphyxia at birth.
We analyzed the absolute values of sFlt-1, PlGF, VEGF, and sFlt-1/PlGF ratio in each group, depending on the gestational age. Gestational age was determined from the date of the last menstrual period and the data of the first-trimester screening. The sFlt-1/PlGF ratios were compared with the reference values recommended as standards obtained for the domestic population of pregnant women [22]. The possible relationship between the level of angiogenesis markers in the third trimester and the PAS depth and the likelihood of early emergency delivery was assessed.
The criterion for emergency delivery was a cesarean section in the presence of a threat to the life of the woman or the fetus. Blood loss during childbirth was assessed visually and gravimetrically, measured as absolute blood loss volume and in ml/kg of maternal weight. Massive blood loss was considered to be at least 1.5 L (25-30% of circulating blood volume) at a time or at least 2.5 L (50% of circulating blood volume) in 3 hours [26]. The Fetal Medicine Foundation's centile tables were used to assess whether birth weight matched gestational age [27].
Statistical analysis
Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) were reported. The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. It was found that numerical variables were not normally distributed, and groups were compared using nonparametric tests. Differences between groups were assessed using the Mann–Whitney U test. Categorical variables were presented as counts and percentages.
The sample size calculation for the primary outcome of the probability of PAS versus PP was based on data reported by Schwickert A. et al. [19]. It is calculated that to identify differences in biomarker values between the two groups (Student's t-test) at α=0.05, test power = 95%, at least 38 patients are required in both groups.
Fisher's exact test was used to compare the incidence of PAS risk factors in PP patients. For all statistical tests, two-sided values of the p-level are presented; the critical level of significance (p) was considered at 0.05 (p<0.05). ROC analysis was used to assess the threshold values of the studied variables.
The predictive value of biomarkers was assessed using logistic regression analysis (SPSS Version 26 software (IBM, Chicago, USA) and MedCalc Version 20.011 (MedCalc Software Ltd, Belgium were used). We measured odds ratio (OR), with inclusion in the model adjusted odds ratio (aOR), sensitivity (Se), specificity (Sp), positive and negative predictive values (PPV, NPV), and area under the ROC curve (AUC) with 95% confidence interval (CI) for these operational characteristics. Biomarker thresholds were calculated using Youden's index.
The dependent variable was the presence/absence of PAS and emergency delivery. Predictor variables were the studied biomarkers and risk factors with statistical significance for assessing the probability of PAS and emergency delivery. At the first stage, odds ratios with 95% confidence intervals (95% CI) were calculated for each variable separately as PAS and emergency delivery (univariate analysis). In the second step, adjusted odds ratios were calculated using multivariate binary logistic regression, including variables showing statistical significance in univariate analysis (p<0.05). Predictors were included in the logit model using the stepwise forward selection. To determine the strength of the relationship between clinical variables, the Spearman correlation coefficients with the confidence intervals were calculated, and the statistical significance of the relationship (p) was reported. A semi-partial correlation was used to determine the relationship between the variables corrected for gestational age at blood sampling.
Results and discussion
PAS was verified in 46 patients (Group 1) and excluded in 42 patients (Group 2). All patients were of the white race. Baseline clinical and demographic characteristics did not differ statistically between the groups (p>0.1) in terms of comorbidity, gestational age at the time of sampling, and delivery (Table 1), which ensured that further analysis of characteristics, including biomarker levels, was clean. Comparative analysis showed that patients with PAS were significantly older, had more past pregnancies, deliveries, and cesarean sections, and had significantly higher blood loss volume at delivery (Table 1).
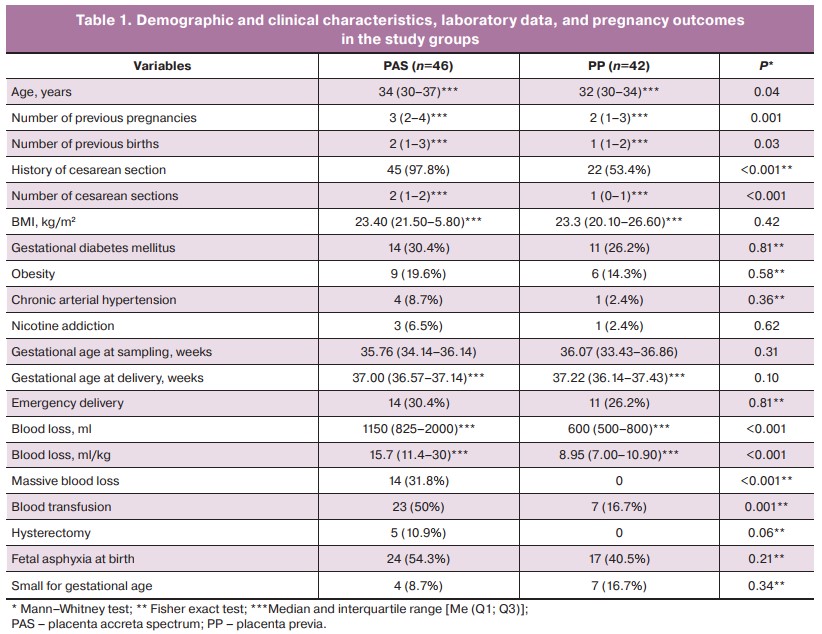
Of the 88 patients enrolled in the study, 25 (28.4%) had emergency deliveries, 11 without, and 14 with PAS. The indications for emergency/non-emergency delivery were antenatal hemorrhage (n=21), fetal distress (n=1), and others (n=3).
Analysis of potential serum PAS biomarkers in the third trimester showed no statistically significant differences between the groups, except for PlGF and the sFlt-1/PlGF ratio (Table 2). Given that in an uncomplicated pregnancy, serum concentration of proangiogenic factors decreases with increasing gestational age in the third trimester [20], we analyzed possible differences between the groups in biomarker levels, taking into account gestational age and the depth of placental invasion in the PAS group (Table 2).
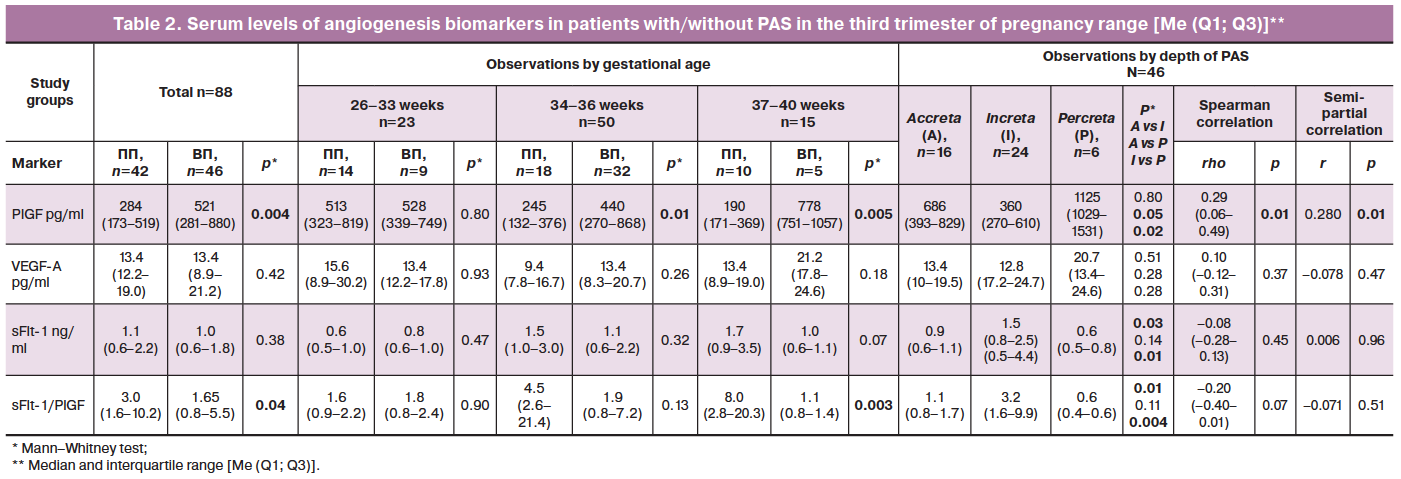
When comparing changes in biomarker concentrations depending on gestational age, it was found that in patients with PP but without PAS, the dynamics of the indices correspond to those in a healthy pregnancy, i.e., PlGF levels decrease by the end of pregnancy, while the sFlt-1/PlGF ratio increases. In PAS, there was an opposite trend: an increase in PlGF with a significant difference between the groups beginning at 34 weeks gestation and a decrease in sFlt-1/PlGF with a significant difference at full-term gestation (Table 2).
Analysis of the probable relationship between depth of PAS and levels of angiogenesis biomarkers showed that with increasing depth of placental invasion, PlGF levels increased and sFlt-1 levels decreased, as did the sFlt-1/PlGF ratio (Table 2). There was a significant direct correlation (Spearman's pairwise correlation method) between PAS depth and PIGF pg/mL concentration (Spearman's rho=0.29; p=0.01), and for sFlt-1/PlGF, at the statistical trend level (Spearman's rho=-0.20; p<0.1). Semi-partial correlation analysis, taking into account the effect of gestational age at blood sampling, showed that the relationship of PAS depth with serum PIGF concentration (pg/ml) remained statistically significant (p=0.01), while the sFlt-1/PlGF relationship completely disappeared (Table 2).
When comparing serum levels of angiogenesis biomarkers between planned and emergency delivery subgroups, a statistically significant difference in sFlt-1/PlGF concentration was found for delivery earlier than 37+0 weeks of pregnancy: sFlt-1/PlGF levels were lower in emergency delivery than in planned delivery (0.9 versus 4.2; p=0.017). At the same time, in emergency cesarean section, serum PlGF serum levels were higher in the PAS group at term (≥37+0 weeks) than in planned cases (Me, pg/mL (Q1–Q3)) (1028.7 (880.2–1190.5) versus 482.9 (242.0–773.6; p=0.045).
To determine the predictive value of clinical predictors and biomarkers in the diagnosis of PAS and the prognosis of emergency delivery, their thresholds were determined by ROC analysis by calculating the Yonden's index (Table 3). For the predictor «maternal age,» the threshold value was «>33 years» (Table 3). As independent predictors of PAS, clinical parameters (history of cesarean section, maternal age) were found to exceed the informative value of biomarkers, but sFlt-1/PlGF<2.5 and PlGF>254.0 pg/ml demonstrated higher combinations of sensitivity and specificity for the prediction of an emergency delivery.
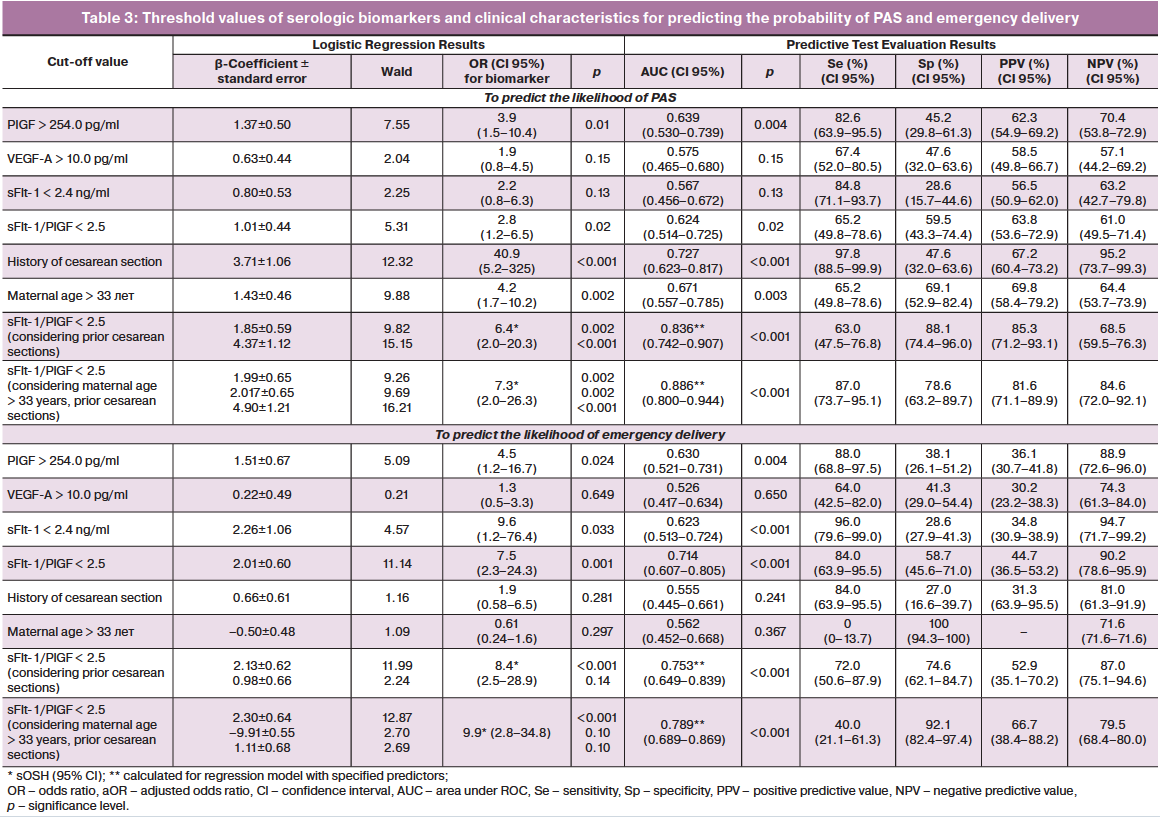
Given the dependence of absolute PlGF level values on the analytical test systems used [13, 14, 22], we regarded the sFlt-1/PlGF ratio as the most promising serological biomarker for use in clinical practice in the third trimester and further evaluated its significance for predicting PAS and emergency delivery in a binary logistic regression model with clinical predictors, which were selected by direct-entry method (Table 3). Inclusion of additional factors (cesarean section; maternal age >33 years) in the regression model increased the predictive significance of the sFlt-1/PlGF marker for assessing the probability of PAS (AUC=0.886, p=0<0.001, PPV=81.6%, NPV=84.6%) (Table 3).
In assessing the probability of an emergency delivery, we found that the resulting model, which takes into account level of biomarkers, maternal age, and a history of cesarean section, is more suitable for predicting the progression of pregnancy to term due to the high specificity and prognostic value of the negative test excluding the probability of an emergency delivery (AUC=0.789, p=0<0.001, Sp=92.1%, NPV=79.5%) (Table 3).
Discussion
Analysis of patient clinical characteristics confirmed that the risk of PAS increases with the number of prior cesarean deliveries and in women of older age with a more significant number of previous pregnancies [3, 5, 8, 9, 19]. The characteristic feature of this study was a comparison group including patients with PP. PP is known to be an independent risk factor for PAS (3, 5, 28), but differences in the pathogenesis of these conditions are poorly understood (13). Thus, the differential diagnosis of PAS in a high-risk group is particularly relevant.
During the implantation and placentation process, the highly invasive nature of trophoblasts is kept in control by the decidua that requires regulation and coordination with angiogenic factors (VEGF, PlGF, sFlt-1) [13, 19, 29]. Ivanets T.Yu. et al. [22], in a study of healthy pregnant women, demonstrated that in uncomplicated pregnancy, the sFlt-1/PlGF ratio has maximum values at 11–14 and 37–40 weeks of pregnancy. The physiological utility of this phenomenon is probably related to the need to prevent uncontrolled chorion invasion during active placentation and in preparation for placental separation with vascular remodeling to prevent massive bleeding in labor. Minimum sFlt-1/PlGF values in uncomplicated pregnancy are observed at 24–33 weeks of gestation. Studies investigating serum angiogenic profiles in patients with PP and PAS are lacking, and the results are controversial (12–14).
VEGF-A is a potent stimulator of angiogenesis and promotes endothelial cell proliferation and migration; its withdrawal can lead to regression of blood vessels (30). Given the application sites of this protein, an increase in its blood level in patients with PAS is to be expected. However, previous studies [12, 14, 19] have found decreased serum VEGF levels in the third trimester in patients with PAS compared with controls (0.8 (0.02–3.4) vs 6.5 (2.7–10.5) pg/ml, p=0.02; 39.18±11.98 vs 85.87±18.05 pg/ml, p<0.001; 285 (95% CI 248–322) vs 391 (95% CI 356–426) pg/ml, p<0.01, respectively). According to Schwickert A. et al. [19], serum VEGF-l level also correlated with the depth of placental invasion (r=-0.32, p<0.01). However, we and Biberoglu E. et al. [13] found no statistically significant differences in this parameter in patients with and without PAS. On the one hand, this controversy can be explained by the different study design. In contrast to the work of Schwickert A. et al. [19], Uyanikoglu H. et al. [14], who compared biomarker concentration between PAS and normal placenta, we chose PP patients as control subjects. On the other hand, despite using the same measuring units, the results in different studies differed by order of magnitude due to differences in the testing procedures used in the various diagnostic panels.
PlGF is involved in placental angiogenesis and spiral artery remodeling [30]. In an uncomplicated pregnancy, its levels change wave-like, with a peak at 30–33 weeks of gestation and a subsequent decrease [22, 30]. Our findings were not consistent with those of Biberoglu E. et al. [13], who found no difference between PlGF levels in the third trimester, regardless of placental position, presence of PAS, and gestational age, and Uyanikoglu H. et al. [14], who found a statistically significant decrease in PlGF levels in patients with placenta percreta compared with patients with normal placenta delivered at the same gestational age. Among our patients with PAS, a paradoxical increase in PlGF was detected at full-term. The higher serum concentration of PlGF compared to patients with PP at full-term (p=0.005) indirectly indicates a persistent proangiogenic phenotype of the placenta accreta spectrum during the third trimester of pregnancy.
sFlt1, a splice variant of the VEGF receptor, acts as a potent VEGF-A and PlGF antagonist by binding these factors from interaction with their receptors [21]. In our data, the differences in sFlt-1 levels between patients with PP in the presence and absence of PAS were less pronounced. Its relatively lower level in patients with PAS revealed only a trend towards statistical significance at full-term (p=0.07). When comparing the findings with reference ranges developed as normative parameters for the domestic population [22], we encountered difficulties interpreting data obtained on different analytical diagnostic tools. At present, the lack of standardized data on maternal blood levels of angiogenesis markers in the third trimester expressed in multiples of the median (MoM) limits their use in daily clinical practice. Therefore, we believe that the most significant interest in terms of practical application is the interpretation of the sFlt-1/PlGF ratio, which provides an integral assessment of the level of placental pro-and anti-angiogenic potential without reference to the absolute values.
In a comparative analysis, the sFlt-1/PlGF ratio was below the standard value for a healthy pregnancy [20] at 34–36 weeks, especially after reaching 37 weeks gestation in both groups. This observation is inconsistent with the opinion of Schwickert A. et al. [19] that PP does not affect serum proangiogenic factors in pregnant women. However, the percentile ranges of the PAS group in our study and the data calculated earlier for a physiological pregnancy at term [22] do not overlap (0.4–1.7 versus 4.4–49.2, respectively), indicating that the differences are statistically significant.
However, one cannot ignore the fact that our patients differed in terms of types of pregnancy course: those carrying a pregnancy to full term and having an elective delivery, and those who underwent emergency cesarean delivery before full-term. Moreover, of the six hysterectomies in PAS patients, five were performed during emergency cesarean section before 37 weeks gestation, indicating the importance of accurate prediction of antenatal hemorrhage. Increased placental proangiogenic potential, resulting in extensive neovascularisation [15], may be associated with an additional risk of antenatal bleeding due to myometrial contractions unrelated to labor activity. In assessing the possible association between the level of placental proangiogenic activity and the likelihood of an emergency delivery, we found a reduced sFlt-1/PlGF ratio in the third trimester of pregnancy in emergency deliveries among the patients included in the study. The predictive accuracy improves when anamnestic factors are included in the model, which can be used for individualized prognosis and timing of delivery in high-risk PAS patients. Although previous studies [28] showed that age was not an independent risk factor for PAS, the inclusion of this predictor in our model improved its predictive power.
Conclusion
The findings of this study suggest that elevated serum angiogenesis markers could be used in the third trimester of pregnancy to predict PAS and emergency delivery in pregnant women with PP.
Study strengths include a control group with PP, which allows the prediction of PAS using angiogenesis biomarkers to be assessed in high-risk patients; the dynamics of the studied parameters at different gestational ages within the third trimester; and the determination of likelihood of an emergency delivery enabling a tailoring treatment for individual patients and the choice of the delivery date.
Limitations of the study: no comparison was made of serum levels of the markers with the local content of the studied proteins in the placenta zone in patients with and without PAS; the limited sample size increases the probability of statistical error.
References
- Abdelhakium A.K. et al. Placenta Accreta Spectrum (PAS) disorders: incidence, risk factors and outcomes of different management strategies in a tertiary referral hospital in Minia, Egypt: a prospective study. BMC Pregnancy Childbirth. 2019; 19(1): 313. https://dx.doi.org/10.1186/s12884-019-2466-5.
- Zabelina T.M., Vasil'chenko O.N., Karimova G.N., Ezhova L.S., Uchevatkina P.V., Shmakov R.G. Delivery of pregnant with the placenta accreta without a scar on the uterus. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 4: 150-6. (in Russian). https://dx.doi.org/10.18565/aig.2021.4.150-156.
- Barinov S.V., Medyannikova I.V., Tirskaya Yu.I., Beznoshchenko G.B., Kadcyna T.V., Lazareva O.V. et al. Prediction of placenta accreta in case of placenta previa. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 1: 61-9. (in Russian). https://dx.doi.org/10.18565/aig.2021.1.61-69.
-
Kurtser M.A., Breslav I. Yu., Grigor'yan A.M., Latyshkevich O.A., Kutakova Yu.Yu., Kondrat'eva M.A. Temporary balloon occlusion of the common iliac arteries during organ-preserving operations in patients with placental accreta. Akusherstvo i ginekologiya: novosti, mneniya, obuchenie/Obstetrics and gynecology: news, opinions, training. 2018; 6(4): 31-7. (in Russian).
https://dx.doi.org/10.24411/2303-9698-2018-14003.
-
Jauniaux E., Hussein А.M., Fox K.A., Collins S.L. New evidence-based diagnostic and management strategies for placenta accreta spectrum disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 2019; 61: 75-88. https://dx.doi.org/10.1016/
bpobgyn.2019.04.006.
- Penzhoyan G.A. Obstetrics service in a large city. Problemy social'noj gigieny, zdravoohraneniya i istorii mediciny/ Problems of social hygiene, health care and the history of medicine. 2003; 3: 37. (in Russian).
- Penzhoyan G.A. The effectiveness of modern perinatal technologies. Problemy social'noj gigieny, zdravoohraneniya i istorii mediciny/ Problems of social hygiene, health care and the history of medicine. 2002; 6: 42. (in Russian).
- Ornaghi S., Maraschini A., Donati S.; Regional Obstetric Surveillance System Working Group. Characteristics and outcomes of pregnant women with placenta accreta spectrum in Italy: A prospective population-based cohort study. PLoS One. 2021; 16(6): e0252654. https://dx.doi.org/10.1371/journal.pone.0252654.
- Lisitsyna O.I., Nizyaeva N.V., Miheeva A.A. Placenta accreta. Evolution of knowledge and skills. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 6: 34-40. (in Russian). https://dx.doi.org/10.18565/aig.2021.6.34-40.
-
Gus A.I., Boykova Yu.V., Yarygina T.A., Yarotskaya E.L. Modern approaches to prenatal diagnosis and screening of placenta accreta (review of recommendations). Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 10: 5-12.
(in Russian). https://dx.doi.org/10.18565/aig.2020.10.5-12.
-
Philips J., Gurganus M., DeShields S., Cunningham T., Sinkovskaya E., Kanaan C. et al. Prevalence of sonographic markers of placenta accreta spectrum in low-risk pregnancies. J. Perinatol. 2019; 36(8): 773-80.
https://dx.doi.org/10.1055/s-0038-1676488.
-
Wehrum M.J., Buhimschi I.A., Salafia C., Thung S., Bahtiyar M.O., Werner E.F. et al. Accreta complicating complete placenta previa is characterized by reduced systemic levels of vascular endothelial growth factor and by epithelial-to-mesenchymal transition of the invasive trophoblast. Am. J. Obstet. Gynecol. 2011; 204(5): 411.e1-411.e11. https://dx.doi.org/10.1016/
ajog.2010.12.027.
- Biberoglu E., Kirbas A., Daglar K., Biberoglu K., Timur H., Demirtas C. et al. Serum angiogenic profile in abnormal placentation. J. Matern. Fetal Neonatal Med. 2016; 29(19): 3193-7.https://dx.doi.org/10.3109/14767058.2015.1118044.
- Uyanıkoğlu H., İncebıyık A., Turp A.B., Çakmak G., Sak S., Hilali N.G. Serum angiogenic and anti-angiogenic markers in pregnant omen with placenta percreta. Balkan Med. J. 2018; 35(1): 55-60. https://dx.doi.org/10.4274/balkanmedj.2016.1890.
- Bartels H.C., Postle J.D., Downey P., Brennan D.J. Placenta Accreta Spectrum: A review of pathology, molecular biology, and biomarkers. Dis Markers. 2018; 2018: 1507674. https://dx.doi.org/10.1155/2018/1507674.
- Timofeeva A.V., Fedorov I.S., Pirogova M.M., Vasilchenko O.N., Chagovets V.V., Ezhova L.S. et al. Clusterin and its potential regulatory microRNAs as a part of secretome for the diagnosis of abnormally invasive placenta: Accreta, increta, and percreta cases. Life (Basel). 2021; 11(4): 270. https://dx.doi.org/10.3390/life11040270.
- Al-Khan A., Youssef Y.H., Feldman K.M., Illsley N.P., Remache Y., Alvarez-Perez J. et al. Biomarkers of abnormally invasive placenta. Placenta. 2020; 91: 37-42. https://dx.doi.org/10.1016/j.placenta.2020.01.007.
-
Makukhina T.B., Penzhoyan G.A., Lebedenko E.S., Khorolsky V.A., Solntseva A.V., Kazanchi F.B. Possibilities of early prediction of perinatal outcomes in the first trimester in patients with placenta previa complicated by its abnormal invasion. Akusherstvo i ginekologiya: novosti, mneniya, obuchenie/ Obstetrics and gynecology: news, opinions, training. 2020; 8 (1): 47-52 (in Russian).
https://dx.doi.org/10.24411/2303-9698-2020-11006.
- Schwickert A., Chantraine F., Ehrlich L., Henrich W., Muallem M.Z., Nonnenmacher A. et al. Maternal serum VEGF predicts abnormally invasive placenta better than NT-proBNP: A Multicenter Case-Control Study. Sci. 2021; 28(2): 361-70. https://dx.doi.org/10.1007/s43032-020-00319-y.
- Vinitskiy A.A., Shmakov R.G. Modern ideas about the etiopathogenesis of placental accreta and the prospects for its prediction by molecular diagnostic methods. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; 2: 5-10. (in Russian). https://dx.doi.org/10.18565/aig.2017.2.5-10.
-
McMahon K., Karumanchi S.A., Stillman I.E., Cummings P., Patton D., Easterling T. Does soluble fms-like tyrosine kinase-1 regulate placental invasion? Insight from the invasive placenta. Am. J. Obstet. Gynecol. 2014; 210(1):
e1-4. https://dx.doi.org/10.1016/j.ajog.2013.08.032.
- Ivanets T.Yu., Alekseeva M.L., Kan N.E., Tyutyunnik V.L., Amiraslanov E.Yu., Nasonova D.M., Fanchenko N.D. Diagnostic significance of determining placental growth factor and soluble fms-like tyrosine kinase-1 as markers of preeclampsia. Problemy reprodukcii / Problems of reproduction, 2015; 4: 129-133 (in Russian). https://dx.doi.org/10.17116/repro2015214129-133.
- Umapathy A., Chamley L.W., James J.L. Reconciling the distinct roles of angiogenic/anti-angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies. Angiogenesis. 2020; 23(2): 105-17. https://dx.doi.org/10.1007/s10456-019-09694-w.
- Gur'eva V.M., Travkina A.A., Matveev M.O., Morokhotova L.S., Budykina T.S., Kotov Yu.B., Semenova T.A. Clinical significance of sFlt-1 / PlGF in the diagnosis and prognosis of preeclampsia. .Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 7: 195-200. (in Russian). https://dx.doi.org/10.18565/aig.2021.7.195-200.
- Jauniaux E., Ayres-de-Campos D., Langhoff-Roos J., Fox K.A., Collins S.; FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. J. Gynaecol. Obstet. 2019; 146(1): 20-4. https://dx.doi.org/10.1002/ijgo.12761.
- Shifman E.M., Kulikov A.V., Protsenko D.N., Ovezov A.M., Zabolotskikh I.B., Artymuk N.V. et al. Anesthesia and intensive care for massive blood loss in obstetrics. clinical guidelines (treatment protocols). Voprosy ginekologii, akusherstva i perinatologii/ Questions of gynecology, obstetrics and perinatology. 2018; 17(3): 81-100. (in Russian).
- Nicolaides K.H., Wright D., Syngelaki A., Wright A., Akolekar R. Fetal medicine foundation fetal and neonatal population weight charts. Ultrasound Obstet. Gynecol. 2018; 52: 44-51. https://dx.doi.org/10.1002/uog.19073.
- Bowman Z.S., Eller A.G., Bardsley T.R., Greene T., Varner M.W., Silver R.M. Risk factors for placenta accreta: a large prospective cohort. J. Perinatol. 2014; 31(9): 799-804. https://dx.doi.org/10.1055/s-0033-1361833.
- Sokolov D.I., Kolobov A.V., Lesnichiya M.V., Kostyuchek I.N., Bolya K.V., Arzhanova O.N. et al. The role of pro-angiogenic and anti-angiogenic factors in the development of the placenta. Medicinskaya immunologiya/Medical immunology. 2008; 10 (4-5): 347-52. (in Russian).
- Palm M., Basu S., Larsson A., Wernroth L., Åkerud H., Axelsson O. A longitudinal study of plasma levels of soluble fms-like tyrosine kinase 1 (sFlt-1), placental growth factor (PlGF), sFlt-1: PlGF ratio and vascular endothelial growth factor (VEGF-A) in normal pregnancy. Acta Obstet. Gynecol. Scand. 2011; 90(11): 1244-51. https://dx.doi.org/10.1111/j.1600-0412.2011.01186.x.
Received 03.09.2021
Accepted 18.11.2021
About the Authors
Tatiana B. Makukhina, Kuban State Medical University, Ministry of Health of Russia; Regional Clinical Hospital No. 2, Ministry of Health of Krasnodar Region, soltatiana@mail.ru, https://orcid.org/0000-0003-0536-4500Gregory A. Penzhoyan, Kuban State Medical University, Ministry of Health of Russia, pga05@mailru, https://orcid.org/0000-0002-8600-0532
Arpine M. Amirkhanyan, Kuban State Medical University, Ministry of Health of Russia; Regional Clinical Hospital No. 2, Ministry of Health of Krasnodar Region, arm2035@yandex.ru, https://orcid.org/0000-0002-4051-6875
Corresponding author: Tatiana B. Makukhina, soltatiana@mail.ru
Authors' contributions: Makukhina T.B., Penzhoyan G.A. - the conception and design of the study; Makukhina T.B., Amirkhanyan A.M. - data collection and analysis; Makukhina T.B. - statistical analysis, manuscript drafting; Penzhoyan G.A. - manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Makukhina T.B., Penzhoyan G.A., Amirkhanyan A.M. Potential of angiogenesis-related serum markers for predicting placenta accreta spectrum i n pregnant women with placenta previa.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 1:62-71 (in Russian)
https://dx.doi.org/10.18565/aig.2022.1.62-71



