Ultrasound criteria of uterine scar secondary to placenta accreta spectrum metroplasty and use of platelet-rich plasma
Objective: To evaluate the effects of platelet-rich plasma (PRP) injections on uterine scar formation following metroplasty in patients with placenta accreta spectrum. Materials and methods: This blinded randomized controlled trial included 100 women who underwent surgery for placenta accreta spectrum between November 2020 and July 2022. Patients who met the inclusion criteria were randomly assigned to receive intramyometrial PRP injections (interventional group, n=48) or not (control group, n=52). Patients in both groups were examined at 12 weeks postoperatively. Clinical evaluation included ultrasound examination according to the Delphi protocol, analysis of clinical data, and assessment of medical history and quality of life. Results: Comparison of the scar characteristics showed a significantly lower incidence of niche formation in the intervention group (31.2 %) compared to control group (65.4 %) (p<0.001). The mean residual myometrium thickness (RMT) in the study and control groups was 7.2 (2.95) and 4.3 (3.5) mm, respectively (p<0.001). The largest niche widths were 7 and 11 mm (p<0.001), the largest depths were 6 and 8 mm (p=0.018), and the largest lengths were 11 and 13 mm. (p=0.101) mm in the study and control groups, respectively. The thickness of the adjacent myometrium was comparable between groups (p=0.143). Conclusion: This pilot randomized trial showed that intraoperative intramyometrial PRP injection is an effective technique for improving myometrial tissue regeneration and enhancing the quality of uterine scars in the metroplasty area. Therefore, this technology can be proposed for use in the routine practice of operative obstetrics, including for metroplasty after placenta accreta spectrum. Authors’ contributions: Mikheeva A.A., Yarygina T.A., Shmakov R.G. – conception and design of the study; Mikheeva A.A., Yarygina T.A., Kostyukov K.V. – material collection and processing, manuscript drafting; Mikheeva A.A., Yarygina T.A. – statistical analysis; Shmakov R.G., Rogachevsky O.V., Pyregov A.V. – manuscript editing. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P. Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors’ Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Mikheeva A.A., Yarygina T.A., Shmakov R.G., Kostyukov K.V., Rogachevsky O.V., Pyregov A.V. Ultrasound criteria of uterine scar secondary to placenta accreta spectrum metroplasty and use of platelet-rich plasma. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (3): 41-50 (in Russian) https://dx.doi.org/10.18565/aig.2023.19Mikheeva A.A., Yarygina T.A., Shmakov R.G., Kostyukov K.V., Rogachevsky O.V., Pyregov A.V.
Keywords
The problem of uterine scarring after operative delivery has been actively debated in the medical literature in recent years due to the steady multifactorial increase in caesarean section rates [1]. In this context, there has been increasing interest in the long-term consequences of operative delivery, such as residual myometrium thickness (RMT) and niche formation in scar areas [2, 3].
The presence of a niche and thin residual myometrium thickness has been associated with obstetric complications, such as scar rupture or dehiscence, adherent placenta, ectopic pregnancy at the cesarean section scar, abnormal uterine bleeding, dysmenorrhea, postmenstrual spotting, pelvic pain, infertility, threatened miscarriage, and/or preterm birth [3–5]. To date, metroplasty has been used to correct a niche in the uterine scar area to reduce the incidence of these complications, but the effectiveness of repeated uterine surgeries still requires further research [6, 7].
A pressing problem today in clinical practice is the development of techniques that prevent the development of myometrial defects in the scar area [8]. The need to find effective integrated approaches to accelerate tissue regeneration is due to the exponential increase in the incidence of placenta accreta at cesarean scars. This results in uterine hernia formation, complete thinning of the myometrium in the uterine scar with pathologic invasion of the placenta into adjacent organs and tissues, and neo-angiogenesis between the uterus and bladder [9, 10].
One of the latest new methods for regenerating the myometrium after caesarean section is the injection of platelet-rich plasma (PRP) into the area of the uterine scar (11). PRP is produced from fresh whole blood obtained from the patient’s peripheral vein 10–15 minutes before surgery and stored in an anticoagulant acid-citrate dextrose solution during transport to the centrifuge. Two-stage centrifugation was then performed in a rotary angle centrifuge, and the ready for injection of PRP was isolated (Fig. 1).
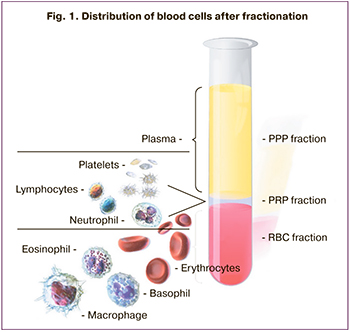
When injected into the wound area, platelets are activated and aggregate to release granules containing growth factors, including vascular endothelial growth factor, transforming growth factor, platelet-derived growth factor, and epidermal growth factor, which regulate cell migration, cell attachment, proliferation, and differentiation and promote extracellular matrix accumulation [12]. Currently, PRP is widely used in various areas of medicine, such as orthopedics, ophthalmology, and surgery, to treat wounds and improve tissue regeneration.
In the reviewed literature, the effectiveness of PRP has been found in surgical interventions in gynecology and obstetrics, including the treatment of patients with infertility due to thin endometrium and improving the quality of healing of uterine and skin scars after caesarean section [12, 13]. The effects of PRP on myometrial regeneration after metroplasty for placenta accreta spectrum have not been investigated.
This study aimed to evaluate the effects of platelet-rich plasma injections on uterine scar formation following metroplasty in patients with placenta accreta spectrum.
Materials and methods
Initially, we calculated the required sample size and determined the statistical power of the study. To determine the required sample size, the following calculations were performed: with an expected niche formation rate in the scar area in the control group of 60% and an expected two-fold decrease in the niche formation rate in patients with PRP (intervention group) for alpha 5% and beta 20% risks (statistical power of the study was 80%). The required sample size was 84 patients, including 42 women in the study and control groups. We recruited 156 pregnant women under the assumption of a potential loss to follow-up.
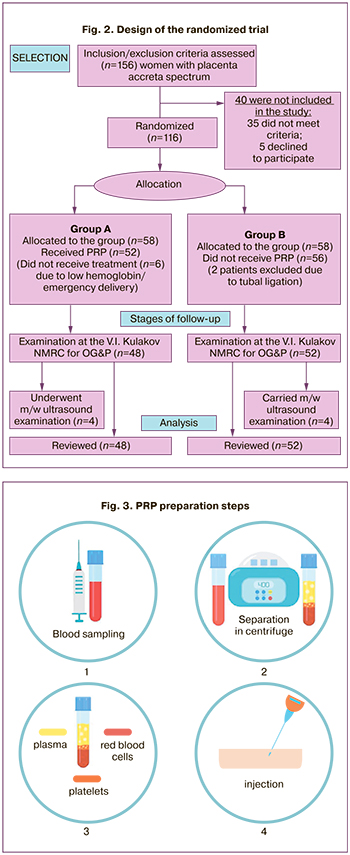
According to the calculation of the required sample size, a blinded randomized clinical trial was conducted at Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation from 2020 to 2022. A total of 156 patients were included in this study. Of these, 40 women were excluded from the study in the first phase:35 did not meet the inclusion criteria and 5 refused to participate.
The inclusion criteria were age 18–45 years, uterine scar after previous caesarean section, placenta accreta detected by ultrasound and MRI, and informed consent to participate in the study.
Exclusion criteria were severe maternal non-obstetric disease, acute infectious disease or exacerbation of chronic disease, large uterine myoma/multiple uterine myomas, current or history of cancer, and absence of consent from the patient. The exclusion criteria for blood sampling and PRP injection were syphilis, HIV, hepatitis B and C, systemic blood diseases and coagulopathies, hemoglobin level < 100 g/l, platelets less than 100×109/l, platelet abnormalities and dysfunction, use of non-steroidal anti-inflammatory drugs, anticoagulation, and antiaggregant therapy.
The exclusion criteria were tubal ligation, inability to return to the center for expert ultrasound, and willingness of the patient to discontinue participation in the study.
The superiority hypothesis was selected as the test hypothesis. The primary outcome was the percentage of niche formation and residual myometrial thickness. Uterine vascular resistance parameters at 12 weeks after surgery were defined as secondary outcomes. Opaque sealed and sequentially numbered envelopes were used to generate a random allocation sequence. One hundred and sixteen opaque envelopes (58 for each group) were prepared in advance, and the scheme was indicated (with and without PRP therapy and intraoperative administration of PRP). To reduce systematic errors, the radiologist was not aware (blinded) to the therapy method. All patients were informed of the aim and objectives of this study, its stages, further monitoring, and gave consent to participate.
All women (n=100) were divided into two groups after selection according to the inclusion criteria. The study group (n=48) consisted of patients who received PRP after organ-sparing surgery and metroplasty for placenta accreta spectrum, and a comparison group (n=52) without PRP injections into the scar area. Four patients from the study group were excluded from the study because of low hemoglobin levels, two due to emergency delivery, and two women from the control group were excluded due to tubal ligation after delivery. In addition, four patients from each group dropped out of the study due to post-metroplasty ultrasound follow-up at their place of residence (Fig. 2).
We analyzed clinical, anamnestic, instrumental and laboratory findings. Clinical evaluation included maternal age, previous uterine surgery, information on previous pregnancies (number of previous caesarean sections), blood loss volume, and duration of surgery [14]. All patients with suspected placenta accrete spectrum underwent surgical treatment with midline laparotomy, lower segment caesarean section, complex compression hemostasis, balloon uterine tamponade, and metroplasty. After the uterine incision was closed, participants in the intervention group received PRP isolated from the patient’s blood in the incision area (both on the upper and lower sides of the hysterotomy between the decidual tissue and myometrium), while the control group did not receive PRP.
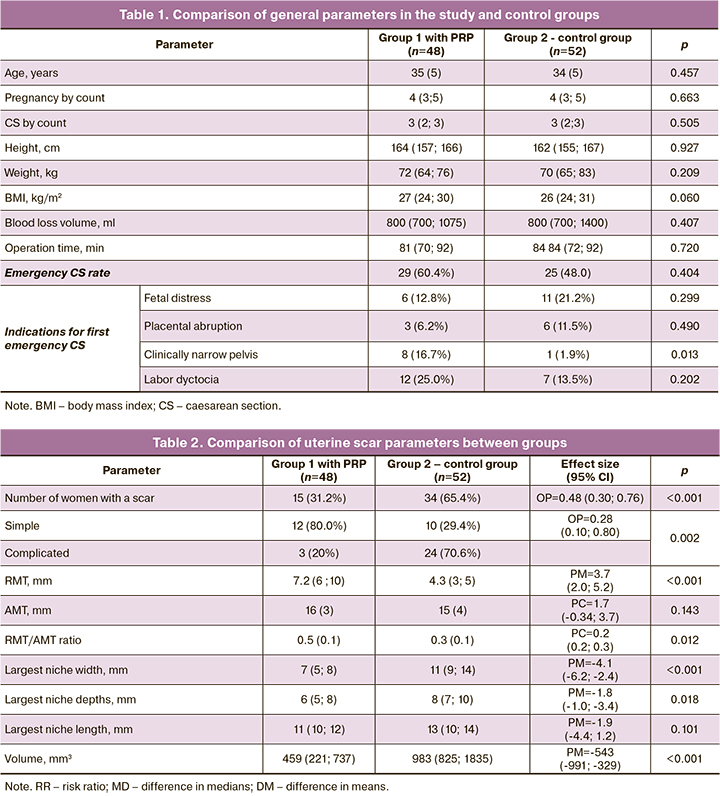
For PRP preparation, venous blood was perfused 10–15 minutes before the operation with 13.5 ml in a syringe containing 1.5 ml of anticoagulant (total volume, 15 ml). Blood with citrate was then transferred to an hourglass-shaped tube and centrifuged for 5–7 minutes at 3500 rpm. Centrifugation resulted in approximately 3-4 ml PRP. In the control group, PRP was not injected into the metroplasty area (Fig. 3).
Furthermore, the aponeurosis, subcutaneous fat, and skin were repaired in the same manner in both groups. All surgeries were performed by the same obstetric team. To maximize the objectivity of the ultrasound assessment of the uterine scar (12 weeks after surgery), the examiner was blinded to the patient group.
It has been scientifically proven that the stimulating effect of concentrated plasma begins when the platelet concentration exceeds the initial values by more than four times. To confirm the effectiveness of PRP, the clinical and diagnostic laboratory of the Centre performed platelet counts in PRP on a hematological analyzer, Sysmex XP-350. The platelet count (765–2738 10*9/l) coincided and was even slightly higher than the results of other studies [15].
Ultrasound was performed using a Voluson S6 Expert (GE Healthcare, Austria) and SAMSUNG MEDISON WS80A (Samsung Medison, South Korea) expert class systems using 3D\4D transvaginal convex multifrequency (2–8 MHz) and 3D\4D intracavitary multifrequency (5-13 MHz) sensors. Twelve weeks after surgery, transvaginal ultrasound of the uterine scar and ultrasound Doppler of blood flow in the uterine arteries were performed. Examination of the uterine scar area was performed according to the criteria previously described in the 2019 Delphi Protocol, and the following concepts were approved by consensus [16]:
1) niche: indentation at the site of the CS scar with a depth of at least 2 mm.
2) branch niche: a thinner part of the main niche, which is directed towards the serosa and has a width smaller than that of the main niche.
There are three subclasses of niches based on the presence of branches:1) simple niche, 2) simple niche with one branch, and 3) complex niche (with more than one branch) [8, 16].
The main parameters used for ultrasound assessment of the uterine scar area are “healing ratio” (residual myometrial thickness (RMT)/adjacent myometrial thickness (AMT)) and niche parameters. Accurate measurement and standardized description of the niche in the ultrasound protocol are fundamentally important for the clinical assessment of gynecological symptoms, planning of surgical treatment, and subsequent pregnancies.
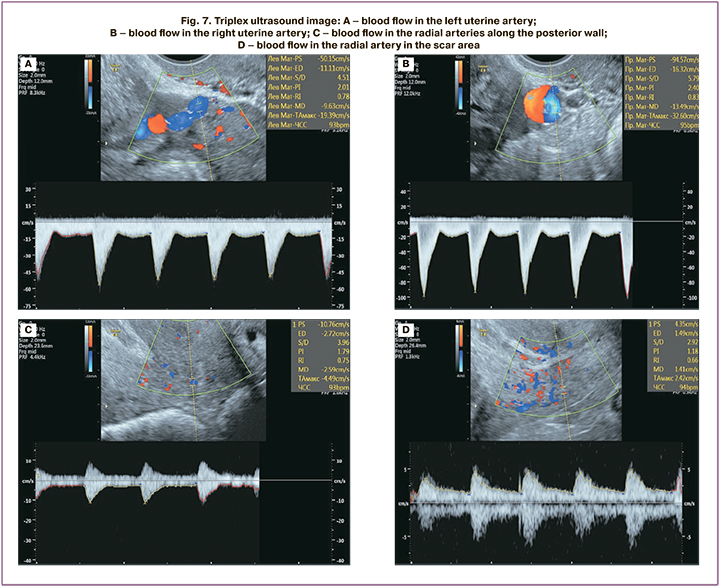
Ultrasound Doppler ultrasound was additionally used to determine the uterine vascular resistance index (left and right uterine arteries, radial arteries in the uterine scar, and posterior uterine wall region).
Statistical analysis
Statistical analysis was performed using StatTech v. 2.8.8 (Stattech Ltd., Russia). The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. Quantitative variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and 95% confidence interval (95% CI); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. Categorical variables were compared using the Fisher’s exact test. Continuous variables were compared using the Student’s t-test. The difference in means (DM) with a 95% confidence interval was used as the effect measure. Variables that did not meet normality assumptions were compared using a nonparametric Mann–Whitney test. The median difference (MD) with 95% confidence interval was used as an effect measure. Categorical variables were reported as frequencies and percentages. Risk ratio (RR) with a 95% confidence interval was used as an effect measure. Differences were considered statistically significant at p<0.05.
Results
The mean age of the study population was 34 (5) years, and the majority of the patients were multipara. The study population was homogeneous, with no significant differences in terms of age, weight, height, parity, degree of placental invasion, and body mass index (Table 1). The emergency caesarean section rate for the first operative delivery was 28/48 (58.3%) in group 1 and 29/52 (55.8%) in group 2. Of these, fetal distress in labor, placental abruption, a clinically narrow pelvis, and labor dystocia were the most common (Table 1).
All surgeries in this study were performed using the tourniquet hemostasis technique by a team of surgeons according to a well-established pattern of surgical stages. Postoperative ultrasound was performed 12 weeks later, and a niche in the uterine scar area was detected in 49% of the women. Among the niche cases, 15/48 (31.2%) and 34/52 (65.4%) were in the PRP-treated and control groups, respectively (p<0.001) (Fig. 4).
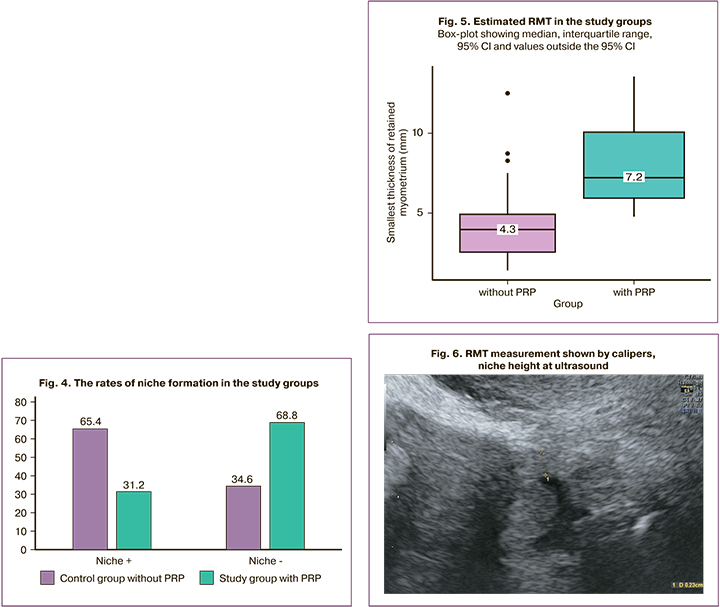
The RMT was significantly higher in the intervention group than in the control group at 7.2 (6; 10) vs. 4.3 (3; 5) mm (p<0.001) (Fig. 5, 6).
There was no difference in the AMT between the two groups (p=0,143). When the niche parameters were analyzed, we obtained the following results: the largest niche widths were 7 and 11 mm (p<0.001), the largest depths were 6 and 8 mm (p=0.018), and the largest lengths were 11 and 13 (p=0.101) mm in the study and control groups, respectively. Niche volume was significantly smaller in the intervention group, 459 versus 983 mm3 in the control group; p<0.001). The myometrial parameters in the uterine scar area after metroplasty are presented in Table 2.
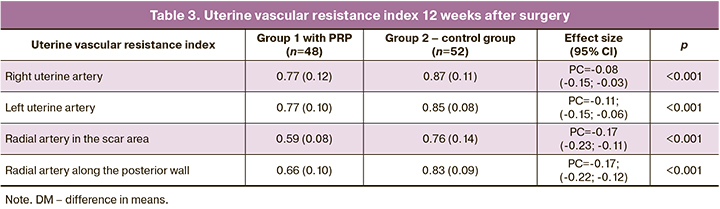
Doppler blood flow parameters at 12 months after operative delivery and metroplasty for placenta accreta spectrum showed lower resistance in the right uterine artery, left uterine artery, radial artery of the scar area, and radial artery at the posterior uterine wall, compared to those without intervention (Table 3).
Complications associated with uterine scar defects after caesarean section have prompted scientists to delve deeper into this problem and study this area more extensively [17]. Recently, some studies have focused on the formation of niches or thin residual myometrium in scar areas [18]. Surgical techniques, including uterine suturing (single- or double-layered sutures) and suture material, are among the important factors determining scar healing and the occurrence of subsequent complications [19, 20]. An even more pressing and understudied problem is the formation of niches after metroplasty, when a large area of the myometrium is excised and, naturally, tissue approximation and regeneration are significantly reduced.
PRP has been considered a safe therapy for tissue healing for many years. PRP consists of growth factors, cytokines, chemokines, and fibrin matrix, which in turn stimulates wound healing. The fact that platelets secrete growth factors and active metabolites indicates that their use can have a positive effect in clinical situations requiring rapid effects and tissue regeneration [21].
In an era of rapid advances in regenerative medicine, the use of PRP in obstetric practice has become particularly relevant. Our results largely coincide with a similar double-blind placebo-controlled randomized clinical trial published by Chaichian S. et al. (2021), where the rate of formation of a healthy scar after caesarean section in the group that received PRP therapy was significantly higher than that in women in the control group. In the study cohort, PRP was administered intramyocardially after uterine incision was closed [21, 22].
Despite several publications on the effect of PRP on myometrial regeneration after caesarean section, the integrity of the scar and RMT after metroplasty for placental accreta spectrum has not been investigated. In this study, we evaluated the efficacy and safety of PRP in terms of uterine scar integrity and thickness. Our results showed a favorable effect of injecting PRP into the uterine scar area after metroplasty on the scar integrity and thickness. Fewer niches, fewer complications, and higher RMT were observed in the intervention group than in the control group. In the present study, niche formation in the PRP group was nearly one-third of that in the control group, and this difference was statistically significant. No side effects of PRP injections were reported by the study participants [23].
These results are consistent with previous studies suggesting a positive effect of injectable PRP as an effective treatment modality to improve wound healing after gynecological surgery because of its ability to stimulate angiogenesis and initiate an inflammatory response [12, 21]. However, according to the authors, no study has evaluated the effectiveness of PRP for uterine scarring after metroplasty for placenta accreta spectrum, which can be compared with our study. Therefore, this issue requires new well-designed, randomized controlled trials in the future.
Conclusion
Randomized trial conducted at the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology has shown high efficacy and safety of PRP injection, as compared to no PRP application. For the first time, the use of PRP after metroplasty for placenta accreta spectrum allows for precise impact on the area of the myometrium, improvement of regeneration processes, quality healing of the scar associated with angiogenesis, and proliferation of cells under the influence of growth factors. Our results, including lower blood flow resistance in the uterine vessels, especially in the scar area, indicate an effective process of vascularization and regeneration in the uterine scar area after PRP injection during metroplasty.
The results of this pilot study confirmed the efficacy of PRP as a promising and safe modality for preventing scar formation and uterine scar dehiscence. Therefore, PRP injection can be used as a potential preventive measure and incorporated into the routine practice of obstetricians and gynecologists. Further studies are required to confirm the efficacy of PRP in various settings.
References
- Soto-Vega E., Casco S., Chamizo K., Flores-Hernÿndez D., Landini V., Guillén-Florez A. Rising trends of cesarean section worldwide: a systematic review. Obstet. Gynecol. Int. J. 2015; 3(2): 00073. https://dx.doi.org/10.15406/ogij.2015.03.00073.
- Stegwee S.I., Jordans I.P.M., van der Voet L.F., Bongers M.Y., de Groot C.J.M., Lambalk C.B. et al. Single-versus double-layer closure of the caesarean (uterine) scar in the prevention of gynaecological symptoms in relation to niche development–the 2Close study: a multicentre randomised controlled trial. BMC Pregnancy Childbirth. 2019; 19(1): 85. https://dx.doi.org/10.1186/s12884-019-2221-y.
- Litwicka K., Greco E. Caesarean scar pregnancy: a review of management options. Curr. Opin. Obstet. Gynecol. 2013; 25(6): 456-61.https://dx.doi.org/10.1097/GCO.0000000000000023.
- Bij de Vaate A.J., van der Voet L.F., Naji O., Witmer M., Veersema S., Brolmann H.A. et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: systematic review. Ultrasound Obstet. Gynecol. 2014; 43(4): 372-82.https://dx.doi.org/10.1002/uog.13199.
- Hou R., Liu C., Li N., Yang T. Obstetric complications and outcomes of singleton pregnancy with previous caesarean section according to maternal age. Placenta. 2022; 128: 62-8. https://dx.doi.org/10.1016/j.placenta.2022.08.060.
- Supermainam S., Koh E.T. Laparoscopic partial bladder cystectomy for bladder endometriosis: a combined cystoscopic and laparoscopic approach. J. Minim. Invasive Gynecol. 2020; 27(3): 575-6. https://dx.doi.org/10.1016/j.jmig.2019.06.020.
- Tower A.M., Frishman G.N. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J. Minim. Invasive Gynecol. 2013; 20(5): 562-72. https://dx.doi.org/10.1016/j.jmig.2013.03.008.
- Jordans I.P.M., De Leeuw R.A., Stegwee S.I., Amso N.N., Barri‐Soldevila P.N., van den Bosch T. et al. Sonographic examination of uterine niche in non‐pregnant women: a modified Delphi procedure. Ultrasound Obstet. Gynecol. 2019; 53(1): 107-15. https://dx.doi.org/10.1002/uog.19049.
- Flood K.M., Said S., Geary M., Robson M., Fitzpatrick C., Malone F.D. Changing trends in peripartum hysterectomy over the last 4 decades. Am. J. Obstet. Gynecol. 2009; 200(6): 632. e1-632. e6. https://dx.doi.org/10.1016/j.ajog.2009.02.001.
- Morlando M., Sarno L., Napolitano R., Capone A., Tessitore G., Maruotti G.M. et al. Placenta accreta: incidence and risk factors in an area with a particularly high rate of cesarean section. Acta Obstet. Gynecol. Scand. 2013; 92(4): 457-60. https://dx.doi.org/10.1111/aogs.12080.
- Dawood A.S., Salem H.A. Current clinical applications of platelet-rich plasma in various gynecological disorders: An appraisal of theory and practice. Clin. Exp. Reprod. Med. 2018; 45(2): 67-74. https://dx.doi.org/10.5653/cerm.2018.45.2.67.
- Tehranian A., Esfehani-Mehr B., Pirjani R., Rezaei N., Sadat Heidary S., Sepidarkish M. Application of autologous platelet-rich plasma (PRP) on wound healing after Caesarean section in high-risk patients. Iran. Red Crescent Med. J. 2016; 18(7): e34449. https://dx.doi.org/10.5812/ircmj.34449.
- Аполихина И.А., Эфендиева З.Н., Федорова Т.А., Белоусов Д.М., Вишнякова П.А., Артемова Д.А., Фатхудинов Т.Х. Обогащенная тромбоцитами аутологичная плазма в комплексной терапии женщин с рефрактерным «тонким» эндометрием. Акушерство и гинекология. 2021; 4: 112-9. [Apolikhina I.A., Efendieva Z.N., Fedorova T.A.,Belousov D.M., Vishnyakova P.A., Artemova D.A., Fatkhudinov T.Kh.Platelet-rich autologous plasma in the complex therapy of women with refractory "thin" endometrium. Obstetrics and Gynecology. 2021; (4): 112-9.(in Russian)]. https://dx.doi.org/10.18565/aig.2021.4.112-119.
- Hamar B.D., Saber S.B., Cackovic M., Magloire L.K., Pettker C.M., Abdel-Razeq S.S. et al. Ultrasound evaluation of the uterine scar after cesarean delivery: A randomized controlled trial of one- and two-layer closure. Obstet. Gynecol. 2007; 110(4): 808-13. https://dx.doi.org/10.1097/01.AOG.0000284628.29796.80.
- Hall M.P., Band P.A., Meislin R.J., Jazrawi L.M., Cardone D.A. Platelet-rich plasma: current concepts and application in sports medicine. J. Am. Acad. Orthop. Surg. 2009; 17(10): 602-8. https://dx.doi.org/10.5435/00124635-200910000-00002.
- Гус А.И., Ярыгина Т.А., Михеева А.А., Воеводина В.И., Шмаков Р.Г. Стандартизированное исследование послеоперационного рубца на матке. Акушерство и гинекология. 2022; 1: 42-7. [Gus A.I., Yarygina T.A., Mikheeva A.A., Voevodina V.I., Shmakov R.G. Standardized study of postoperative scar on the uterus. Obstetrics and Gynecology. 2022; (1): 42-7.(in Russian)]. https://dx.doi.org/10.18565/aig.2022.1.42-47.
- Кабатин Н.А., Калинин В.В., Щерина А.В., Полонецкий А.Я., Смирнов А.В. Синдром Огилви как редкое хирургическое осложнение после операции кесарева сечения. Акушерство и гинекология. 2021; 5: 199-203. [Kabatin N.A., Kalinin V.V., Shcherina A.V., Polonetsky A.Ya., Smirnov A.V. Ogilvie's syndrome as a rare surgical complication after caesarean section. Obstetrics and Gynecology. 2021; (5): 199-203. (in Russian)].https://dx.doi.org/10.18565/aig.2021.5.199-203.
- Fiocchi F., Petrella E., Nocetti L., Curra S., Ligabue G., Costi T. et al. Transvaginal ultrasound assessment of uterine scar after previous caesarean section: comparison with 3T-magnetic resonance diffusion tensor imaging. Radiol. Med. 2015; 120(2): 228-38. https://dx.doi.org/10.1007/s11547-014-0431-y.
- van der Voet L.L.F., Limperg T., Veersema S., Timmermans A., Bij de Vaate A.M.J., Brolmann H.A.M., Huirne J.A.F. Niches after cesarean section in a population seeking hysteroscopic sterilization. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017; 214: 104-8. https://dx.doi.org/10.1016/j.ejogrb.2017.05.004.
- Anitua E., Andia I., Ardanza B., Nurden P., Nurden A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Tromb. Haemost. 2014; 91: 4-15. https://dx.doi.org/10.1160/TH03-07-0440.
- Chaichian S., MirgaloybayatS., Tahermanesh1 K., Hossein Mohammadi M., Mostafavi R.S., Mehdizadehkashi A. et al. Effect of autologous platelet–rich plasma on cesarean section scar; a randomized, double-blinded pilot study. Shiraz E-Med. J. 2022; 23(1): e114072. https://dx.doi.org/10.5812/semj.114072.
- Marx R. What determines the therapeutic effectiveness of the injectable form of PRP? Cortexil PRP. 2022; 22: 12-8.
- Gentile P., Garcovich S. Systematic review – the potential implications of different platelet-rich plasma (Prp) concentrations in regenerative medicine for tissue repair. Int. J. Mol. Sci. 2020; 21(16): 5702. https://dx.doi.org/10.3390/ijms21165702.
Received 26.01.2023
Accepted 08.02.2023
About the Authors
Alexandra A. Mikheeva, Postgraduate Student, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia, shuratora@mail.ru, https://orcid.org/0000-0002-8159-6373,117997, Russia, Moscow, Oparina str., 4.
Tamara A. Yarygina, PhD, Diagnostic Medical Sonographer, Perinatal Cardiology Center, A.N. Bakulev NMRC of Cardiovascular Surgery of Minzdrav of Russia,
+7(495)414-78-75, tamarayarygina@gmail.com, https://orcid.org/0000-0001-6140-1930, 121552, Russia, Moscow, Roublyevskoe Shosse, 135.
Roman G. Shmakov, Dr. Med. Sci., Professor, Director of the Institute of Obstetrics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia, +7(495)438-72-00,
r_shmakov@oparina4.ru, https://orcid.org/0000-0002-2206-1002, 117997, Russia, Moscow, Oparina str., 4.
Kirill V. Kostyukov, Dr. Med. Sci., Head of the Ultrasound Department, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia, +7(926)214-97-84, kostyukov_k@yahoo.com, https://orcid.org/0000-0003-3094-4013, 117997, Russia, Moscow, Oparina str., 4.
Oleg V. Rogachevsky, Dr. Med. Sci., Head of the Department of Extracorporal Methods of Treatment and Detoxification, Professor at the Department of Anesthesiology
and Resuscitation, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia, +7(495)438-71-35, о_rogachevskiy@oparina4.ru, https://orcid.org/0000-0002-4332-430X,
117997, Russia, Moscow, Oparina str., 4.
Aleksey V. Pyregov, Dr. Med. Sci., Director of the Institute of Anesthesiology and Intensive Care, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia,
+7(495)438-78-30, pyregov@oparina4.ru, https://orcid.org/0000-0001-8382-9671, 117997, Russia, Moscow, Oparina str., 4.



