The potential of magnetic resonance imaging in the diagnosis of placenta accreta
Ralnikova A.Yu., Arakelyan B.V., Morozov A.N., Voydak I.V., Bezhenar V.F.
Placenta accreta spectrum (PAS) is one of the most serious complications of pregnancy and the postpartum period. Despite this, a considerable number of PAS cases remain undetected in the prehospital stage. Ultrasonography (US) and magnetic resonance imaging (MRI) are commonly used for PAS diagnosis. A correct prenatal diagnosis is necessary to reduce maternal and fetal complications associated with this pathology. This article provides an analysis of the MRI parameters used to identify PAS.
Objective: This study aimed to investigate the potential of magnetic resonance imaging (MRI) in optimizing the surgical treatment strategy in patients with placenta accreta spectrum.
Materials and methods: This study retrospectively analyzed the medical records of 87 patients who gave birth at the obstetrics and gynecology clinic of Pavlov First St. Petersburg State Medical University, Ministry of Health of Russia. Pregnant women with placenta accreta (n=45, 52%) were divided into two groups based on the outcome of surgical delivery. Group 1 included 30 (66.7%) patients who underwent cesarean section and metroplasty, whereas Group 2 comprised 15 patients (33.3%) who had to undergo hysterectomy. The diagnosis was confirmed postoperatively in all the patients through histopathological examination. The control group included 42 pregnant women (48 %) with no placental pathology.
Results: The study found that the most common MRI marker of placenta accreta, present in 82.2% of cases, was an abnormal structure of the uteroplacental interface. The second most common marker was the presence of hypointense stripes in placental tissues, observed in 73.3% of cases. When analyzing the sensitivity (Se) and specificity (Sp) indicators, the following results were obtained: disruption of the structure of the uteroplacental interface: Se 82.2%, Sp 76.2%; deformation of the bladder wall: Se 85.7%, Sp 81.6%; the presence of wide hypointense bands in the placenta: Se 73.3%, Sp 100%; changes in the structure of placental tissue and thinning of the myometrium in the area of the lower segment: Se 64.4%, Sp 66.7%; intramural and parametric hypervascularization: Se 62.2%, Sp 83.3%. Significantly, in forms of placenta accreta characterized by the deepest invasion into the cervix and underlying structures, such as the parametrium, the MRI marker «naked vessel» was encountered more frequently. In all cases of this sign during MRI studies in our sample of pregnant women, surgical delivery had to be completed with hysterectomy.
Conclusion: MRI is an adjunct to the diagnosis of placenta accreta. Initially, all at-risk patients should undergo US of the uterus and placenta. However, when it is necessary to assess pathological changes in the uterine blood supply and topography of placental invasion, MRI is of greater interest than ultrasound diagnostics.
Authors’ contributions: Bezhenar V.F., Arakelyan B.V., Ralnikova A.Yu. – conception and design of the study; Ralnikova A.Yu., Voydak I.V., Morozov A.N. – material collection and processing; Ralnikova A.Yu. – statistical analysis; Ralnikova A.Yu., Arakelyan B.V. – drafting of the manuscript; Arakelyan B.V., Bezhenar V.F. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical approval: The study was reviewed and approved by the Research Ethics Committee of Pavlov First SPSMU.
Patient consent for publication: All patients provided informed consent for the publication of their data.
Authors’ data sharing statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Ralnikova A.Yu., Arakelyan B.V., Morozov A.N., Voydak I.V., Bezhenar V.F.
The potential of magnetic resonance imaging in the diagnosis of placenta accreta.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (12): 125-132 (in Russian)
https://dx.doi.org/10.18565/aig.2023.227
Keywords
Placenta accreta spectrum (PAS) is an urgent challenge in light of advances in uterine surgery and the increase in cesarean section (CS) rates. CS commonly leads to acute massive bleeding in patients with placenta accreta. Obstetric hemorrhage is a primary cause of maternal mortality, complicating up to 8% of all deliveries [1].
Approximately 10% of obstetric bleeding cases arise from placenta accreta and its separation disorders [2]. However, the etiology and pathogenesis of this condition have not been adequately explored. The average incidence of placenta accreta is approximately 1 case per 1000–2500 births [3, 4]. With the escalating trend in CS and uterine surgeries globally, the risk of placenta accreta has increased proportionately [5]. Methods involving organ-sparing interventions using various angiographic and surgical regional blood flow block techniques are being actively developed and implemented [2, 5–8]. However, implementing this approach requires specialized facilities and multidisciplinary departments, which are not typically found in regular city maternity hospitals.
Managing a pregnant woman with PAS mandates planned delivery, assembling an operating team comprising specialists from diverse fields: obstetricians-gynecologists, vascular surgeons, interventional surgeons, anesthesiologists, transfusiologists, neonatologists, and, in cases where the placenta infiltrates adjacent organs and structures, an expanded roster of related specialists [2]. Timely prenatal diagnosis is crucial to ensure proper management of such cases [8–11]. Diagnosing placenta accreta is challenging due to its vague clinical presentation and the absence of specific ultrasound signs in cases where the placenta remains normally located and minimally invades the myometrium.
Ensuring the timely detection of placenta accreta necessitates increased clinical vigilance in women with a history of CS, myomectomy, or placenta previa. Ultrasound imaging is the "gold standard" for primary diagnosis [9]. However, the accuracy of ultrasound findings depends significantly on the expertise of the clinician; detecting the region of interest and reliably assessing invasion signs is not always feasible.
Magnetic resonance imaging (MRI) is essential for precise visualization and assessment of topography and tissue relationships, especially when the abnormal placentation area lies along the posterior uterine wall and is inaccessible to ultrasound imaging. Typically, optimal visualization is achieved on T2-weighted images. The timing of the MRI is crucial, considering the physiological thinning of the normal myometrium by full-term gestation and the heterogeneous structure of the placenta. Additionally, fetal movement complicates distinguishing the pathology from the norm. Consequently, the optimal timing for MRI falls between 30 and 35 weeks of pregnancy [8, 9, 12, 13].
A joint consensus statement by the Society of Abdominal Radiology (SAR) and the European Society of Urogenital Radiology (ESUR) has outlined seven primary MRI signs of placenta accreta, including T2-dark bands, placental bulge, Loss of T2 hypointense interface, myometrial thinning/rupture, bladder wall interruption, focal exophytic mass, and abnormal vascularization of the placental bed [11, 13]. Among the additional prognostic indicators is the naked vessel, characterized by a linear structure of at least 5 mm in diameter and approximately 2 cm in length. Hypervascularity on MRI presents a specificity of up to 80%, with a sensitivity ranging from 42 to 69% [14]. Macroscopic examination revealed a network of subchorionic branched stem vascular structures, typically 3–5 mm in diameter and 2–8 cm in length [15]. These vessels, minimally surrounded by chorionic tissue, penetrate the placental thickness, giving rise to a few branches. Studies have suggested that vessel diameter correlates with PAS severity and the risk of periportal bleeding [16].
In diagnosing placenta accreta, the challenge lies not only in choosing delivery tactics, timing, access, surgical techniques, and devascularization methods but also in minimizing false-positive results. Overdiagnosis may prompt premature delivery and increase perinatal morbidity [17].
This study aimed to investigate the potential of magnetic resonance imaging in optimizing the surgical treatment strategy in patients with placenta accreta spectrum.
Materials and methods
The study retrospectively analyzed the medical records of patients (mean age 36.4 (5.1) years) who gave birth at the Obstetrics and Gynecology Clinic of Pavlov First St. Petersburg State Medical University, Ministry of Health of Russia from 2017 to 2021.
Pregnant women with placenta accreta (n=49) were divided into two groups based on the outcome of surgical delivery. Group 1 included 34 patients who underwent CS and metroplasty, Group 2 included 15 patients who had to undergo hysterectomy. The control group included 42 pregnant women without placental pathologies. The reasons for organ-sparing surgery (hysterectomy) in group 2 were the technical inability to perform metroplasty due to invasion of placental tissue in the parametrium and in the area of the internal os and/or total circular invasion of cervical tissue.
All patients delivered between 34 and 36 weeks of gestation, at a time sufficient for a favorable prognosis for newborns, but also early enough to minimize the risk of emergency surgery due to the development of labor. The fetus was removed through a classical corporal uterine incision or the Fritsch approach, depending on the placentation area. After placing the cut umbilical cord into the uterine cavity, the incision was sutured with a double-row suture, followed by metroplasty in the invasion area. Regional blood flow was blocked in all cases by applying tourniquets to the common iliac arteries and ovarian suspensory ligaments before metroplasty. The mean blood loss in cases completed by metroplasty was 2253 (729) ml according to gravimetry. In patients requiring hysterectomy, blood loss was 3500.0 (2375; 5230) mL; Cell-Saver technology was used in all cases.
Before surgery, all the patients underwent US and MRI. MRI was performed from 20 to 36 weeks of gestation (median 32 weeks) on MRI scanners with a magnetic field strength of 1.5 Tesla, using standard sequences (T1, T2-weighted images) with fat suppression, DWI with b=1000, delayed and without breath holding in three orthogonal planes, oriented along the uterine and placental axes, with the patient in the supine position. Two patients with PAS and multiple pregnancies, one with a metal skull structure, and one with claustrophobia were excluded from the study.
Statistical analysis
Statistical analysis was performed using StatTech v software. 3.1.8, and Microsoft Excel. The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. The Mann–Whitney U test was used to compare the two groups for continuous variables with a non-normal distribution. Categorical variables were compared using contingency tables and Pearson’s chi-squared test. Analysis of sensitivity, specificity, and positive and negative predictive values was performed using the 2×2 contingency tables.
Results
Analysis of the MRI findings included the following:
- disruption of the uteroplacental interface (loss of the hypointense line);
- homogeneity/heterogeneity of the placenta structure;
- thickness of the placental disc;
- presence of intraplacental dark bands;
- intramural and parametrial hypervascularity;
- bladder wall interruption;
- myometrial thickness.
Special diagnostic findings were also noted, such as bridge-like vessels, which are perpendicular vessels at the border of the placenta with the myometrium, and the symptom of a naked vessel–a vessel with a diameter of 3–4 mm, more than 2 cm long, not giving collateral branches along its path (Table 1).
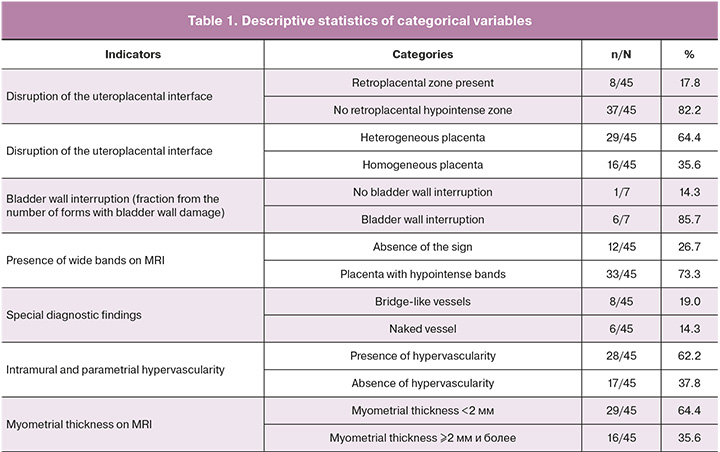
For the described qualitative features, we calculated sensitivity (Se), specificity (Sp), and predictive value. An analysis of the frequency of occurrence according to the morphological type of PAS and its correlation with surgical outcome was also carried out. When calculating these indicators, we compared the MRI data of pregnant women with true placenta accreta (histologically verified, n=45) with the MRI data of patients whose diagnosis was not histologically confirmed (control group, n=42) (Table 2).
Based on the data obtained, we can conclude that the most specific sign for diagnosing placenta accreta is the presence of hypointense bands in the placental tissue, corresponding to the presence of pathological vascularization in these tissues.
According to the data obtained, when comparing invasions at different depths, significant differences in changes in the bladder wall were established (p=0.005, Pearson Chi-square). Bladder wall interruptions were detected in 85.7% of the cases of placenta percreta; however, the sign also occurred with less deep placental invasion that did not reach the bladder wall. The presence of bladder wall interruption and suspicion of exophytic inclusions in the lumen of the bladder often give false-positive results due to bullous edema detected on preoperative cystoscopy and dilation of the submucosal vessels.
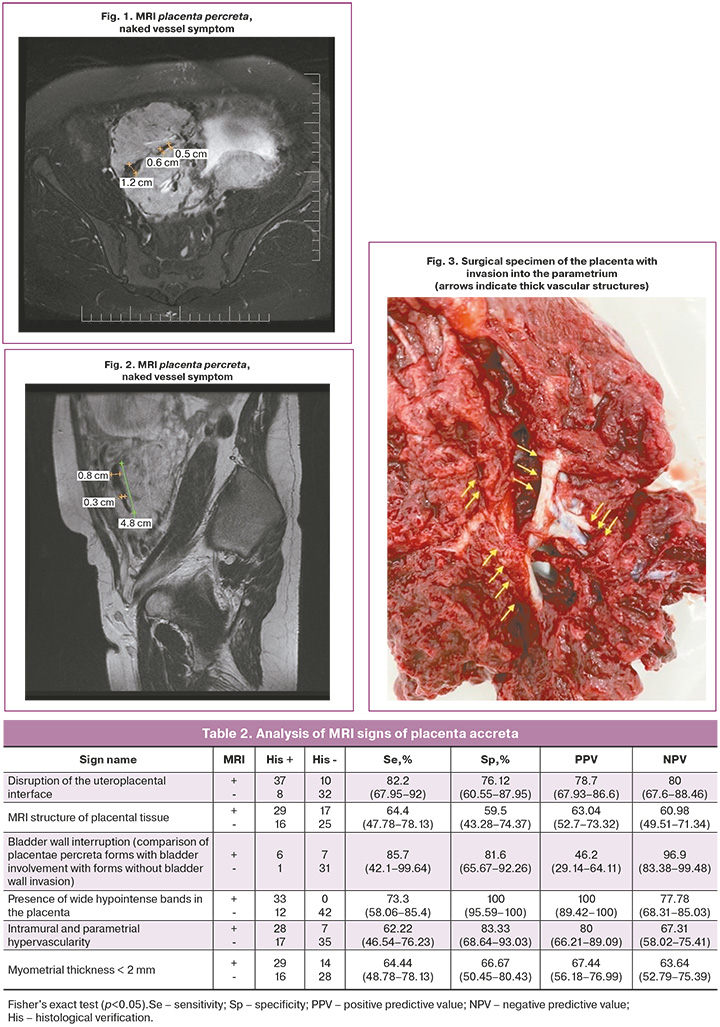
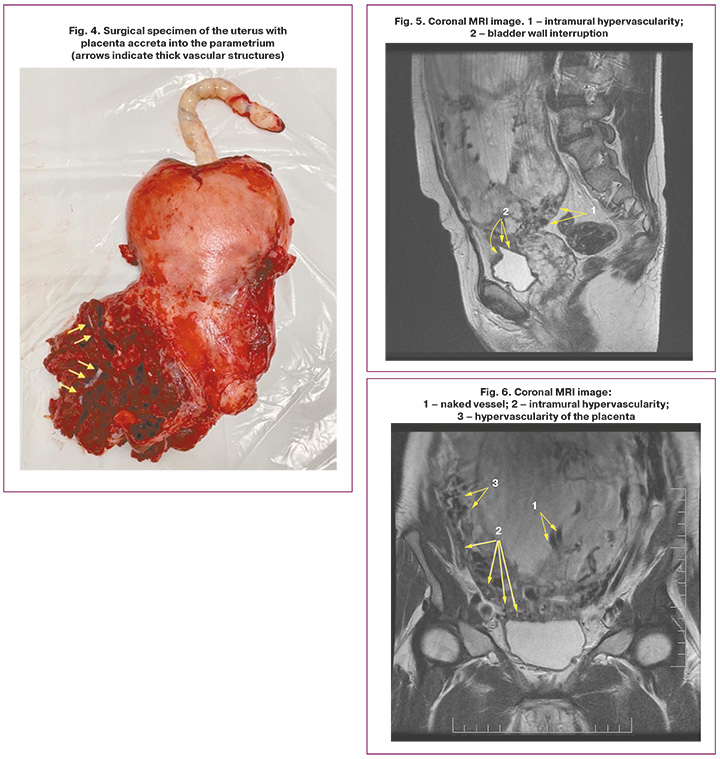
It is also worth highlighting a special type of placental hypervascularity, namely, the inclusion of naked vessel-type changes in the placental tissue (Fig. 1, 2). We analyzed the occurrence of this symptom in our patients. Interestingly, this symptom in our sample occurred only in the presence of placenta percreta – 6 cases, namely, with damage to the parametrium, and was not encountered in placental invasion limited to the myometrium. Bridge-like vessels were less specific; however, their presence was accompanied by severe forms of PAS caused by deeper invasion of the chorionic villi (n=8).
Whether the naked vessel sign is pathognomonic for parametrial invasion remains to be determined; however, this study established its association with the rate of hysterectomies in groups with the presence of the naked vessel, which is likely due to a strong vascular network of collaterals and neoangiogenesis. All patients with this sign subsequently had to undergo hysterectomy (Fig. 3–6, patient N., 37 years old).
The median placental thickness was 49 (16) mm, ranging from 22 mm to 79 mm. Myometrial thickness is also not a reliable diagnostic marker of invasion, since at the end of the third trimester, the normal thickness of the myometrium of a healthy uterus can reach approximately 2 mm. This sign is misleading and has not been found to correlate with the morphology of abnormal placentation and surgical outcomes.
Conclusion
MRI is an adjunct diagnostic modality for placenta accreta, and all at-risk patients should initially undergo ultrasound of the uterus and placenta. However, when it is necessary to assess pathological changes in the uterine blood supply and topography of placental invasion, MRI is more useful than ultrasound diagnostics.
Disruption of the uteroplacental interface (82.2%) and the presence of wide hypointense bands in the placenta (73.3%) (Sp, 100%) are indicative of pathological invasion. Additionally, markers such as intramural and parametrial hypervascularity (Se, 62.2%; Sp, 83.3%) also suggest pathological invasion of the placenta. Comparing these data may assist in predicting the delivery strategy, including the selection of the level and method of blocking regional blood flow, composition of the surgical team, and determining the final extent of surgical intervention.
It is possible that, in cases of expected deep invasion of the cervix and parametrium, when it is technically impossible to restore normal uterine anatomy with a high probability, it may be advisable to delay the timing of the intervention closer to full-term gestation.
References
- Баев О.Р. Послеродовое кровотечение. Что нового? Медицинский оппонент. 2018; 1(4): 58-64. [Baev O.R. Postpartum bleeding. What's new? Medical opponent. 2018; 1(4): 58-64. (in Rusian)].
- Либова Т.А., Аракелян Б.В., Резник В.А., Рухляда Н.Н., Прохорович Т.И. Способ уменьшения объема кровопотери при врастании плаценты. 2 698 051 C1 Российская Федерация. [Libova T.A., Arakelyan B.V., Reznik V.A., Rukhlyada N.N., Prokhorovich T.I. A way to reduce the volume of blood loss during placental growth. 2,698,051 C1 Russian Federation. (in Russian)].
- Palacios-Jaraquemada J.M., D'Antonio F., Buca D., Fiorillo A., Larraza P. Systematic review on near miss cases of placenta accreta spectrum disorders: correlation with invasion topography, prenatal imaging, and surgical outcome. J. Matern. Fetal. Neonatal. Med. 2020; 33(19): 3377-84. https://dx.doi.org/10.1080/14767058.2019.1570494.
- Kayem G., Seco A., Beucher G., Dupont C., Branger B., Crenn Hebert C. et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021; 128(10): 1646-55. https://dx.doi.org/10.1111/1471-0528.16647.
- Куликов И.А., Шмаков Р.Г., Белоусова Т.Н., Плахотина Е.Н., Низяева Н.В., Гейлис И.А., Искаков Д.Д., Милютина Е.Р., Вдовиченко Е.А., Прочаковский Д.В. Эффективность метода дистального компрессионного гемостаза с применением турникетов в сочетании с баллонной тампонадой влагалища вагинальным катетером Жуковского при родоразрешении беременных с врастанием плаценты. Акушерство и гинекология. 2022; 10: 58-66. [Kulikov I.A., Shmakov R.G., Belousova T.N., Plakhotina E.N., Nizyaeva N.V., Geilis I.A., Iskakov D.D., Milyutina E.R., Vdovichenko E.A., Prochakovsky D.V. Efficacy of the tourniquet hemostasis combined with vaginal balloon tamponade using a Zhukovsky vaginal catheter during delivery in placenta accreta spectrum Obstetrics and Gynecology. 2022; (10): 58-66(in Russian)]. https://dx.doi.org/10.18565/aig.2022.10.58-66.
- Ральникова А.Ю., Беженарь В.Ф., Аракелян Б.В., Линде В.А., Габелова К.А., Молчанов О.Л. Успешное органосохраняющее хирургическое лечение у пациентки с врастанием предлежащей плаценты в область рубца на матке. Акушерство и гинекология. 2020; 2; 183-9. [Ralnikova A.Yu., Bezhenar V.F., Arakelyan B.V., Linde V.A., Gabelova K.A., Molchanov O.L. Successful organ-sparing surgical treatment in a patient with the placenta previa growing into the uterine scar region. Obstetrics and Gynecology. 2020; (2): 183-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.2.183-189.
- Курцер М.А., Григорьян А.М., Бреслав И.Ю., Амбарцумян Г.А., Мкртычян Б.Т., Сергачева А.С. Непосредственные результаты временной баллонной окклюзии общих подвздошных артерий при врастании плаценты. Эндоваскулярная хирургия. 2021; 8(2): 176-84. [Kurtser M.A., Grigoryan A.M., Breslav I.Yu., Ambartsumyan G.A., Mkrtychyan B.T., Sergacheva A.S. Immediate results of temporary balloon occlusion of the common iliac arteries in placenta accreta. Russian Journal of Endovascular Surgery. 2021; 8(2): 176-84 (in Russian)]. https://dx.doi.org/10.24183/2409-4080-2021-8-2-176-184.
- Chanclud J., Nguyen T., Alison M., Thomassin-Naggara I., Adamsbaum C., Bazot M. et al. Imagerie des anomalies d’insertion placentaire : le rôle du radiologue. Journal D’imagerie Diagnostique et Interventionnelle. 2020; 10: 1-12. doi:10.1016/j.jidi.2020.10.013.
- Expert Panel on Women’s Imaging; Poder L., Weinstein S., Maturen K.E., Feldstein V.A., Mackenzie D.C., Oliver E.R. et al. ACR Appropriateness Criteria® Placenta Accreta Spectrum Disorder. J. Am. Coll. Radiol. 2020; 17(5S): S207-S214. https://dx.doi.org/10.1016/j.jacr.2020.01.031.
- Palacios-Jaraquemada J.M., Fiorillo A., Hamer J., Martínez M., Bruno C. Placenta accreta spectrum: a hysterectomy can be prevented in almost 80% of cases using a resective-reconstructive technique. J. Matern. Fetal. Neonatal. Med. 2022; 35(2): 275-82. https://dx.doi.org/10.1080/14767058.2020.1716715.
- Jha P., Pōder L., Bourgioti C., Bharwani N., Lewis S., Kamath A. et al. Society of Abdominal Radiology (SAR) and European Society of Urogenital Radiology (ESUR) joint consensus statement for MR imaging of placenta accreta spectrum disorders. Eur. Radiol. 2020; 30(5):2604-15. https://dx.doi.org/10.1007/s00330-019-06617-7.
- Horowitz J.M., Berggruen S., McCarthy R.J., Chen M.J., Hammond C., Trinh A. et al. When timing is everything: are placental MRI examinations performed before 24 weeks' gestational age reliable? AJR Am. J. Roentgenol. 2015; 205(3): 685-92. https://dx.doi.org/10.2214/AJR.14.14134.
- Kilcoyne A., Shenoy-Bhangle A.S., Roberts D.J., Sisodia R.C., Gervais D.A., Lee S.I. MRI of placenta accreta, placenta increta, and placenta percreta: pearls and pitfalls. AJR Am. J. Roentgenol. 2017; 208(1): 214-21. https://dx.doi.org/10.2214/AJR.16.16281.
- Bourgioti C., Konstantinidou A.E., Zafeiropoulou K., Antoniou A., Fotopoulos S., Theodora M. et al. Intraplacental fetal vessel diameter may help predict for placental invasiveness in pregnant women at high risk for placenta accreta spectrum disorders. Radiology. 2021; 298(2): 403-12. https://dx.doi.org/10.1148/radiol.2020200273.
- Konstantinidou A.E., Bourgioti C., Fotopoulos S., Souka E., Nikolaidou M.E., Zafeiropoulou K., Moulopoulos L.A. Stripped fetal vessel sign: a novel pathological feature of abnormal fetal vasculature in placenta accreta spectrum disorders with MRI correlates. Placenta. 2019; 85: 74-7. https://dx.doi.org/10.1016/j.placenta.2019.07.005.
- Dighe M. Intraplacental fetal vessels: an additional sign for placenta accreta spectrum. Radiology. 2021; 298(2): 413-4. https://dx.doi.org/10.1148/radiol.2020204118.
- Morlando M., Collins S. Placenta accreta spectrum disorders: challenges, risks, and management strategies. Int. J. Womens Health. 2020; 12: 1033-45.https://dx.doi.org/10.2147/IJWH.S224191.
Received 04.10.2023
Accepted 14.11.2023
About the Authors
Anna Yu. Ralnikova, Obstetrician-Gynecologist at the Department of Pregnancy Pathology, Clinic of Obstetrics and Gynecology, I.P. Pavlov First St. Petersburg State Medical University, Ministry of Health of Russia, Anna.ralnikova1510@gmail.com, https://orcid.org/0000-0003-1875-4567,197022, Russia, St. Petersburg, Lev Tolstoy str., 6-8.
Buzand V. Arakelyan, Dr. Med. Sci., Professor, Department of Obstetrics, Gynecology and Neonatology, Deputy Head of the Clinic of Obstetrics and Gynecology,
I.P. Pavlov First St. Petersburg State Medical University, Ministry of Health of Russia, byuzand@mail.ru, https://orcid.org/0000-0002-2868-7997,
197022, Russia, St. Petersburg, Lev Tolstoy str., 6-8.
Vitaly F. Bezhenar, Dr. Med. Sci., Professor, Head of the Department of Obstetrics, Gynecology and Neonatology/Department of Obstetrics, Gynecology and Reproductology, Head of the Clinic of Obstetrics and Gynecology, I.P. Pavlov First St. Petersburg State Medical University, Ministry of Health of Russia, bez-vitaly@yandex.ru,
https://orcid.org/0000-0002-7807-4929, 197022, Russia, St. Petersburg, Lev Tolstoy str., 6-8.
Alexey N. Morozov, Head of X-ray Computed Tomography Department No. 1, I.P. Pavlov First St. Petersburg State Medical University, Ministry of Health of Russia, morozovan1983@gmail.com, 197022, Russia, St. Petersburg, Lev Tolstoy str., 6-8.
Inna V. Voydak, Radiologist, X-ray Computed Tomography Department No.1, I.P. Pavlov First St. Petersburg State Medical University, Ministry of Health of Russia,
voydak@spbgmu.ru, https://orcid.org/0000-0001-8203-3305, 197022, Russia, St. Petersburg, Lev Tolstoy str., 6-8.
Corresponding author: Anna Yu. Ralnikova, Anna.ralnikova1510@gmail.com



