The role of angiogenic factors in the pathogenesis of placenta accreta spectrum in women with placenta previa
Objective: To investigate the contribution of angiogenic factors to the pathogenesis of placenta accreta spectrum (PAS) in women with placenta previa to identify additional biomarkers of abnormal placental invasion. Materials and methods: Baseline evaluation included measurement of serum concentrations of PlGF, VEGF, sFlt-1, Doppler ultrasound in women with placenta previa (n=42) and with PAS (n=46), immunohistochemistry of local expression of VEGF and Flt-1in placenta previa (n=17), pl. accreta (n=16), pl. increta (n=18), pl. percreta (n = 6) groups. Results: Women with PAS had lower expression of Flt-1 in chorionic villi than those without PAS. This was found both in (p=0.02; <0.001; 0.003 for pl. accreta, increta, percreta, respectively) and outside of the invasion zone for invasive forms of PAS (p=0.005; 0.003 for pl. increta, percreta, respectively). These changes were associated with a decrease in serum sFlt-1/PlGF in PAS (1.65 vs 3.0, p=0.035). The increase in local VEGF expression in PAS was not in parallel with the changes in serum concentrations of this biomarker. Women with PAS were more likely to have increased subplacental vascularization according to color Doppler; a combination of ≥ 3 US signs (91.3% vs 40.5%; 67.4% vs 11.9%, p<0.001<0.001, respectively). There were no differences in uteroplacental hemodynamics between groups according to spectral Doppler (p=0.323). Ultrasound for the diagnosis of PAS demonstrated sensitivity of 95.7%, specificity of 54.8%, positive predictive value of 69.8%, negative predictive value of 92.0%, and accuracy of 76.1%. Conclusion: The decrease in serum sFlt-1/PlGF in pregnant women with PAS in the third trimester is consistent with a decrease in local Flt-1 expression in the chorionic villi and can be used as an additional biomarker of PAS in women with placenta previa. Assessment of multiple ultrasound signs of PAS may improve the specificity and accuracy of antenatal diagnosis in patients at risk.Makukhina T.B., Penzhoyan G.A., Morozova R.V., Zadornaya O.I., Dontsova M.V., Krivonosova N.V., Amirkhanyan A.M.
Keywords
The placenta accreta spectrum (PAS) is a complex process that involves various mechanisms [1–4]. Understanding their determining patterns could allow early diagnosis and subsequent prevention of abnormal placentation [3–6].
Ultrasound is the main diagnostic tool among methods of antenatal diagnosis of PAS [7], but its diagnostic accuracy is significantly reduced in patients without a history of surgery and when the placenta is located on the posterior uterine wall [8]. The addition of biochemical markers to these imaging techniques appears promising in improving the accuracy of PAS diagnosis [9–13], especially in high-risk patients, including pregnant women with placenta previa and uterine scars. Among numerous biochemical markers of PAS, angiogenetic factors have been actively studied in recent years [14–19]. This group of proteins affects the morphological phenotype of the placenta [14, 20], and disruption of the serum balance of proangiogenic factors (vascular endothelial growth factor (VEGF), placental growth factor (PlGF)) and their antagonists (soluble fms-like tyrosine kinase-1 (sFlt-1)) precede the clinical manifestation of preeclampsia, which makes their serum concentrations increasingly used in routine clinical practice [21]. A number of studies have found changes in the blood levels of angiogenetic factors in pregnant women with PAS [18, 19, 22]. However, it remains unclear whether these changes are associated or related predictors of abnormal placentation. To clarify the presence of a direct relationship, it is necessary to compare the level of angiogenetic factors in the blood with immunohistochemical analysis of their expression in the placental zone.
This study aimed to investigate the contribution of angiogenic factors to the pathogenesis of the placenta accreta spectrum in women with placenta previa to identify additional biomarkers of abnormal placental invasion.
Materials and methods
This is a case-control study that was conducted in the Perinatal Center of the Regional Clinical Hospital No. 2 (Krasnodar) according to the ethical principles of the Declaration of Helsinki. The study included 88 consecutive patients with placenta previa who sought specialized medical care at the Perinatal Center between 01.12.2019 and 01.03.2021.
On admission to the hospital for delivery, all patients underwent a transabdominal and transvaginal uterine ultrasound performed by a diagnostic medical sonographer using Medison AV10, Voluson S8, Voluson E6, Voluson E8 scanners equipped with multifrequency transducers for abdominal and endocavitary studies. Placenta previa was diagnosed according to generally accepted criteria [23] with a targeted search for ultrasound signs of PAS [24].
PAS was subsequently verified at delivery and by pathological and histological examination (PHE) of surgical material.
The study was approved by the Research Ethics Committee of the Regional Clinical Hospital No. 2 (Ref. No: 90 of 13-Nov-2019). After informed consent to study blood samples and histological material, venous blood samples were collected from all patients with placenta previa for subsequent determination of serum levels of angiogenetic markers (PlGF, VEGF, sFlt-1,) before steroids and blood products (if needed) were administered.
Inclusion criteria were antenatal diagnosis O.44.0–O44.1 (ICD X) according to ultrasound and/or magnetic resonance imaging, gestational age 26–38 weeks, informed consent of a pregnant woman to participate in the study.
Exclusion criteria were preeclampsia, antenatal fetal death, multiple pregnancies, congenital fetal malformations that were indications for early delivery, absence of pregnancy outcome data with histological and/or clinical verification of the diagnosis.
Clinical groups were formed according to the clinical diagnosis at delivery and according to the PHE data of the surgical material (if available).
The specialists who performed the serum enzyme immunoassay (ELISA) had no data on the clinical diagnosis of the patients. Pathomorphologists who performed immunohistochemical (IHC) analysis of the surgical material were unaware of the ELISA results.
Serum concentrations of PlGF, VEGF, sFlt-1 were determined taking into account gestational age, clarified by combined screening of the 1st trimester, according to the ELISA technique described earlier [22].
Histological and IHC study
Surgical material (placenta, fragments of myometrium dissected during metroplasty or uterus in cases of hysterectomy) after removal was immediately fixed in 10% neutral buffered formalin. After fixation, the material was excised with the necessary volume taken for the study, including the area of the placental pad with the underlying uterine wall to its full depth, which was performed through a vacuum histological processor, embedded in paraffin and followed by histological staining with hematoxylin-eosin. We used a Tissue-Tek VIPtm5Jr closed-type histological guide apparatus, a Ventana Bench Mark XT immunohistostainer, and a Nikon Есlirse 80i direct light optical microscope. The histological criteria for PAS were determined according to FIGO recommendations [25]. For IHC analysis, we used antibody sets, including FLT-1/VEGFR1 (Vascular endothelial growth factor receptor 1) rabbit polyclonal, VEGF (Vascular endothelia growth factor) mouse monoclonal, and CD34 (QB End/10) mouse monoclonal.
The IHC study was performed in 57 patients, including 17 samples after metroplasty in the group with placenta previa group without PAS, 40 cases in the PAS group (depth of invasion: grade 1 – in 16; grade 2 – in 18; grade 3 – in 6). Histological examination of surgical material after hysterectomy (due to uterine atony with a normal placenta position at 38 weeks of gestation, n=1; for myoma outside pregnancy, n=2) was used as an external control in patients who were operated on at the Children's Regional Clinical Hospital, Ministry of Health of the Krasnodar Krai and gave their informed consent to use their anonymized data for research purposes.
Methods: IHC was performed on 3μm thick deparaffinized sections after antigen unmasking (high temperature treatment method in acidic medium, pH 6.0) using an avidin-biotin enzyme detection system. Histological contrasting was performed using chromogenic substrates of two colors, the Ventana Medical Systems UltraView Universal DAB Detection Kit and the XT UltraView RED v3 detection system with Mayer hematoxylin. The histological pattern and immunoperoxidase binding results wereevaluated on a Nikon Eclipse 80i direct light optical microscope.
Flt-1 (Flt-1/VEGFR1 Rabbit Polyclonal Antibody), OptiView DAB IHC Detection Kit. Sample preparation included a 1:50 dilution of primary antibodies (without regard to sample clinical group). Deparaffinization of the slice; Cell Conditioning 1 increased (72 minutes); primary incubation for 120 minutes at 37°C; Hematoxylin II contrast staining, 16 minutes; subsequent staining with Bluing Reagent, 8 minutes. Hand microphotography was used to stain areas of normal and excessive reagent fixation. The villi in the studied areas did not contain perivillous fibrinoid and had no pathological changes (e.g., infarction).
VEGF (anti-VEGF Primary Antibody), UltraView Universal DAB Detection Kit: deparaffinization of the slice; conditioning of the slice (antigen demasking), Cell Conditioning 2 standard (60 minutes); primary incubation for 120 minutes at 37°C; contrast staining with Hematoxylin II, 20 minutes; subsequent staining with Bluing Reagent, 4 minutes.
CD34 (anti-CD34 (QBEnd/10) Primary Antibody), UltraView Universal DAB Detection Kit: deparaffinized slice; primary incubation for 16 minutes at 37°C; Hematoxylin II contrast staining, 16 minutes; subsequent staining with Bluing Reagent, 4 minutes.
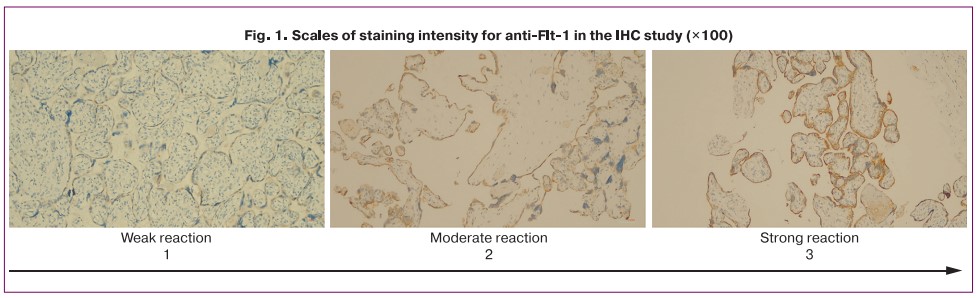
The semi-quantitative evaluation of the intensity of Flt-1 fixation by chorionic villi in the invasion zone and by decidual cells was carried out by two independent specialists. When using the 5-point grading method of staining intensity [14], there was a discrepancy in assessment between the specialists in 18 preparations out of 57. To increase the reproducibility of data interpretation, we developed an original ordinal three-point scale that included the following categories: intense >70% (3 points); moderate = 30–70% (2 points); weak < 30% (1 point) (Fig. 1), to which each sample was further compared. There were no discrepancies in the interpretation of data on the three-point scale between specialists. The mean values for the scale scores are reported as the median and interquartile range.
The clinical and demographic characteristics of the patients were compared (race, age, number of pregnancies, parity, number of cesarean sections, body mass index (BMI), somatic comorbidities (diabetes, chronic arterial hypertension, obesity), obstetric comorbidities (gestational diabetes), nicotine addiction, gestational age at the time of biomaterial sampling for analysis).
Statistical analysis
Quantitative variables were expressed as mean (Me) with interquartile range (Q1; Q3), frequencies and percentages for categorical variables
All statistical tests were two-tailed, statistical significance was assumed for p<0.05. Proportions of categorical variables were compared using chi-square test (χ2), with Yates correction when the expected frequency of one or more cells was less than five. The normality of the distribution was tested by the Shapiro–Wilk test. The statistical significance of between-group differences (placenta previa and PAS) for continuous variables was tested with Mann–Whitney test, and the Wilcoxon test was used between paired samples. A box plot with floating points (jitter plot) was used for a graphical representation of differences in groups according to the depth of invasion, which visually represents the distribution of categorical data. Statistical analysis was performed using R version 4.1.2 (2021-11-01, R Foundation for Statistical Computing) in the R Studio application (2021.09.0 2009-2021 R Studio, PBC).
Calculation of the sample size to enable comparison of groups for IHC (placenta previa, pl. accreta, pl. increta, and pl. percreta), assuming a sufficient level of difference between them, was based on the publications of Cohen J. [26] and Kabakov R.I. [27] (power=0.80; p≤0.05, ANOVA). Based on the calculation results, it was determined that approximately 18 cases in each group should be included in the sample.
Results
According to the clinical diagnosis at delivery and PHE of the surgical material, 46 of the 88 patients were included in the group with PAS (Group 1) and 42 were included in the group with placenta previa without PAS (Group 2). All the patients were of Caucasian race. Comparative clinical and demographic characteristics revealed homogeneity of the groups in terms of gestational age at the time of blood sampling, BMI, and comorbidity. Patients in the PAS group were significantly older, had higher parity and a greater number of previous cesarean sections (data have been published previously) [22].
Analysis of serum levels of angiogenesis markers
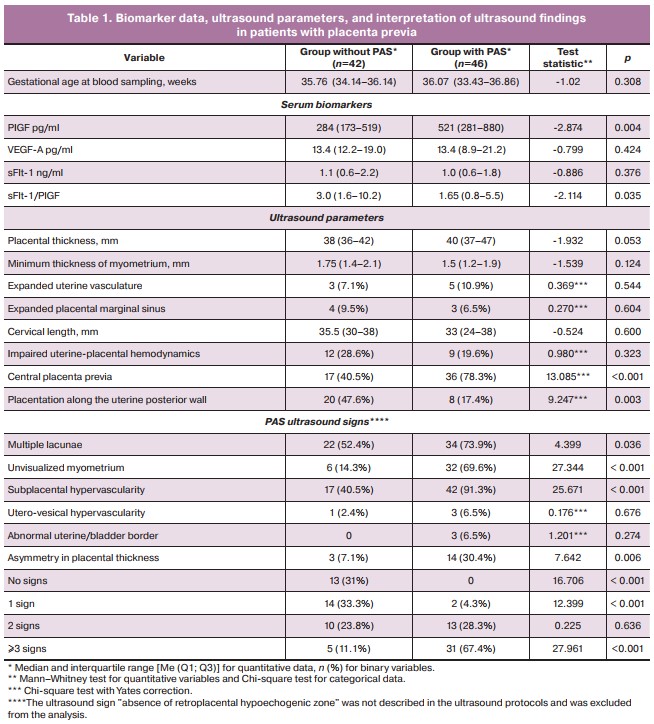
There was a statistically significant increase in PlGF concentration and a decrease in the sFlt-1/PlGF ratio in pregnant women with PAS in the third trimester. There were no statistically significant differences in absolute VEGF and sFlt-1 levels (Table 1).
Analysis of ultrasound findings.
Each of the PAS signs was observed in both groups, except for «uterine and bladder border abnormalities,» but given the rarity of this sign in PAS, the difference in its occurrence between the groups did not reach the level of statistical significance (Table 1). No «exophytic masses in the bladder lumen» were observed in any patient. The most common features were «subplacental hypervascularity», «multiple placental lacunae», and «no visualizable myometrium in the placental area». Less common were «asymmetry in placental thickness» with thickening in the area of placental invasion [28] and «hypervascularity of the vesicoureteral zone» (Table 1). Patients with PAS were more likely to have placenta previa along the anterior uterine wall, central placenta previa. A combination of at least three ultrasound signs was present in 67.4% of patients with PAS versus 11.9% without PAS (p<0.001). Overall, ultrasound showed high sensitivity in the antenatal diagnosis of PAS, including the location of the placenta along the posterior uterine wall, but low specificity due to a significant number of false positive findings in patients with placenta previa and uterine scars (Table 2).
Histological findings
The morphology of PAS was typical for an abnormal placentation. In the areas of invasion, microscopic examination showed the absence of decidual tissue in the basal plate area, underdevelopment of fibrinoid in the placental bed area, and chorionic villi sprouting in the myometrium. In most cases, there was edema and chronic inflammation in the myometrium adjacent to the placenta.
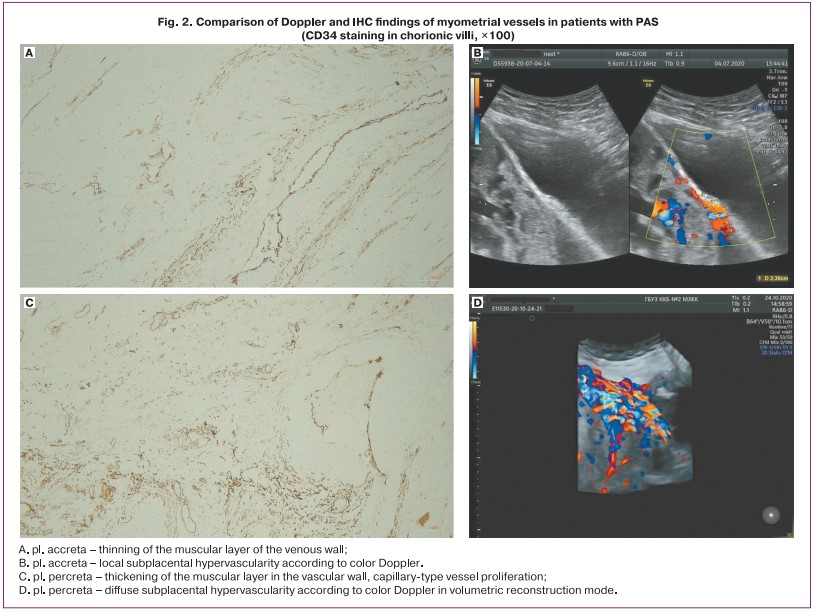
In the cases of PAS, there was a more pronounced proliferation of capillary-type vessels and a predominance of thick-walled muscular-type vessels in the pl. percreta, while in pl. increta, muscular-type vessels, both small and large caliber with a thin muscular wall, prevailed (see Fig. 2).
IHC findings
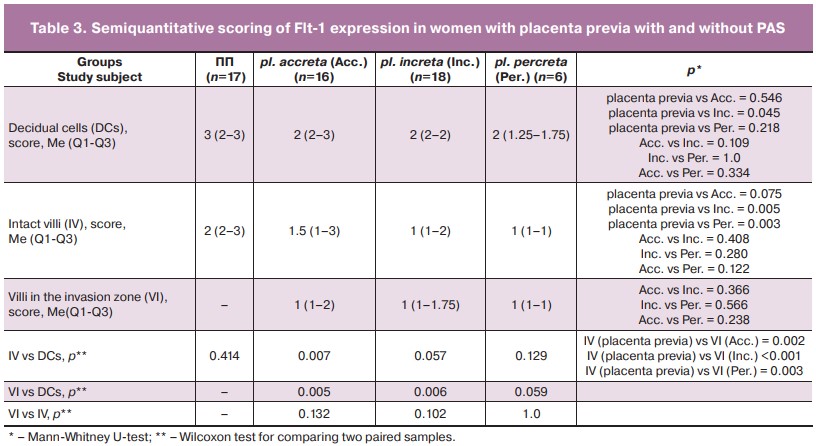
IHC examination of the myometrial vessels revealed zones of excessive vascularization with increased density of the vascular network, which coincided with the description of the presence of «subplacental hypervascularity» according to Doppler in patients with PAS (Fig. 2). The intensity of CD34 vascular marker staining increased with the depth of invasion.
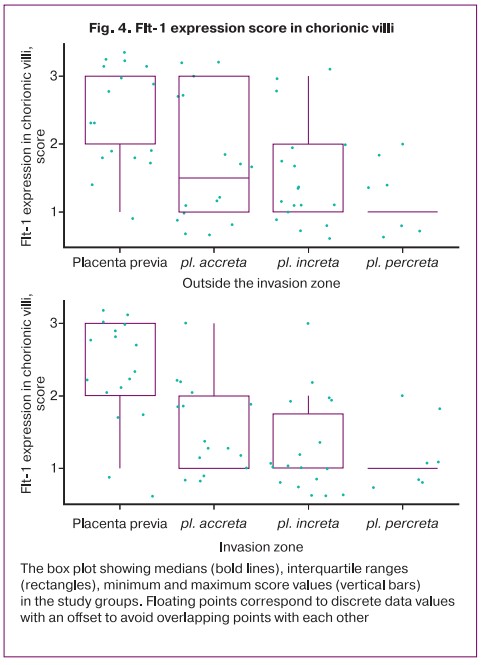
The Flt-1 expression score in the decidual cells and in the chorion villi in patients with placenta previa had no statistically significant differences (Table 3, Fig. 3). Although the mean values of the intensity of the staining in the decidual cells of patients with PAS were lower than in the placenta previa group, the difference compared with the placenta previa group and between groups with different depths of invasion did not reach statistical significance, except for pl. increta (p=0.045) (Table 3). At the same time, in intact chorionic villi, the level of Flt-1 expression in patients with invasive forms of PAS (increta/percreta) was lower than in the absence of PAS (Table 3, Fig. 3, 4). When comparing the level of Flt expression in the villi of patients with placenta previa and in the villi of the placental invasion area in patients with PAS, a lower expression of the marker studied in the placental invasion area was found regardless of the depth of invasion with a statistically significant difference in stain intensity according to IHC (Table 3, Fig. 3, 4).
Although the intensity of staining, according to the mean values of the score between preparations with different depths of placental invasion in intact chorionic villi decreased, but this trend did not reach statistical significance (differences between pl. accreta/pl. percreta; p=0.122). In the area of invasion, no differences depending on the depth of invasion were found. At the same time, differences with high statistical significance when comparing Flt-1 expression between decidual cells and the invasion area of pl. accreta and pl. increta. The absence of such marked differences for pl. percreta can be explained by the small number of observations in the group (n=6). According to IHC, there were no statistically significant differences in decrease in Flt-1 expression between intact villi and the invasion area in patients with PAS (Table 3).
Enhanced staining was observed indicating an increase in VEGF content in PAS in interstitial trophoblast cells, vascular endothelium, extracellular matrix, decidual cells, as well as between muscle fibers and connective tissue. The number of VEGF-positive cells in the myometrium was higher in pl. percreta (Fig. 3).
Discussion
The ability of antenatal instrumental diagnosis of PAS has been under review in recent years. According to a meta-analysis, about 20% of invasive forms of PAS remain missing if all mandatory screening ultrasounds are performed [29]. Diagnostic accuracy of the method is higher in case of a combination of several (at least 3) ultrasound signs, which was confirmed by our analysis. The lack of description of the widely known ultrasound signs «loss of retroplacental clear zone», «bridging vessels» in color Doppler and single cases of «uterovesical hypervascularity» sign can explain the low possibility of pathology exclusion in the presence of risk in our sample.
Angiogenic factors appear to be one of the most promising areas for study to improve antenatal diagnosis of PAS, since they are already in demand in clinical practice for early preeclampsia prediction. However, to date, most studies are limited to small samples, studies comparing local and systemic levels of angiogenic factors in PAS are few, and the results are ambiguous. It is logical to expect an increase in proangiogenic factors and a corresponding decrease in antiangiogenic factors in the blood of women with this pregnancy complication as opposed to preeclampsia. However, Biberoglu et al. [16] found no suspected changes, while other works revealed the opposite phenomenon: a decrease in serum VEGF in PAS [14, 17, 18].
Faraji A. et al. [19] confirmed increased PlGF, but not VEGF in pregnant women with PAS. This work is consistent with our results. Determination of serum VEGF level is technically difficult due to its low concentration. In our sample, three patients had marker levels below the threshold; in [14] the number of such non-informative samples was higher. Plasma protein concentration is higher, but serum analysis is generally accepted in clinical practice.
The absolute level of sFlt-1 in our study did not differ between the study groups, but the analysis of the pro- and anti-angiogenic factors showed statistically significant differences with a decrease in the sFlt-1/PlGF ratio in pregnant women with PAS. Assessment of the diagnostic potential of the ratio appears to be the most promising, since it does not depend on absolute marker values and is applicable regardless of equipment and diagnostic kits. However, when interpreting serum biomarker concentration data, it is unclear whether the decrease in the sFlt-1/PlGF ratio is due to a change in the levels of both markers or whether the index works only due to an increase in PlGF levels. Furthermore, it cannot be certain that a possible change in sFlt-1 levels is due to pathological placentation rather than an associated factor. Only a comparison of ELISA serological data with IGC expression of angiogenic factors in the placental area in normal and PAS can help to understand the identified phenomenon.
Published studies investigating local expression of sFlt-1 are few and have significant differences in design. Thus, McMahon K. et al. [15] studied the level of the marker in the material after hysterectomy at 20 to 40 weeks of gestation, while it is known that the level of angiogenic factors significantly changes from II to the end of III trimester of pregnancy. Shainker S.A. et al. [30] studied the local expression of sFlt-1 as a control in patients with normal placentation and chorioamnionitis. The authors did not compare local Flt-1 level with serum sFlt-1 concentration. McMahon et al. [15], as in our study, found a decrease in the local level of sFlt-1 expression with increasing depth of placental invasion in the chorionic villi with a tendency to statistical significance (p=0.11) between patients with pl. accreta and pl. рercreta. Statistically significant differences were found in the level of expression of the studied anti-angiogenic factors in the villi between patients with invasive forms of PAS and placenta previa. However, our study did not confirm statistically significant differences in the level of Flt-1 between intact villi and the zone of invasion in patients with PAS. These indices are consistent with the data of Shainker S.A. et al. [30]. Therefore, according to our results, even in intact villi of patients with PAS, there is a decrease in Flt-1 concentration, which indicates the systemic nature of the identified differences, not limited to the invasion zone.
This phenomenon is important to understand the pathogenesis of abnormal placental invasion. To date, two theories of the development of this process coexist. According to Knöfler M. [1], an internal differentiation program, independent of the local environment, triggers the proliferation and invasion of extracellular trophoblasts. Maternal stromal decidual cells, macrophages, and decidual killer cells (dNK) modulate the invasive activity of the trophoblast [1]. According to Jauniaux et al. [31], the uterine factors that lead to the development of PAS are primary and changes in syncytiotrophoblasts and extravillous trophoblasts are secondary in nature. One of the arguments is the absence of morphological changes in the chorion villi in PAS compared with normal placenta [31]. However, in morphological studies by Tantbirojn P. et al. [32] a clear correspondence of the placental invasion to the area was determined only in 8% of the cases. In the rest of the cases the abnormal invasion spread to the anterior uterine wall in the zone of supposed scarring after the cesarean section. It should also be taken into account that routine histological analysis is associated with multiple systematic error due to the heterogeneity of the findings [33]. The revealed changes in the systemic level of angiogenic markers combined with impaired expression in patients with PAS, both in the zone of abnormal invasion and outside it, indicate that impaired angiogenic processes are not limited to the local zone of maternal factors in the scar area. Consequently, changes in the systemic level of biomarkers can be used in clinical practice for the antenatal diagnosis of PAS.
Shainker S.A. et al. [30] suggested that the reduced level of sFlt-1 may be associated with increased perfusion of the invasive placenta. However, increased local expression of VEGF in the absence of differences at the systemic level (Faraji A. et al. [30], our data), or even with a decrease in the peripheral blood flow [14, 17, 18] contradicts this hypothesis. According to the IHC study, increased local expression of VEGF was fully consistent with previously published works indicating a proangiogenic phenotype of the placenta [14, 20, 31].
In our sample, color Doppler data recorded «subplacental hypervascularity» in accordance with the morphological characteristics of the vascular bed in the zone of abnormal invasion. At the same time, this echographic sign in the form of areas of local hypervascularity was often found (40.5% of cases) in pregnant women in the absence of placental invasion of the uterine wall. When analyzing standard spectral Doppler findings, we found no differences in uteroplacental hemodynamics between the groups with and without PAS (p=0.323) (Table 1). Therefore, the specific characteristics of hemodynamics in PAS require further study, possibly using more sensitive methods to assess blood flow parameters at the local level.
Increased serum levels of PlGF and local expression of VEGF indicate a contribution of angiogenic factors to the pathogenesis of PAS. The reduced sFlt-1/PlGF ratio in the third trimester in patients with placenta previa is associated with a decrease in local Flt-1 expression in the chorionic villi in patients with PAS. Although subplacental vascularity in PAS is more frequent, isolated color Doppler data are insufficient for a reliable antenatal diagnosis of PAS. The parameters of standard spectral Doppler imaging of uterine-placental blood flow are not informative for PAS diagnosis. It is possible to improve the accuracy of prenatal diagnosis of PAS by creating a comprehensive program that includes medical imaging methods and biochemical markers. To develop such a program, studies with greater statistical power are needed.
Limitations of the study include the small sample size. We aimed to reduce the probability of error by forming proportions of groups with sufficient power; determination of different splice variants of Flt-1 by ELISA and IGC, although antibodies to sFlt-1 recognize both variants and do not differentiate them [15]. The ultrasound finding reports did not register all common signs of PAS, which may have affected the accuracy of the antenatal diagnosis.
The advantage of the study is that we investigated a combination of ultrasound and Doppler data with local and systemic levels of angiogenic markers. This approach allowed us to expand our understanding of the pathogenesis and potential biomarkers of abnormal placentation to improve antenatal diagnosis.
Conclusion
Color Doppler data reflecting vascularity of the pathological invasion zone should be evaluated in combination with other ultrasound signs of PAS. Consideration of additional signs of PAS can expand the diagnostic accuracy of ultrasound in patients at risk by increasing the specificity and positive predictive value. The decrease in serum sFlt-1/PlaGF ratio in pregnant women with PAS in the third trimester is consistent with decreased local expression of Flt-1 in the chorionic villi and can be used as an additional biomarker of abnormal placental invasion in women with placenta previa.
References
- Knöfler M., Pollheimer J. IFPA Award in Placentology lecture: molecular regulation of human trophoblast invasion. Placenta. 2012; 33(Suppl. 2): S55-62. https://dx.doi.org/10.1016/j.placenta.2011.09.019.
- Bartels H.C., Postle J.D., Downey P., Brennan D.J. Placenta Accreta spectrum: a review of pathology, molecular biology, and biomarkers. Dis Markers. 2018; 2018: 1507674. https://dx.doi.org/10.1155/2018/1507674.
- Barinov S.V., Medyannikova I.V., Tirskaya Yu.I., Beznoshchenko G.B., Kadcyna T.V., Lazareva O.V. et al. Prediction of placenta accreta in case of placenta previa. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 1: 61-9. (in Russian). https://dx.doi.org/10.18565/aig.2021.1.61-69.
- Lisicyna O.I., Nizyaeva N.V., Miheeva A.A. Placenta accreta. Evolution of knowledge and skills. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 6: 34-40. (in Russian). https://dx.doi.org/10.18565/aig.2021.6.34-40.
- Penzhoyan G.A. The effectiveness of modern perinatal technologies. Problems of social hygiene, health care and the history of medicine. 2002; 6: 42. (in Russian).
- Kurtser M.A., Breslav I.Yu., Grigor'yan A.M., Latyshkevich O.A., Kutakova Yu.Yu., Kondrat'eva M.A. Temporary balloon occlusion of the common iliac arteries during organ-preserving operations in patients with placental accreta. Akusherstvo i ginekologiya: novosti, mneniya, obuchenie/Obstetrics and gynecology: news, opinions, training. 2018; 6(4): 31-7. (in Russian). https://dx.doi.org/10.24411/2303-9698-2018-14003.
- Demidov V.N., Gus A.I., Yarygina T.A. On the possibility of high-precision diagnosis of placenta accreta into the scar on the uterus after cesarean section by ultrasound. Prenatal'naya diagnostika/Prenatal diagnosis. 2020; 19(4): 336-42 (in Russian). https://dx.doi.org/10.21516/2413-1458-2020-19-4-336-342.
- Tinari S., Buca D., Cali G., Timor-Tritsch I., Palacios-Jaraquemada J., Rizzo G. et al. Risk factors, histopathology and diagnostic accuracy in posterior placenta accreta spectrum disorders: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2021; 57(6): 903-9. https://dx.doi.org/10.1002/uog.22183.
- Vinickij A.A., Shmakov R.G. Modern ideas about the etiopathogenesis of placental accreta and the prospects for its prediction by molecular diagnostic methods. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; 2: 5-10. (in Russian). https://dx.doi.org/10.18565/aig.2017.2.5-10.
- Jauniaux E., Bhide A., Kennedy A., Woodward P., Hubinont C., Collins S.; FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. J. Gynaecol. Obstet. 2018; 140(3): 274-80. https://dx.doi.org/10.1002/ijgo.12408.
- Penzhoyan G.A., Makukhina T.B. Significance of the routine first-trimester antenatal screening program for aneuploidy in the assessment of the risk of placenta accreta spectrum disorders. fPerinat. Med. 2019; 48(1): 21-6. https://dx.doi.org/10.1515/jpm-2019-0261.
- Makukhina T.B., Penzhoyan G.A., Lebedenko E.S., Khorolsky V.A., Solntseva A.V., Kazanchi F.B. Possibilities of early prediction of perinatal outcomes in the first trimester in patients with placenta previa complicated by its abnormal invasion. Akusherstvo i ginekologiya: novosti, mneniya, obuchenie/Obstetrics and gynecology: news, opinions, training. 2020; 8(1): 47-52. (in Russian). https://dx.doi.org/10.24411/2303-9698-2020-11006.
- Borovkov V.A, Igitova M.B., Korenovskij Yu.V, Dudareva Yu.A. Prognostic value of specific pregnancy proteins in women with uterine scar and placenta ingrowth. Klinicheskaya laboratornaya diagnostika/Clinical laboratory diagnostics. 2020; 65(6): 353-7 (in Russian). https://dx.doi.org/10.18821/0869-2084-2020-65-6-353-357.
- Wehrum M.J., Buhimschi I.A., Salafia C., Thung S., Bahtiyar M.O., Werner E.F. et al. Accreta complicating complete placenta previa is characterized by reduced systemic levels of vascular endothelial growth factor and by epithelial-to-mesenchymal transition of the invasive trophoblast. Am. J. Obstet. Gynecol. 2011; 204(5): 411.e1-411.e11. https://dx.doi.org/10.1016/j.ajog.2010.12.027.
- McMahon K., Karumanchi S.A., Stillman I.E., Cummings P., Patton D., Easterling T. Does soluble fms-like tyrosine kinase-1 regulate placental invasion? Insight from the invasive placenta. Am. J. Obstet. Gynecol. 2014; 210(1): 68.e1-4. https://dx.doi.org/10.1016/j.ajog.2013.08.032.
- Biberoglu E., Kirbas A., Daglar K., Biberoglu K., Timur H., Demirtas C. et al. Serum angiogenic profile in abnormal placentation. J. Matern. Fetal Neonatal Med. 2016; 29(19): 3193-7. https://dx.doi.org/10.3109/2015.1118044.
- Uyanıkoğlu H., İncebıyık A., Turp A.B., Çakmak G., Sak S., Hilali N.G. Serum angiogenic and anti-angiogenic markers in pregnant women with placenta percreta. Balkan Med. J. 2018; 35: 55-60. https://dx.doi.org/10.4274/balkanmedj.2016.1890.
- Schwickert A., Chantraine F., Ehrlich L., Henrich W., Muallem M.Z., Nonnenmacher A. et al. Maternal serum VEGF predicts abnormally invasive placenta better than NT-proBNP: a multicenter case-control study. Reprod. Sci. 2021; 28(2): 361-370. https://dx.doi.org/10.1007/s43032-020-00319-y.
- Faraji A., Akbarzadeh-Jahromi M., Bahrami S., Gharamani S., Raeisi Shahraki H., Kasraeian M. et al. Predictive value of vascular endothelial growth factor and placenta growth factor for placenta accreta spectrum. J. Obstet. Gynaecol. 2022; 42(5): 900-5. https://dx.doi.org/10.1080/01443615.2021.1955337.
- Duzyj C.M., Buhimschi I.A., Laky C.A., Cozzini G., Zhao G., Wehrum M. et al. Extravillous trophoblast invasion in placenta accreta is associated with differential local expression of angiogenic and growth factors: a cross-sectional study. 2018; 125(11):1441-8. https://dx.doi.org/10.1111/1471-0528.15176.
- Gur'eva V.M., Travkina A.A., Matveev M.O., Morohotova L.S., Budykina T.S., Kotov Yu.B. et al. Clinical significance of sFlt-1/PlGF in the diagnosis and prognosis of preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 7: 195-200. (in Russian). https://dx.doi.org/10.18565/aig.2021.7.195-200.
- Makukhina T.B., Penzhoyan G.A., Amirkhanyan A.M. Potential of angiogenesis-related serum markers for predicting placenta accreta spectrum i n pregnant women with placenta previa. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 1: 62-71 (in Russian). https://dx.doi.org/10.18565/aig.2022.1.62-71.
- Jauniaux E., Alfirevic Z., Bhide A.G., Belfort M.A., Burton G.J., Collins L. et al. Placenta Praevia andPlacenta Accreta: diagnosis and management: green-top guideline no. 27a. BJOG. 2019; 126(1): e1-e48. https://dx.doi.org/10.1111/1471-0528.15306.
- Savel'eva, G.M., Sukhikh G.T., Serov V.N., Radzinskiy V.E., eds. Obstetrics: a national guide. M.: GEOTAR-Media; 2019. 1080 p. (in Russian).
- Jauniaux E., Ayres-de-Campos D.; FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Introduction. Int. J. Gynaecol. Obstet. 2018; 140(3): 261-4. https://dx.doi.org/10.1002/ijgo.12406.
- Cohen J. Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum; 1988. 567p.
- Kabakov R.I. R In action. Analysis and visualization of data in the program R. M.: DMK Press; 2016. 348 p. (in Russian).
- Bhide A., Laoreti A., Kaelin Agten A., Papageorghiou A., Khalil A., Uprichard J. et al. Lower uterine segment placental thickness in women with abnormally invasive placenta. Acta Obstet. Gynecol. Scand. 2019; 98(1): 95-100. https://dx.doi.org/10.1111/aogs.13422.
- Pagani G., Cali G., Acharya G., Trisch I.T., Palacios-Jaraquemada J., Familiari A. et al. Diagnostic accuracy of ultrasound in detecting the severity of abnormally invasive placentation: a systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2018; 97(1): 25-37. https://dx.doi.org/10.1111/aogs.13238.
- Shainker S.A., Dannheim K., Gerson K.D., Neo D., Zsengeller Z.K., Pernicone E. et al. Down-regulation of soluble fms-like tyrosine kinase 1 expression in invasive placentation. Arch. Gynecol. Obstet. 2017; 296(2): 257-62. https://dx.doi.org/10.1007/s00404-017-4432-7.
- Jauniaux E., Burton G.J. Pathophysiology of placenta accreta spectrum disorders: a review of current findings. Clin. Obstet. Gynecol. 2018; 61(4): 743-54. https://dx.doi.org/10.1097/GRF.0000000000000392.
- Tantbirojn P., Crum C.P., Parast M.M. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta. 2008; 29(7): 639-45. https://dx.doi.org/10.1016/j.placenta.2008.04.008.
- Jauniaux E., Hussein A.M., Zosmer N., Elbarmelgy R.M., Elbarmelgy R.A., Shaikh H. et al. A new methodologic approach for clinico-pathologic correlations in invasive placenta previa accreta. Am. J. Obstet. Gynecol. 2020; 222(4): 379.e1-379.e11. https://dx.doi.org/10.1016/j.ajog.2019.11.1246.
Received 10.06.2022
Accepted 09.09.2022



