Diagnosis of fetal congenital anomalies in the first trimester of pregnancy
Piskulina A.A., Kostyukov K.V.
Objective: To investigate the incidence and types of fetal congenital anomalies (FCA) detected during first-trimester screening in pregnant women from various risk groups.
Materials and methods: This retrospective study included 10,044 patients who underwent first-trimester screening at 11–14 weeks of pregnancy at V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia from 2018 to 2023. Based on baseline medical history and established recommendations, patients were categorized into low- and high-risk groups for fetal pathology. The incidence and types of FCA were analyzed for each group. Pregnancy outcomes including diagnostic verification were assessed using archived electronic patient records. Ultrasound examinations were performed by expert physicians of the Ultrasound and Functional Diagnostics Department with appropriate qualifications. Statistical analysis was conducted using the generalized D'Agostino–Pearson test, Mann–Whitney U test, and Kruskal–Wallis test, with differences considered significant at p<0.05.
Results: Of the study population, 61.6% were in the low-risk group, and 38.3% were in the high-risk group. The mean age of high-risk patients was 37.6 (3.5) years, compared to 29.7 (3.2) years for low-risk patients (p<0.001). Although the median body mass index (BMI) did not differ significantly between the groups, obesity
(BMI>30 kg/m²) was more common in the high-risk group (8.7%) than in the low-risk group (4.8%). Additionally, assisted reproductive technologies (ART) were used more frequently in the high-risk group (19.2%) than in the low-risk group (8.4%; p<0.01). In the low-risk group, most women were primigravidae (64.6%) and primiparae (63.2%), whereas in the high-risk group, 41.5% were primigravidae and 44% were primiparae. The overall incidence of FCA in the cohort was 4.3%, with fetal defects occurring in 1.9% of the low-risk patients and 8.1% of the high-risk patients (p<0.01). Combined fetal defects were the most common, accounting for 55% of all the detected anomalies. The most frequently isolated FCAs were congenital heart, limb anomalies, genitourinary, anterior abdominal wall, and gastrointestinal tract defects. Statistically significant differences in isolated FCA incidences were observed between the groups, specifically for facial, chest, and genitourinary pathologies in low-risk patients (p<0.05). Of the 194 isolated defects, anomalies consistently detected before 14 weeks of pregnancy accounted for 35%, those sometimes detected constituted 58.3%, and those practically not detected made up 6.7%.
Conclusion: Prenatal ultrasound diagnostics enables the detection of a significant proportion of fetal anomalies at 11–14 weeks of gestation. The higher incidence of FCA in the high-risk group than in the low-risk group supports the efficacy of risk-based stratification in prenatal screening.
Authors' contributions: Piskulina A.A. – review of relevant literature, obtaining data for analysis, statistical analysis, drafting of the manuscript; Kostyukov K.V. – conception and design of the study, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study is a retrospective analysis of data obtained as a result of a standardized screening study in V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia and does not require approval from the ethics committee.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Piskulina A.A., Kostyukov K.V. Diagnosis of
fetal congenital anomalies in the first trimester of pregnancy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (11): 90-97 (in Russian)
https://dx.doi.org/10.18565/aig.2024.182
Keywords
In recent decades, health care professionals have focused much attention on reducing perinatal losses in the context of a worsening demographic situation. A key approach to minimizing adverse pregnancy outcomes is the early detection of pathological conditions in the fetus [1, 2]. According to the literature and the World Health Organization, about 3–7% of children in Russia are born with developmental defects every year, and about 16% of them have multiple defects. The incidence of genetic pathology is 1 case per 100 births [1]. Birth of children with developmental abnormalities caused by chromosomal pathology is the main cause of severe disability and mortality within the first five years of life [1, 3, 4]. Each woman faces a different risk of giving birth to a child with chromosomal abnormalities; therefore, during the first trimester screening, patients are stratified into groups based on high and low baseline and individual risks. The baseline risk of fetal genetic pathology can be assessed through patient questioning and is influenced by the patient's age and medical history of complications [5, 6]. Identifying high-risk patients is crucial for prenatal screening. For these patients, the likelihood of adverse outcomes that pose a threat to the health and life of both the mother and child is higher than that in a normal pregnancy, including an elevated risk of fetal pathology.
Timely diagnosis facilitates the implementation of necessary measures as early as possible and the adjustment of pregnancy and childbirth management strategies, thereby increasing the chance of a favorable outcome [2, 4]. Owing to significant advancements in prenatal ultrasound diagnostics over the past 40 years, the fetus can be thoroughly examined in the early stages of pregnancy, enabling the identification of fetal congenital anomalies (FCA) [7, 8].
During the first-trimester screening, in addition to confirming fetal viability, determining gestational age, assessing the risk of aneuploidies, and predicting major obstetric syndromes, fetal anatomy is evaluated to identify malformations. However, the accuracy of FCA diagnosis in the first trimester of pregnancy varies considerably. The factors influencing the success of anomaly detection in the first trimester and the optimal screening approach have not been sufficiently studied.
In January 2023, the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) presented new practice guidelines for first-trimester screening, proposing a detailed assessment of fetal anatomical structures [9]. The authors advocate for a differentiated approach: a simplified study that assesses the main organs and systems to diagnose fetal congenital anomalies (FCA) and an in-depth study that thoroughly examines all anatomical structures. However, clear guidelines for implementing this approach have not been established, and they are not routinely used by ultrasound diagnostic physicians [5].
Traditionally, most fetal abnormalities are diagnosed in the second trimester of pregnancy. However, the early detection of structural abnormalities at 11–14 weeks of gestation has undeniable advantages [10]. Some authors categorize fetal FCA in the first trimester into three groups: defects that can always be detected, defects that may be detected, and defects that cannot be diagnosed during the first screening [11]. This variation can be attributed to several factors that affect the accuracy of early diagnosis. These factors include modifiable elements, such as the class of ultrasound machine used and the absence of a systematic protocol for investigating fetal anatomy, as well as non-modifiable factors, such as the anatomical and pathophysiological characteristics of the defect, difficulties in visualization during examination, and discrepancies in fetal size.
A more comprehensive examination of fetal anatomy in high-risk groups of pregnant women may enhance the detection rate of FCA in the first trimester, as prenatal diagnostic specialists are likely to be more vigilant regarding the potential complications in this cohort. Therefore, studying the incidence and types of fetal FCA detected during first-trimester screening in pregnant women from different risk groups is highly relevant.
This study aimed to investigate the incidence and types of congenital fetal anomalies detected during first-trimester screening in pregnant women from various risk groups.
Materials and methods
The study involved a retrospective assessment of data from 10,044 patients with singleton pregnancies who underwent combined first-trimester screening at 11+0 to 14+0 weeks of gestation (crown-rump length [CRL] 45–84 mm) and were followed until delivery or termination of pregnancy at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia from 2018 to 2023. According to Order 1130n, first-trimester screening includes ultrasound examination, analysis of maternal serum markers (pregnancy-associated plasma protein A and free β-subunit of human chorionic gonadotropin), medical history review, and measurement of blood pressure, height, and weight of the patient [12]. Based on their baseline medical history and recommendations, the patients were divided into groups with low and high baseline risk of fetal genetic pathology. Patients with non-viable pregnancies were excluded from the study. Data on pregnancy outcomes were gathered from the archive of electronic patient records in the Medialog database.
Ultrasound examinations were performed using expert ultrasound systems Voluson Expert E8 and E10 (General Electric, USA), employing transabdominal scanning with a convex sensor operating at a frequency of 2.0–9.0 MHz and a linear sensor operating at 4.0–10.0 MHz, as well as transvaginal scanning with a microconvex intracavitary sensor at 5.0–9.0 MHz. During ultrasound examination, fetal viability was confirmed by the presence of a heartbeat and the location of the pregnancy within the uterine cavity. Gestational age was determined based on CRL measurements, and markers of genetic pathology and fetal anatomy were assessed. The study utilized a standard protocol for assessing fetal anatomy and examined the following anatomical regions and organs: head (presence and ossification of bones, "butterfly" sign), neck (nuchal fold thickness [NFT]), face (normal profile, nasal bone), spine (correct shape and continuity), chest (correct shape, symmetrical lungs), heart (four-chamber view, correct position in the chest), anterior abdominal wall and abdominal cavity (absence of space-occupying lesions and defects, normal positioning of organs), limbs (four limbs, three segments), placenta, and umbilical cord [12]. A detailed examination of markers for chromosomal abnormalities (CAs) was performed, including an NFT greater than the 95th percentile, nasal bone hypoplasia, reverse blood flow in the venous duct, tricuspid regurgitation, and fetal heart rate abnormalities (bradycardia or tachycardia). A diagnosis was made if abnormal findings were present in any anatomical section. Fetal hydrops was diagnosed in the presence of edema of the scalp and trunk, as well as fluid accumulation in more than one cavity [13, 14]. All cases of fetal developmental anomalies detected during the first-trimester scan were reviewed in the context of perinatal consultation by ultrasound diagnostic experts and related specialists. In most cases, an invasive diagnosis was recommended. The diagnosis was verified based on pathological examination in cases of pregnancy termination, subsequent ultrasound examinations in the second and third trimesters, and after birth.
Statistical analysis
Parametric and nonparametric tests were employed to compare the means of continuous variables depending on the type of distribution. The sample was evaluated for normality using the Shapiro–Wilk test. If the data exhibited a normal distribution, a t-test was applied for independent samples. Statistical analysis included the generalized D'Agostino-Pearson test, Mann–Whitney U test, and Kruskal-Wallis test. Differences were considered statistically significant at p<0.05. Continuous variables with a normal distribution are reported as M (SD), where M represents the arithmetic mean and SD denotes the standard deviation. Statistical analysis was conducted using GraphPad Prism software, version 9.5.1 (528), and Microsoft Excel.
Results
A total of 61.6% (6,188/10,044) of the patients were in the low baseline risk group, while 38.3% (3,856/10,044) were in the high-risk group. The mean age of high-risk patients was 37.6 (3.5) years compared to 29.7 (3.2) years for low-risk patients (p<0.001). The median body mass index (BMI) in the high-risk group was 23.0 (21.0–25.0) kg/m², while in the low-risk group it was 21.0 (20.0-24.0) kg/m². The percentage of obese patients (BMI>30 kg/m²) was higher in the high-risk group than that in the low-risk group (8.7%). The smoking rates in both study groups were not statistically different. Patients with a high baseline risk were more likely to achieve pregnancy using assisted reproductive technologies, with 19.2% of cases compared to 8.4% in the low-risk group (p<0.01). In the low baseline-risk group, patients were more often primigravidas, with 64.6% versus 41.5% in the high-risk group. A greater proportion of patients expecting their first birth was in the low-risk group (63.2%), while 44% of the high-risk group fell into this category (Table 1). The overall incidence of fetal defects detected in the first trimester was 4.3% (429/10,044). Among patients with fetal congenital anomalies (FCA), 73.2% (314/429) had a high baseline risk, whereas 26.8% (115/429) had a low risk (p<0.01). In the high-risk group, the incidence of fetal anomalies was 8.1% (314/3,856), while in the low-risk group, it was 1.9% (115/6,188) (p<0.01).
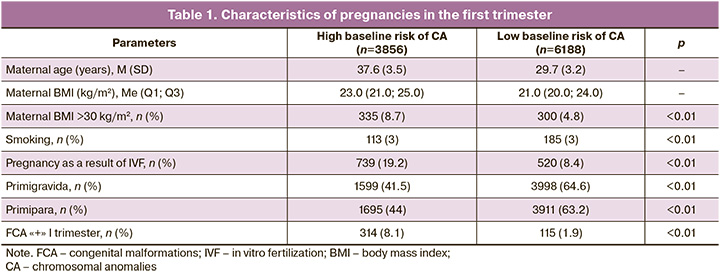
Fetal anomalies were identified in 55% (235/429) of cases (Fig. 1). The most common combinations of defects include anomalies of the skull, central nervous system (CNS), face and neck, heart defects, fetal hydrops, anterior abdominal wall defects, and gastrointestinal tract (GIT) anomalies. The most prevalent defects in combined pathology, defined as the presence of defects in different organs or systems, were fetal heart abnormalities, which accounted for 72% (170/235) of the cases, including hypoplastic left heart syndrome (HLHS), atrioventricular canal defects, and Fallot's tetrad. Anomalies of the abdominal cavity and anterior abdominal wall represented 49% (115/235) of the cases, including omphalocele and gastroschisis. Chest and lung defects were relatively rare, occurring in 3% (13/429) of the cases, comprising congenital diaphragmatic hernias and cystic adenomatous lung malformation (CAMP). Genitourinary system abnormalities (GUS) were observed in 22.5% (53/235) of the cases, with conditions such as megacystis and hyperechoic kidneys. Fetal limb anomalies were present in 49% of the cases (115/235), including reduction anomalies, syndactyly, polydactyly, and clubfoot. Spinal anomalies were found in 4.3% (10/235) of cases, including curvature and additional hemivertebrae. Head anomalies occurred in 10.2% (24/235) of the cases, facial anomalies in 20.9% (49/235), and CNS defects in 26.8% (63/235), which included acrania, anencephaly, exencephaly, holoprosencephaly, posterior fossa cysts, spina bifida, micrognathia, cheiloschisis, proboscis, and hypertelorism. The structure of the isolated defects is shown in Figure 2 and Table 2.
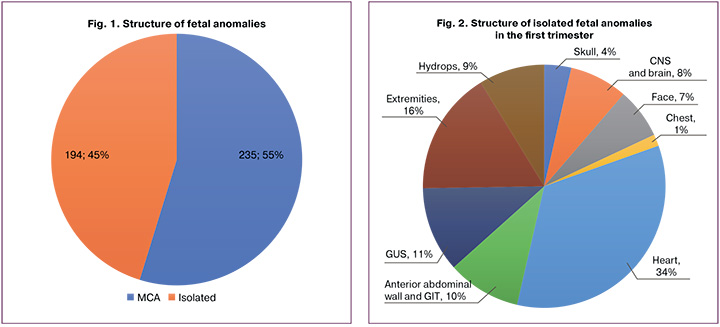
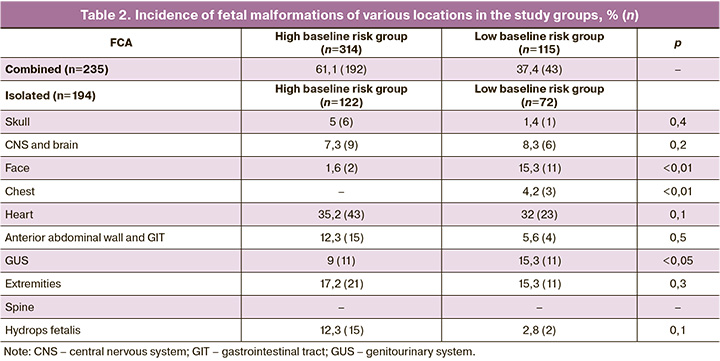
Isolated fetal pathology occurred in 45% of the cases. Within the high-risk group, it was present in 63%, while in the low-risk group, it was observed in 37%. Isolated pathology was more commonly detected in heart defects (34% or 66/194), limb anomalies (16% or 32/194), and GUS defects (11% or 22/194). Defects of the anterior abdominal wall and GIT, as well as fetal hydrops, were found in 10% (19/194) and 9% (17/194) of the cases, respectively. CNS and brain defects accounted for 8% (15/194) and facial defects for 7% (13/194). Fetal skull anomalies were identified in isolation in 4% (7/194) of the cases and chest defects in 1% (3/194). The incidence of isolated fetal limb anomalies relative to the two risk groups showed statistically significant differences in facial, chest, and GUS pathologies in low-risk patients (p<0.05).
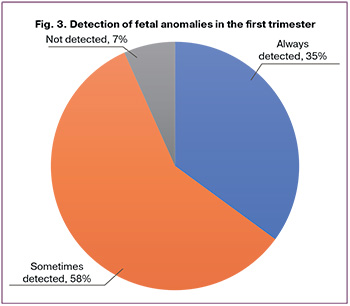
Figure 3 illustrates the incidence of FCA based on detection during the first trimester. Among the 194 isolated defects, the group of always detected anomalies includes acrania, anencephaly, holoprosencephaly, ectopia cordis, encephalocele, omphalocele, gastroschisis, and megacystis, which together accounted for 35% (68/194). The detected anomalies included facial clefts, proboscis, spina bifida, polydactyly, limb reduction defects, large ventricular septal defects (VSD), atrial septal defects (ASD), transposition of the great arteries, and HLHS, representing 58.3% (113/194). Finally, FCAs that are practically not detected before the 14th week of pregnancy include CCAM, hydronephrosis, and renal agenesis, totaling 6.7% (13/194).
Discussion
Chromosomal abnormalities and malformations are among the leading causes of mortality in infants and children. Unfortunately, every woman is at a risk of giving birth to a child with a pathology. Therefore, it is crucial to stratify pregnant women into at-risk groups during their first trimester. Women in the high-risk group had a higher probability of developing adverse outcomes, including fetal pathology.
In this study, all women who underwent examinations as part of the first-trimester screening were divided into high- and low-risk groups to develop fetal genetic pathology. The predominance of women in the high-risk group is likely due to the trend toward later marriages and delayed implementation of reproductive functions. In the high-risk group, pregnancies were more often achieved with the help of assisted reproductive technologies (19.2% of cases) than in the low-risk group (8.4%). This difference can be attributed to both the older age of the patients in the high-risk group and the presence of related reproductive issues. Pinborg et al. revealed an increased prevalence of chromosomal abnormalities (CA) and fetal chromosomal abnormalities (FCA) in children conceived through in vitro fertilization (IVF) compared with those conceived naturally (1.6–3.5% and 0.0–0.9%, respectively) [15]. However, the assertion regarding the influence of assisted reproductive technologies on the development of CA and fetal defects is questionable, as it is difficult to determine which factors, such as parental age and concomitant diseases, are more predisposed to the occurrence of pathology.
In this study, the incidence of defects in the first trimester of pregnancy was 4.3%, 8.1%, and 1.9% in the high-and low-risk groups, respectively. This finding was consistent with the results reported by Nicolaides et al. Among 100,997 low-risk patients with singleton pregnancies examined in the first trimester, 1.7% had FCA [16]. The difference in the incidence of FCA detection may be attributed to the large number of patients evaluated as well as the fact that the V.I. Kulakov NMRC for OG&P often receives patients for verification and differential diagnostics of pathologies previously detected at their place of residence.
The authors of the aforementioned study classified fetal defects in the first trimester into three groups. The first group consists of defects that are always detected: gross FCA, which includes acrania, holoprosencephaly, encephalocele, ectopia cordis, omphalocele, gastroschisis, megacystis, and body stem anomaly; their detection rate is 90–100%. The second group includes anomalies that are occasionally detected, such as facial clefts, hydrocephalus, spina bifida, skeletal dysplasia, reduced limb defects, polydactyly, VSD, ASD, transposition of the great arteries, HLHS, and Dandy–Walker syndrome, with detection rate of 10–90%. The third group comprises defects that are rarely detected in the first trimester and occur at a rate of less than 10%. These include CCAM, bladder exstrophy, pulmonary sequestration, duodenal atresia, kidney duplication, hydronephrosis, ovarian cysts, tumors, cerebellar hypoplasia, and corpus callosum agenesis. Nicolaides K.H. et al. identified the incidence these defects by group in a 2019 study. According to their data, the overall incidence of defects was 1.09% among the 44,859 pregnant women. Anomalies were subsequently categorized by detection rate, yielding distributions of 31%, 43%, and 26%. These results are corroborated in the present study, which also divided isolated defects into three groups and analyzed their incidence in the first trimester of pregnancy: the FCA group, which can always be detected, constituted 35%; the second group, consisting of defects that are sometimes detected, amounted to 58.3%; and the incidence of defects that are extremely rarely diagnosed was found to be 6.7%.
Our data on the higher incidence of defect detection in the high-risk group (73.2%) are comparable to the results of a meta-analysis led by Papageorghiou A.T. (61.2%). In the aforementioned study, obesity was identified as a factor that worsens the quality of diagnosis. The data from the present study indicate that, among high-risk patients, where the incidence of defects was higher, there was a higher prevalence of obesity than in the low-risk group (8.7% [335/3856] and 4.8% [300/6188], respectively; P<0.01). This can be attributed to the widespread use of transvaginal scanning in the Russian Federation, where obesity is not always a complicating factor, in contrast to England, where this type of examination is rarely used [17]. The higher incidence of FCA in the high-risk group is likely due to doctors being more cautious about the potential complications in these patients. Several studies have shown that employing a single systematic examination method, including a new standardized anatomical protocol, can increase the detection rate of anomalies in early pregnancy [18–20]. The advantages of diagnosing FCA and genetic pathology during first-trimester screening include early anomaly detection, availability of time to perform all necessary confirmatory diagnostic tests, and safer pregnancy termination options during early pregnancy.
The statistically significant difference in the incidence of isolated defect detection in the low-risk group is most likely due to the fact that multiple defects and syndromic pathology are more commonly associated with chromosomal aberrations, which are more typical for high-risk patients.
This study included several factors that may have influenced the results. As it was retrospective, based on the data obtained, it is impossible to conclude that the examination of high-risk pregnant women was more thorough or that the patients in this cohort were genuinely more likely to have a defect. Therefore, it is advisable to conduct a further prospective study that considers all the advantages and limitations of V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Conclusion
Every year, the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia conducts between 2,500 and 3,000 first-trimester screening studies. Prenatal ultrasound screening allows for the identification of a significant proportion of fetal anomalies at 11–14 weeks of pregnancy. The prevalence of FCA in high-risk patients compared to the low-risk group underscores the appropriateness of this stratification.
References
- Айламазян Э.К., Баранов В.С., ред. Пренатальная диагностика наследственных и врожденных болезней. М.: МЕДпресс-информ; 2007. 416с. [Ailamazyan E.K., Baranov V.S., eds. Prenatal diagnosis of hereditary and congenital diseases. Moscow: MEDpress-inform; 2007. 416p. (in Russian)].
- AIUM Practice Parameter for the performance of detailed diagnostic obstetric ultrasound examinations between 12 weeks 0 days and 13 weeks 6 days. J. Ultrasound Med. 2021; 40(5): E1-E16. https://dx.doi.org/10.1002/jum.15477.
- Лазюк Г.И. Этиология и патогенез врожденных пороков развития. Гл. 2. В кн.: Лазюк Г.И., ред. Тератология человека. 2-e изд. М.: Медицина; 1991: 18-46. [Lazyuk G.I. Etiology and pathogenesis of congenital malformations. Ch. 2. In: Lazyuk G.I., ed. Teratology of man. 2nd ed. Moscow: Medicine; 1991: 18-46. (in Russian)].
- Медведев М.В., ред. Пренатальная эхография. М.: Реальное Время; 2005. 560с. [Medvedev M.V., ed. Prenatal echography. Moscow: Real Time; 2005. 560p. (in Russian)].
- Esteves K.M., Tugarinov N., Lechmann G., Abi Habib P., Cagliyan E., Goetzinger K.R. et al. The value of detailed first-trimester ultrasound in the era of noninvasive prenatal testing. Am. J. Obstet. Gynecol. 2023; 229(3): 326.e1-326.e6. https://dx.doi.org/10.1016/j.ajog.2023.05.031.
- Wagner P., Sonek J., Hoopmann M., Abele H., Kagan K.O. First‐trimester screening for trisomies 18 and 13, triploidy and Turner syndrome by detailed early anomaly scan. Ultrasound Obstet. Gynecol. 2016; 48(4): 446-51. https://dx.doi.org/10.1002/uog.15829.
- Abu-Rustum R.S., Daou L., Abu-Rustum S.E. Role of first-trimester sonography in the diagnosis of aneuploidy and structural fetal anomalies. J. Ultrasound. Med. 2010; 29(10): 1445-52. https://dx.doi.org/10.7863/jum.2010.29.10.1445.
- Timor-Tritsch I.E., Bashiri A., Monteagudo A., Arslan A.A. Qualified and trained sonographers in the US can perform early fetal anatomy scans between 11 and 14 weeks. Am. J. Obstet. Gynecol. 2004; 191(4): 1247-52. https://dx.doi.org/10.1016/j.ajog.2004.03.007.
- Bilardo C.M., Chaoui R., Hyett J.A., Kagan K.O., Karim J.N., Papageorghiou A.T. et al.; International Society of Ultrasound in Obstetrics and Gynecology. ISUOG Practice Guidelines (updated): performance of 11-14-week ultrasound scan. Ultrasound Obstet. Gynecol. 2023; 61(1): 127-43. https://dx.doi.org/10.1002/uog.26106.
- Minnella G.P., Crupano F.M., Syngelaki A., Zidere V., Akolekar R., Nicolaides K.H. Diagnosis of major heart defects by routine first-trimester ultrasound examination: association with increased nuchal translucency, tricuspid regurgitation and abnormal flow in ductus venosus. Ultrasound Obstet. Gynecol. 2020; 55(5): 637-44. https://dx.doi.org/10.1002/uog.21956.
- Buijtendijk M.F., Bet B.B., Leeflang M.M., Shah H., Reuvekamp T., Goring T. et al. Diagnostic accuracy of ultrasound screening for fetal structural abnormalities during the first and second trimester of pregnancy in low-risk and unselected populations. Cochrane Database Syst. Rev. 2024; 5(5): CD014715. https://dx.doi.org/10.1002/14651858.CD014715.pub2.
- Приказ Министерства здравоохранения Российской Федерации от 20.10.2020 N 1130н «Об утверждении Порядка оказания медицинской помощи по профилю "акушерство и гинекология"». [Order of the Ministry of Health of the Russian Federation of 20.10.2020 No. 1130n "On approval of the Procedure for the provision of medical care in the field of obstetrics and gynecology". (in Russian)].
- Kim S.A., Lee S.M., Hong J.S., Lee J., Park C.W., Kim B.J. et al. Ultrasonographic severity scoring of non-immune hydrops: a predictor of perinatal mortality. J. Perinat. Med. 2015; 43(1): 53-9. https://dx.doi.org/10.1515/jpm-2013-0208.
- Vanaparthy R., Vadakekut E.S., Mahdy H. Nonimmune hydrops fetalis. In: StatPearls. StatPearls Publishing: Treasure Island (FL); 2024.
- Gjerris A.C., Tabor A., Loft A., Christiansen M., Pinborg A. First trimester prenatal screening among women pregnant after IVF/ICSI. Hum. Reprod. Update. 2012; 18(4): 350-9. https://dx.doi.org/10.1093/humupd/dms010.
- Syngelaki A., Hammami A., Bower S., Zidere V., Akolekar R., Nicolaides K.H. Diagnosis of fetal non-chromosomal abnormalities on routine ultrasound examination at 11-13 weeks’ gestation. Ultrasound Obstet. Gynecol. 2019; 54(4): 468-76. https://dx.doi.org/10.1002/uog.20844.
- Karim J.N., Roberts N.W., Salomon L.J., Papageorghiou A.T. Systematic review of first-trimester ultrasound screening for detection of fetal structural anomalies and factors that affect screening performance. Ultrasound Obstet. Gynecol. 2017; 50(4): 429-41. https://dx.doi.org/10.1002/uog.17246.
- Liao Y., Wen H., Ouyang S., Yuan Y., Bi J., Guan Y. et al. Routine first-trimester ultrasound screening using a standardized anatomical protocol. Am. J. Obstet. Gynecol. 2021; 224(4): 396.e1-396.e15. https://dx.doi.org/10.1016/j.ajog.2020.10.037.
- Kenkhuis M.J.A., Bakker M., Bardi F., Fontanella F., Bakker M.K., Fleurke-Rozema J.H. et al. Effectiveness of 12-13-week scan for early diagnosis of fetal congenital anomalies in the cell-free DNA era. Ultrasound Obstet. Gynecol. 2018; 51(4): 463-9. https://dx.doi.org/10.1002/uog.17487.
- Kagan K., Schmid M., Hoopmann M., Wagner P., Abele H. Screening performance and costs of different strategies in prenatal screening for trisomy 21. Geburtshilfe Frauenheilkd. 2015; 75(03): 244-50. https://dx.doi.org/10.1055/s-0035-1545885.
Received 31.07.2024
Accepted 14.10.2024
About the Authors
Alexandra A. Piskulina, PhD student, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(904)888-36-22, piskulinaalexandra@yandex.ru, https://orcid.org/0009-0005-7845-690XKirill V. Kostyukov, Dr. Med. Sci., Head of the Department of the Ultrasound and Functional Diagnosis, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(926)214-97-84, kostyukov_k@yahoo.com, https://orcid.org/0000-0003-3094-4013



