Sacrococcygeal fetal teratoma
Relevance: Fetal sacrococcygeal teratoma (SCT) is a serious malformation, and predicting its outcome remains an unsolved problem. SCT is associated with postnatal and antenatal complications. A review of the literature has identified the risk factors for adverse outcomes in fetal SCT; when present, fetal surgery is performed. Objective: To investigate SCT outcomes and identify and analyze the main factors influencing perinatal outcomes. Materials and methods: The study included 59 pregnant women with fetal SCT who received perinatal consultation in the V.I. Kulakov NMRC for OG&P over 5 years. The analyzed data included medical history, fetal ultrasound (size, structure, and degree of SCT vascularization, Altman type, tumor volume/fetal weight ratio, and fetal complications), and pregnancy outcomes. In the final part of the study, prenatal factors that could be considered as predictors of disease outcomes were analyzed. Results: The median gestational age at SCT diagnosis was 19 weeks. There was a predominance of female fetuses (3.5:1). The most common type was type II (56.9%) according to the Altman classification, and the rarest was type III (10.3%). Most SCTs had a predominant solid component (54.2%). Non-immune hydrops fetalis (NIHF) was detected in eight (13.6%), cardiomegaly in 16 (27.6%), and polyhydramnios in 23 (39%) cases. In 38 (64.4%) cases, live births occurred, including 29 (76.3%) by caesarean section and 9 (23.7%) by vaginal delivery. Adverse prenatal risk factors included solid SCT structure and pronounced tumor vascularization, which led to the development of NIHF and cardiomegaly (p<0.005). Tumor volume/fetal weight ratio >0.12 was more than three times more common in the adverse outcome group (76.9% vs. 21.4%). Conclusion: Early detection of SCT, predominance of the solid component and abundant vascularization of SCT, and tumor volume/fetal weight ratio >0.12 before 24 weeks of gestation, can be considered as predictors of poor outcome. Authors' contributions: Kadyrberdieva F.Z., Syrkashev E.M., Podurovskaya Yu.L., Shmakov R.G. – conception and design of the study; Kadyrberdieva F.Z., Syrkashev E.M. – data collection and analysis, manuscript drafting; Podurovskaya Yu.L., Shmakov R.G. – manuscript editing. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Kadyrberdieva F.Z., Syrkashev E.M., Podurovskaya Yu.L., Shmakov R.G. Sacrococcygeal fetal teratoma. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (6): 80-88 (in Russian) https://dx.doi.org/10.18565/aig.2023.85Kadyrberdieva F.Z., Syrkashev E.M., Podurovskaya Yu.L., Shmakov R.G.
Keywords
Fetal sacrococcygeal teratoma (SCT) is one of the most common germ cell tumors, with an incidence of 1 in 10,000–35,000 live births and female predominance (3:1 ratio) [1, 2]. It has been shown that SCTs in fetuses complicate the course of the antenatal and postnatal period [3]; at the same time, the prediction of the perinatal outcome of this pathology remains an unsolved problem. Early diagnosis of SCT, regular dynamic ultrasound monitoring, and identification of factors that may affect perinatal outcomes will allow timely determination of pregnancy management strategies, including pregnancy termination or prolongation. In case of prolonged pregnancy, the appropriateness of fetal surgery and its method can be determined.
In previous publications, we have presented different types of fetal surgery options for SCT worldwide [4–8]:
1) Open fetal surgery.
2) Percutaneous minimally invasive procedures for SCT devascularization, including laser coagulation, thermocoagulation, and embolization (alcohol) of the vessels supplying SCT.
A promising direction is the use of open fetal surgery, where the survival rate is higher than that with percutaneous minimally invasive procedures (approximately 55 %) [5]. This method is a more radical surgical procedure with a lower incidence of possible complications (recanalization of coagulated vessels, aggressive growth of collateral vessels, hemorrhage into the tumor, tumor rupture, and tumor disintegration) arising from SCT devascularization.
This study aimed to investigate SCT outcomes and to identify and analyze the main factors influencing perinatal outcomes.
Materials and methods
The study was conducted in the obstetric and neonatal departments of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (hereinafter referred to as the Center).
The study included 59 pregnant women with fetal SCT who received perinatal consultation in the V.I. Kulakov NMRC for OG&P over 5 years.
The primary endpoints of this study were perinatal outcomes in antenatally diagnosed fetal SCT, including the incidence of antenatal and postnatal pregnancy loss and survival for fetuses with SCT. The secondary end points were antenatal factors that could potentially influence perinatal outcomes.
All patients underwent ultrasound examination (ultrasound) of the fetus using the VOLUSON E8 GE ultrasound system and echocardiography (EchoCG).
Clinical evaluation included gestational age at the time of SCT diagnosis; SCT size, structure, and degree of vascularization; location according to the Altman classification; tumor volume to fetal weight ratio (Vtumor/mfetus) when contacting the Center before the 24th week of pregnancy; signs of non-immune hydrops fetalis (NIHF), cardiomegaly, and polyhydramnios; fetal sex; and pregnancy outcomes.
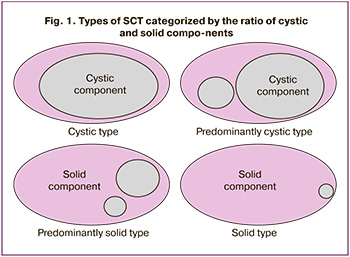
During ultrasound, the structure of the SCT was determined, and four variants were distinguished depending on the ratio of the cystic and solid components, 4 variants were distinguished (Fig. 1) [9]:
- Cystic SCT: more than 90% of the tumor is represented by cysts;
- Predominantly cystic SCT: 50–90% of the tumor is represented by cysts;
- Predominantly solid SCT: 50–90% of the tumor is represented by a solid component;
- Solid SCT: more than 90% of the tumor is represented by a solid component.
The degree of tumor vascularization was assessed using color Doppler and was defined as minimal, moderate, or severe.
The Altman classification was used to describe the location and extent of tumor spread [10]:
- Type I: entirely external;
- Type II: The external component prevails over the internal component;
- Type III: the internal component prevails over the external component;
- Type IV: completely internal with no external presentation.
According to some authors, a tumor volume to fetal weight ratio >0.12 has been associated with poor outcomes in fetuses with SCT [11]. To calculate the tumor volume to fetal weight ratio, the tumor volume was determined by multiplying the three largest tumor sizes in the frontal, sagittal, and vertical projections and multiplying this value by 0.52. Fetal weight was calculated using the Hadlock formula [12], and the tumor volume to fetal weight ratio was calculated in cases where the gestational age at the time of contact with the Center was less than 24 weeks (the optimal gestational age for calculating this indicator) [13].
The following classification was used for the morphological assessment of SCT after its removal [14]:
I. Benign teratomas
A. Mature teratoma:
- Grade 0 (all component tissues appear well differentiated);
- Grade 1 (Occasional microscopic foci contain incompletely differentiated tissues, not exceeding 10 percent of the sampled surface).
B. Immature teratoma:
- Grade 2 (immature tissues make up between 10 and 50 percent of the sampled tumor surface);
- Grade 3 (over half the surface examined is composed of undifferentiated tissues of uncertain metastatic potential).
II. Malignant teratomas
A. With areas of germ cell tumor (tumors of the yolk sac, embryonic cancer, germinoma, choriocarcinoma).
B. Non-germinal malignant tumor pattern (cancer or sarcoma)
C. Malignant immature teratoma (a teratoma that would otherwise be classified as a benign immature teratoma, but which subsequently becomes metastatic).
Pregnancy outcomes were analyzed according to gestational age at the end of pregnancy and the delivery mode. In the case of live birth, the newborn's length and weight growth parameters were analyzed, and the newborn's condition was assessed using the Apgar scale.
In the final part of the study, prenatal factors were analyzed, which, according to the literature, can be considered predictors of disease outcomes.
Statistical analysis
Statistical analysis was performed using StatTech v3.0.5 software (Russia). The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. Continuous variables were expressed as median (Me) with interquartile range (Q1; Q3). Differences in continuous variables between the groups were assessed using the Mann–Whitney nonparametric test. The statistical significance of the differences between the two values was assessed using p-values. Differences were considered statistically significant at p≤0.05.
Results and discussion
From 2018 to 2022, 63 SCT patients were treated at the Center. The distribution of the cases by year is shown in Figure 2.
This study did not include 4 pregnant women. Three patients with ongoing pregnancies remained under outpatient medical supervision, and the outcome of the pregnancy was unknown in one case due to the lack of communication with the patient. Figure 3 shows the outcomes of prenatally diagnosed SCTs.
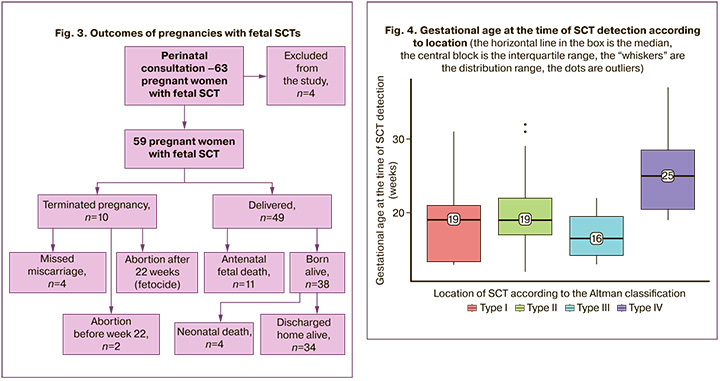
In 10 cases, the pregnancy was terminated: in 4 cases because of missed miscarriage and in 6 cases at the patient's request because of an unfavorable prognosis, of which 4 cases occurred before 22 weeks of ‘gestation and 2 cases after 22 weeks’ of gestation (fetocide).
There were 49 births, of which 11 were induced by fetal death before birth and 38 were live births.
Typically, fetal SCT is diagnosed at the 2nd prenatal screening; according to our data, the median gestational age at SCT detection is 19 (12–37) weeks. Table 1 shows the distribution of fetal SCT detection according to gestational age.
Female fetuses (n=46) predominated over male fetuses (n=13), with a ratio of 3.5:1. Table 2 presents the ultrasound characteristics of the SCTs and fetal complications.
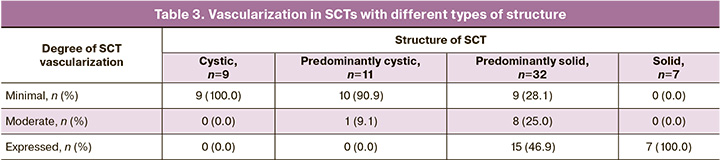
The study results showed that the most common type of SCT (56.9% of cases) according to the Altman classification was type II (predominantly external location) and the rarest type III (predominantly internal location) was observed in 10.3% of cases. The gestational age at the time of SCT detection, according to the type of location, did not differ significantly (p=0.061, Kruskal–Wallis test). However, in type IV (pelvic location), as seen in Figure 4, SCT was diagnosed later, with a median gestational age of 25 weeks, which is consistent with previously published data. It is known that the internal type of SCT is the most difficult location for diagnosis; against the background of an acoustic shadow from the pelvic bones, clear visualization according to ultrasound, especially in early pregnancy, can be difficult.
Cystic SCT (Fig. 5a) and predominantly cystic (Fig. 5b) occurred with almost equal frequency of 15.3 and 18.6% of cases, respectively. Most often (54.2%), SCTs were predominantly represented by a solid component (Fig. 5c), while SCTs with a solid structure (Fig. 5d) were the rarest, accounting for 11.9% of cases. Figure 5 shows examples of different types of SCTs.
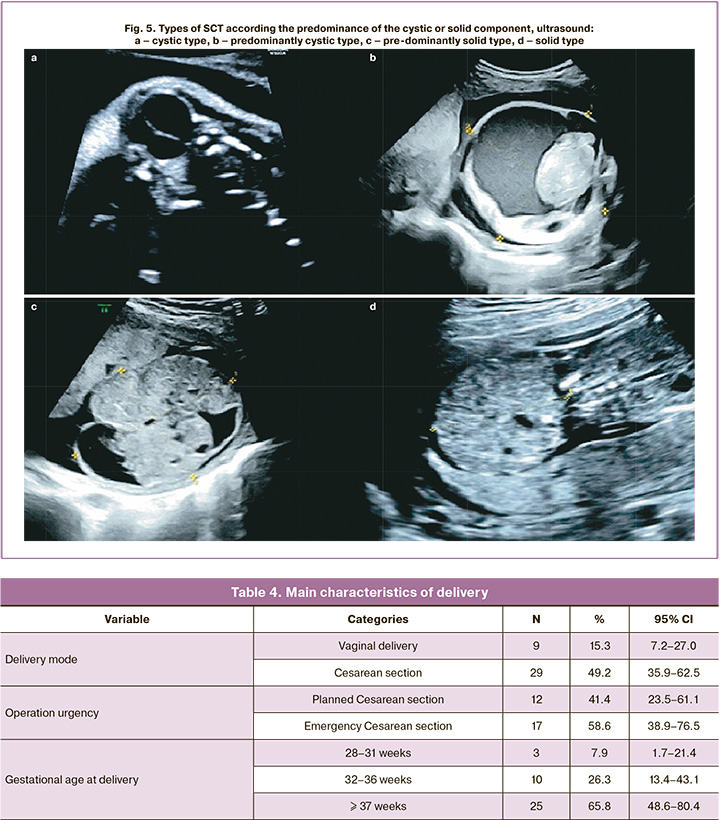
It is known that the more solid the component in the tumor structure, the more pronounced its vascularization, which was confirmed by our data (Table 3). The greatest vascularization was observed in SCT with a solid structure – 7/7 (100%) and the smallest in SCT with a cystic structure – 9/9 (100%).
In several cases, pregnancy complications (Table 4) included NIHF in eight (13.6%), cardiomegaly in 16 (27.6%) (according to echocardiography), and polyhydramnios in 23 (39%) patients.
It should be noted that in six cases, attempts were made to improve perinatal outcomes by performing interstitial laser photocoagulation of the vessels feeding the SCT of the fetus. The outcomes of these pregnancies were unfavorable, including 4 antenatal fetal deaths and two missed miscarriages.
Live births occurred in 38 (64.4%) patients. Most of them – 29 (76.3%) –underwent caesarean section, and 9 (23.7%) had vaginal delivery (with SCT sizes less than 5 cm) (Table 4).
Interestingly, there were significant differences between the gestational ages at delivery (p=0.002) in patients with different delivery modes (Fig. 6). The median gestational age for vaginal delivery was higher than that for delivery by caesarean section at 39 (38;39) weeks versus 37 (35;38) weeks, respectively (Kruskal¬–Wallis test).
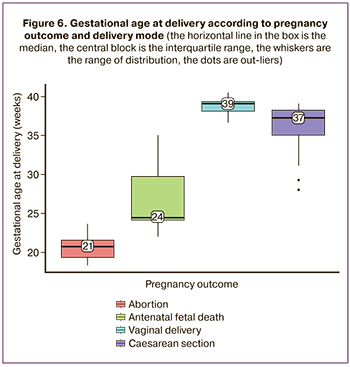
The presence of an external SCT component > 5 cm in the fetus is an indication for operative delivery. At the same time, as can be seen in Table 4, operative delivery was most often carried out as an emergency procedure (58.6%), the indication for which in most cases was the onset of labor and premature rupture of membranes in 58.8% (10/17) of cases. Such a high incidence of spontaneous labor and premature rupture of membranes in these patients may be due to the large size of the SCT as well as the high rate of concomitant polyhydramnios, which leads to excessive stretching of the myometrium. Therefore, polyhydramnios occurred in 22.2% (2/9) of cases during vaginal delivery, while almost every second case of operative delivery was accompanied by polyhydramnios in 48.3% (14/29) of cases.
The characteristics of newborns, despite the difference in gestational age at delivery, were not significantly different; however, the range of the values of length and weight growth parameters and the condition of newborns in children born by caesarean section were wider (Table 5).
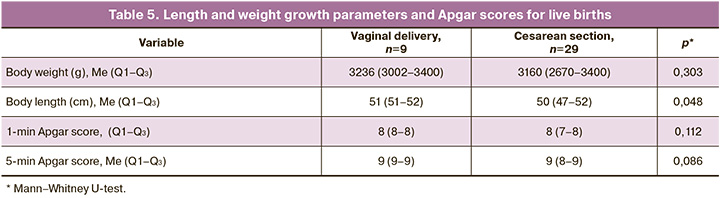
In one case, a child in a stable condition after birth was transferred to another specialized hospital. In 35 patients, surgical removal of SCT was performed after birth, of which 2 died postoperatively. One child died on the 1st day of life, and surgery was not performed because of the severely unstable condition of the newborn.
The results of the histological study of SCTs were available for 39 patients. SCT was mostly benign in 32 cases, whereas malignant SCT was observed in 7 cases. Thus, the outcomes in malignant SCTs had the following structure: four cases resulted in childbirth, two cases ended in abortion at the request of the family, and one resulted in antenatal fetal death.
The literature reports prenatal ultrasound factors in the fetus during the natural course of pregnancy that are associated with adverse outcomes [5, 15]. Some authors recommend considering fetal surgery in these cases to increase the likelihood of good outcome [16]. To assess the factors potentially influencing the perinatal outcome, all cases (with the exception of cases of pregnancy termination at the request of the patients (n=6)) were divided into two groups. Group I (n=19) included patients with an adverse perinatal outcome (missed miscarriage, antenatal fetal death, neonatal death), and group II (n=34) included patients with a good outcome (discharged home alive). Although the overall survival rate was 64.1%, it should be noted that one in three pregnancies with fetal SCT had a poor outcome.
The results of our study suggest that adverse prenatal risk factors included solid SCT structure (p<0.001) and pronounced tumor vascularization (p<0.001), which in most cases led to the development of NIHF (p<0.001) and cardiomegaly (p<0.003) (Table 6).
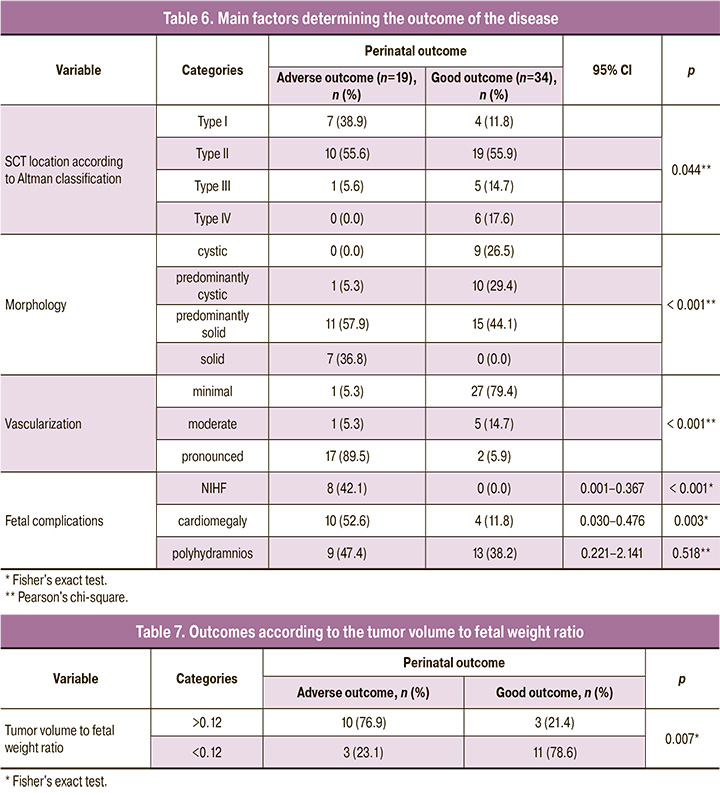
There was no difference in the incidence of polyhydramnios (p=0.518), which complicated the course of almost every second (47.4%) and every third case (38.2%) in the two groups. The distribution in groups by sex also had no differences; the male to female ratio in the group of adverse outcomes was 1:2.8 (5 male versus 14 female fetuses), and in the good outcome group – 1:5.8 (5 male versus 29 female fetuses (p=0.465).
There was a statistically significant difference between the groups in terms of gestational age at the time of SCT detection. In the group with poor outcomes, SCT was detected using US at 18 (15–20) weeks versus 20 (19–25) weeks in the group with good outcomes (p=0.014).
The tumor volume to fetal weight ratio was analyzed only in pregnant women who underwent fetal ultrasound at the Center before 24 weeks of gestation (n=33). A tumor volume to fetal weight ratio >0.12 was more than three times more common in the poor outcome group (76.9% versus 21.4%, respectively; Table 7).
The relative risk (RR) of the tumor volume to fetal weight ratio <0.12 in the good outcome group was 3.59 times higher than that in the adverse outcome group, and the differences in RR were statistically significant (95% CI 1.990–75.060).
Conclusion
Thus, early detection of SCT with a solid structure, abundant vascularization, and a tumor volume to fetal weight ratio > 0.12 before the 24th week of pregnancy can be considered as predictors of an adverse outcome.
The detection of SCT with cystic structure and unexpressed vascularization after the 20th week of pregnancy will allow for prolongation of the pregnancy and a more favorable outcome.
The current failures of fetal surgery should not impede the advancement of medicine in this field, and could be attributed to its initial implementation in a complex population. Therefore, it is crucial to accumulate expertise and evaluate outcomes not only in pregnancy outcomes, but also long-term newborn developmental outcomes when utilizing fetal surgery in various SCT categories. This approach will help to identify a narrow niche in which fetal surgery is essential.
Our experience has shown that percutaneous minimally invasive procedures with SCT devascularization can have an unpredictable course and lead to an aggressive response from the tumor (recanalization of coagulated vessels, rapid growth of collateral vessels), which in turn dictates the need for a differentiated approach to the choice of fetal surgery technique and the use of open interventions.
References
- Pauniaho S.L., Heikinheimo O., Vettenranta K., Salonen J., Stefanovic V., Ritvanen A. et al. High prevalence of sacrococcygeal teratoma in Finland - a nationwide population-based study. Acta Paediatr. 2013; 102(6): e251-6. https://dx.doi.org/10.1111/apa.12211.
- Hambraeus M., Arnbjörnsson E., Börjesson A., Salvesen K., Hagander L. Sacrococcygeal teratoma: a population-based study of incidence and prenatal prognostic factors. J. Pediatr. Surg. 2016; 51(3): 481-5. https://dx.doi.org/10.1016/j.jpedsurg.2015.09.007.
- Кадырбердиева Ф.З., Шмаков Р.Г., Бокерия Е.Л. Неиммунная водянка плода: современные принципы диагностики и лечения. Акушерство и гинекология. 2019; 10: 28-34. [Kadyrberdieva F.Z., Shmakov R.G., Bokeria E.L. Nonimmune hydrops fetalis: modern principles of diagnosis and treatment. Obstetrics and Gynecology. 2019; (10): 28-34. (in Russian)].https://dx.doi.org/10.18565/aig.2019.10.28-34.
- Adzick N.S., Crombleholme T.M., Morgan M.A., Quinn T.M. A rapidly growing fetal teratoma. Lancet. 1997; 349(9051): 538. https://dx.doi.org/10.1016/S0140-6736(97)80088-8.
- Van Mieghem T., Al-Ibrahim A., Deprest J., Lewi L., Langer J.C., Baud D. et al. Minimally invasive therapy for fetal sacrococcygeal teratoma: case series and systematic review of the literature. Ultrasound Obstet. Gynecol. 2014; 43(6): 611-9. https://dx.doi.org/10.1002/uog.13315.
- Adzick N.S. Open fetal surgery for life-threatening fetal anomalies. Semin. Fetal Neonatal Med. 2010; 15(1): 1-8. https://dx.doi.org/10.1016/j.siny.2009.05.003.
- Peiró J.L., Sbragia L., Scorletti F., Lim F.Y., Shaaban A. Management of fetal teratomas. Pediatr. Surg. Int. 2016; 32(7): 635-47. https://dx.doi.org/10.1007/s00383-016-3892-3.
- Кадырбердиева Ф.З., Сыркашев Е.М., Костюков К.В., Шмаков Р.Г. Крестцово-копчиковая тератома у плода: новое о старой проблеме. Акушерство и гинекология. 2023; 2: 12-7. [Kadyrberdieva F.Z., Syrkashev E.M., Kostyukov K.V., Shmakov R.G. Fetal sacrococcygeal teratoma: new about an old problem. Obstetrics and Gynecology. 2023; (2): 12-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.267.
- Usui N., Kitano Y., Sago H., Kanamori Y., Yoneda A., Nakamura T. et al. Outcomes of prenatally diagnosed sacrococcygeal teratomas: the results of a Japanese nationwide survey. J. Pediatr. Surg. 2012; 47(3): 441-7. https://dx.doi.org/10.1016/j.jpedsurg.2011.08.020.
- Altman R.P., Randolph J.G., Lilly J.R. Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section Survey-1973. J. Pediatr. Surg. 1974; 9(3): 389-98. https://dx.doi.org/10.1016/s0022-3468(74)80297-6.
- Akinkuotu A.C., Coleman A., Shue E., Sheikh F., Hirose S., Lim F.Y.,Olutoye O.O. Predictors of poor prognosis in prenatally diagnosed sacrococcygeal teratoma: a multiinstitutional review. J. Pediatr. Surg. 2015; 50(5): 771-4.https://dx.doi.org/10.1016/j.jpedsurg.2015.02.034.
- Blue N.R., Savabi M., Beddow M.E., Katukuri V.R., Fritts C.M., Izquierdo L.A., Chao C.R. The hadlock method is superior to newer methods for the prediction of the birth weight percentile. J. Ultrasound Med. 2019; 38(3): 587-96.https://dx.doi.org/10.1002/jum.14725.
- Rodriguez M.A., Cass D.L., Lazar D.A., Cassady C.I., Moise K.J., Johnson A. et al. Tumor volume to fetal weight ratio as an early prognostic classification for fetal sacrococcygeal teratoma. J. Pediatr. Surg. 2011; 46(6): 1182-5.https://dx.doi.org/10.1016/j.jpedsurg.2011.03.051.
- Щеголев А.И., Подгорнова М.Н., Дубова Е.А., Павлов К.А., Кучеров Ю.И.Клинико морфологическая характеристика крестцово копчиковых тератом у новорожденных. Акушерство и гинекология. 2011; 1: 42-6. [Shchegolev A.I., Podgornova M.N., Dubova E.A., Pavlov K.A.,Kucherov Yu.I. The clinical and morphological characteristics of neonatal sacrococcygeal teratomas. Obstetrics and Gynecology. 2011; (1): 42-6.(in Russian)].
- Kleijer W.J., van der Sterre M.L.T., Garritsen V.H., Raams A., Jaspers N.G.J. Evolution of prenatal detection of neural tube defects in the pregnant population of the city of Barcelona from 1992 to 2006. Prenat. Diagn. 2011; 31(12): 1184-8. https://dx.doi.org/10.1002/pd.2863.
- Sananes N., Javadian P., Schwach Werneck Britto I., Meyer N., Koch A., Gaudineau A. et al. Technical aspects and effectiveness of percutaneous fetal therapies for large sacrococcygeal teratomas: cohort study and literature review. Ultrasound Obstet. Gynecol. 2016; 47(6): 712-9. https://dx.doi.org/10.1002/uog.14935.
Received 05.04.2023
Accepted 01.06.2023
About the Authors
Faina Z. Kadyrberdieva, PhD, obstetrician-gynecologist, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology,Ministry of Health of Russia, +7(909)916-58-52, f_kadyrberdieva@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Egor M. Syrkashev, PhD, Researcher at the Radiology Department, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, e_syrkashev@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Yulia L. Podurovskaya, Ph.D., Head of the Department of Neonatal Surgery, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-14-24, y_podurovskaya@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Roman G. Shmakov, Dr. Med. Sci., Professor of the Russian Academy of Sciences, Director of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-72-00, r_shmakov@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.



