Prenatal ultrasound diagnosis and outcomes of congenital lung malformations: the Center’s 10-year experience with 363 cases
Objective: To investigate the feasibility of prenatal ultrasound diagnosis and to evaluate the outcomes of fetal congenital lung malformations.Mashinets N.V., Demidov V.N., Dorofeeva E.I., Podurovskaya Yu.L., Burov A.A., Filippova E.A., Kozlova A.V., Kulabukhova E.A., Gus A.I.
Materials and methods: The study included 363 pregnant women admitted to the Center from 2010 to 2019, fetal lung malformations were diagnosed prenatally in all the patients.
Results: Lung malformations were most frequently diagnosed at 20–22 weeks’ gestation (96.7%). The ultrasound examination revealed the main signs of the disease, namely, an increase in the lung tissue echogenicity (100%), enlarged pathological lung, the presence of cysts (71.9%), and mediastinal shift to the contralateral side. Positive changes in the course of the disease were observed in 77.7% of patients, previously detected signs disappeared completely in 12.3% of them; no changes were noted in 18.2% of cases. Fetal hydrops was identified in 4.4% of fetuses. A total of 271 pregnant women had a delivery at the Center, and 271 children were born alive. The accuracy of ultrasound diagnosis of lung malformations was 92.6%. Surgical treatment was performed in 84.9% of newborns; postoperative mortality was 0.5% (one preterm newborn).
Conclusion: Ultrasound examination provides a highly accurate intrauterine diagnosis of lung malformations. Total fetal and child mortality from this pathology was 4.7%, including 4.4% of deaths which occurred preoperatively and 0.3% postoperative deaths.
Keywords
Congenital pulmonary airway malformation (CPAM) (formerly known as congenital cystic adenomatoid malformation CCAM) and pulmonary sequestration (PS) are the most common congenital lung malformations in fetuses and newborns. CPAM occurs due to impaired terminal bronchiole development with the formation of lung cysts of various diameters [1–4]. PS is a bronchopulmonary mass without a normal bronchial communication; it receives blood supply through abnormal vessels from the systemic circulation, that is the aorta and its branches [3–5].
The estimated incidence of CPAM ranges from 1per 2000–3000 to 1per 35000 cases [6–10]. The incidence of PS is not precisely known due to the fact that this lesion occurs relatively seldom [5].
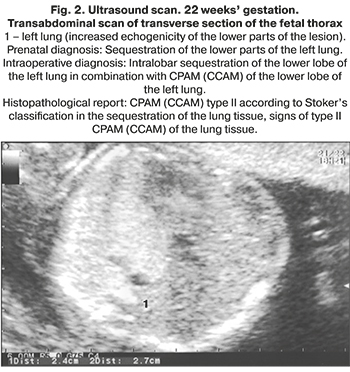
The morphological classification of CPAM, proposed by J. Stocker, is based on the size of the cysts and the nature of the lining epithelium. It is equally convenient for describing both lesions of the lung tissue and sequesters of the lung. Stocker et al. classified CPAM into the following groups: type I with single or multiple large cysts of size 2–10 cm containing mucus cells; type II with multiple medium sized cysts of 0.5–2 cm, lined with columnar epithelium; type III with micro-cysts, less than 0.5 cm in diameter, lined with cubic epithelium. Types 0 and IV were added later to this original classification. Type 0 refers to solid tissue involving both lungs due to congenital acinar dysplasia, and type IV is a large peripheral cyst. These two types account for only 5% of congenital lung malformations [11]. Currently, there is an opinion that CPAM and PS cannot always be considered as independent nosological entities. Morphological studies indicate that about 50% of CPAM and PS are hybrid forms with signs of an abnormal structure of the lung tissue and blood supply characteristic of sequestration [3, 11–14]. In general, the origin, morphological features, and clinical data of these diseases are very similar, therefore, it is possible to combine them into the congenital lung malformations group (Fig. 1–4).
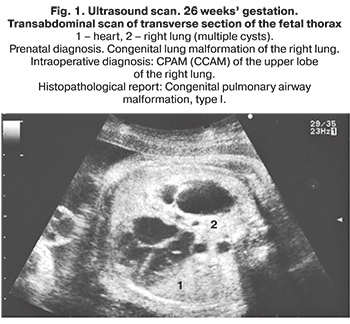
Comorbidity with congenital lung malformations is detected in 3–22% of cases. These are most common cardiovascular and urinary system abnormalities and congenital diaphragmatic hernia (СDH) [1, 8, 11, 12, 15]. Chromosomal pathology is extremely rare [8].
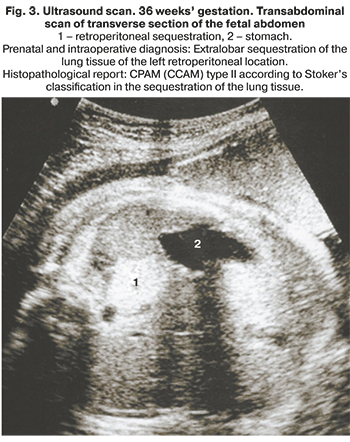
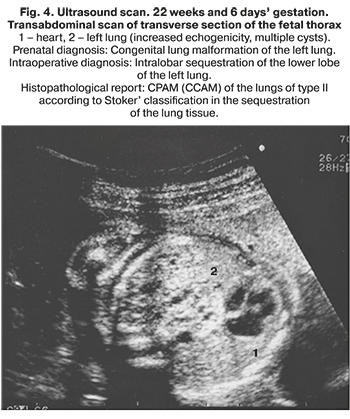
PS can be either intralobar or extralobar, depending on whether the mass is within or outside the normal lung lobe. Intralobar sequestration occurs when the accessory bud arises before the development of the pleura and can be found within the normal lung. Extralobar sequestration is wholly separated from the lung and has its pleura. Retroperitoneal (abdominal) sequestration is located below the diaphragm [5].
Lung tissue abnormalities can be diagnosed prenatally at 20–22 weeks’ gestation [1, 3, 8, 11, 16]. In case of such a malformation fetal chest ultrasound reveals an increase in the size and echogenicity of the entire lung or its part with the presence or absence of cystic lesions. In the overwhelming number of observations, the changes are unilateral and can be detected in only one of the lung lobes (in more than 95% of cases) [1, 3, 8, 11]. At 26–28 weeks’ gestation, there is a peak in the growth of pulmonary pathology [1, 8]. Later, usually after 32 weeks, there may be a decrease in size or complete disappearance of intrauterine echographic signs of this pathology [4, 6, 9, 12, 17–20].
Outcomes are favorable for the newborn in case of an uncomplicated intrauterine course of the disease. The survival rate of newborns is 88–97% [8, 11, 12]. An unfavorable variant of the course of pregnancy may result in fetal hydrops in fetuses with pulmonary malformation [1, 2, 11]. In the prenatal period, the co-occurrence of this complication with lung defects was noted in 5–9% of cases [8, 12], which is significantly lower than in the population. According to the literature, the incidence of non-immune fetal hydrops is 1 per 3000–4000 newborns [20–22]. Mortality rate varies within 50–100% after conservative management of fetal hydrops [11, 12]. Currently, if such a complication occurs in a fetus with a lung defect, possible surgical interventions include thoracoamniotic shunting [8, 12], sclerotherapy [23], and intrauterine laser coagulation of the feeding artery in PS [12].
As noted earlier, the signs of the lung malformation spontaneously resolve by end of the third trimester in 15–50% of cases [1, 8, 11, 12]. However, after examination of newborns (contrast CT or MRI), the pathology that disappeared during pregnancy is detected again in 60–100% of cases [6, 11, 12]. At the same time, the authors note the low diagnostic accuracy of chest radiography (about 60%) in comparison with CT.
Pregnancy management strategy for lung pathology depends on the size of lung lesions. The large lesions and the displacement of the mediastinum require delivery in a specialized hospital with the possibility of providing surgical care [1, 7].
The management of asymptomatic newborns is controversial. Some specialists adhere to conservative management [1, 7, 16, 24]. However, most pediatric surgeons believe it is safer and better to perform elective surgery before symptom manifestation. Patients with this lung pathology diagnosed in utero, despite the absence of symptoms after birth, should be examined at the age of 4–6 weeks, and at the age of 3–6 months, if necessary, they undergo resection of the affected area of the lung or sequesterectomy [23]. It is important to note that early resection of the pathological portion of the lung can also contribute to compensatory growth and enlargement of the normal lung [3, 4, 25–28].
Materials and Methods
The study included 363 pregnant women admitted to the Center from 2010 to 2019, fetal lung malformations were diagnosed prenatally in all the patients.
An expert ultrasound examination of the fetus was carried out at all visits according to the standard technique with the necessary measurements and assessment using the Astraia system and expert fetal echocardiography. Ultrasound examination was performed with the help of Siemens SONOLINE Elegra (Germany), Alfa 10, Aloka (Japan), Voluson E8 (GE) instrument using a 3.5 MHz transabdominal convex probe and a 7.5 MHz linear probe. Along with this, all patients underwent Doppler sonography and cardiotocography using a fully automated cardiac monitor from the Unicos Company (Moscow).
When fetal lung abnormalities were detected, clinical assessment included determining the location, size, and echo structure of the pathological lesion and echographically healthy areas of the affected and contralateral lung, the presence and extent of cystic cavities, their number, and also the signs and extend of mediastinal displacement. The blood supply to the abnormal area of the lung was studied using Doppler mapping. A general examination of all organs and systems of the fetus was performed to exclude other congenital anomalies and complications such as heart failure and non-immune hydrops.
During the examination in the third trimester of pregnancy, changes in ultrasound images of the fetal lungs were assessed and compared with the baseline data (own data or according to medical documentation).
After the birth of a child with the affected lung, the doctors of the Center examined the newborn to clarify the diagnosis and identify indications for surgical treatment.
Results
Over ten years, 363 pregnancies with prenatally diagnosed congenital fetal lung defects were analyzed. The primary diagnosis was made at the Ultrasound and Functional Diagnostics Department of the Centre in 48/363 (13.2%) cases. The signs of congenital malformation of the lung tissue were found on ultrasound screening in other clinics in the remaining cases (315/363 (86.8%)).
Gestational age at the time of diagnosis of congenital lung disease was 20-22 weeks in 351/363 (96.7%) cases and 13–19 weeks in 12/363 (3.3%) patients.
At gestational age from 13 to 28 weeks (mean 23±2 weeks), 192/363 (52.9%) women had a prenatal consultation at the Center. In 171/363 (47.1%) observations, the primary consultation council took place in the third trimester of pregnancy. This can be due to the large territory of the country; however, the early consultation of these women using telemedicine communication was very helpful to inform parents about the problem, to choose management strategy, and arrange an in-person consultation after 32 weeks’ gestation.
During an ultrasound examination, the pathological process was more often diagnosed in the left lung (95/192 (49.5%)) than in the right one (85/192 (44.3%)), as well as in the retroperitoneal space (12/192 (6, 2%)).
An increase in echogenicity and greater size of the affected lung was found in all cases (192/192 (100%)). The cysts were found in the pathologically altered lung in 138/192 (71.9%) fetuses. Among them, the cysts were multiple in 107/138 (77.5%) cases, and single in (31/138 (22.5%)) patients. The diameter of the cysts varied from 0.5 cm to 10 cm. The most common cysts were small (less than 2 cm) in 87/138 (63%) cases, while large (2–5 cm) and giant (more than 5 cm) were detected much less often, namely in 45/138 (32.6%) and 6/138 (4.3%) observations, respectively. Mediastinum displacement of the enlarged lung was seen in the overwhelming majority of cases in the second trimester (156/192 (81.3%). In comparison, a significant increase in the size of the affected part of the lung was more often associated with its cystic structure (121/156 (77.6%)) than solid one (35/156 (22.4%)). PS of retroperitoneal localization was observed in 12/192 (6.2%) cases.
It should be noted that when detecting a retroperitoneal mass, it is necessary to make a differential diagnosis of adrenal gland lesions. Retroperitoneal PS is characterized by increased echogenicity, clear-cut contours and a separate feeding artery. Clear visualization of the ipsilateral adrenal gland separately from the mass is also required.
A complicated course of lung disease in the second trimester of pregnancy was detected in 11/192 (5.7%) cases, including hydropericardium (1), hydropericardium and myocardial hypertrophy (1), ascites (1), polyhydramnios (2), general fetal tissue edema with an accumulation of fluid in the cavities, that is non-immune fetal hydrops (6).
In the third trimester of pregnancy (both baseline and follow-up), ultrasound examination was performed in 318/363 (87.6%) patients. At the same time, the echographic image of the fetal lungs was compared with the data of the second trimester obtained in the course of their baseline examination (with a repeated visit to the doctors in 145/363 (39.9%) cases), or with the data from the medical documents from their clinics (with the initial visit in the latest period in173/363 (47.7%) cases). In 45/363 (12.4%) cases, the data could not be obtained due to the fact that the women failed to come for the second examination. Positive dynamics of fetal lung pathology was observed in 247/318 (77.7%) cases. Its manifestation was a decrease in the size of the pathological lesion, the number and size of cystic cavities, a reduction of mediastinal displacement, and disappearance of fluid in the pericardial cavity. The absence of changes was noted in 58/318 (18.2%) patients with lung defects. All of them were retroperitoneal lesions that retained their shape, size, and ultrasound characteristics. Negative dynamics in the form of an increase in the size of the affected part of the lung and the occurrence of non-immune fetal hydrops were found in 12/318 (3.8%) patients. Intrauterine shunting of the cystic cavity was performed in one case with a primary detected giant cyst and signs of an increase in heart failure and the appearance of ascites in the fetus; thus, it was possible to reduce intrathoracic tension and achieve positive dynamics of the fetus (1/318 (0.3%)).
At the prenatal stage CPAM (CCAM), lung sequestration, retroperitoneal lesions, congenital lung malformation, and lung cyst were found in 181/363 (49.9%), 66/363 (18.2%), 22/363 (6.1 %), 89/363 (24.4%), and 5/363 (1.4%) patients, respectively.
Concomitant anomalies of other organs and systems were diagnosed in 26/363 fetuses (7.2%), including congenital diaphragmatic hernia (CDH) – 8/363 (2.2%), cardiovascular defects – 8/363 (2.2%), urinary system – 4/363 (1.1%), gastrointestinal tract – 5/363 (1.4%), external genital organs – 1/363 (0.3%). In 4 cases, lung defect was found in multiple pregnancies (4/363 (1.1%)), while in 3 cases it was revealed in one of twin fetuses, in one case it was in both fetuses.
A complicated course of fetal lung disease (accumulation of fluid in the cavities, development of non-immune hydrops) during pregnancy was detected in 16/363 (4.4%) patients: 11 patients had it at the beginning of the second trimester and 5 patients had it at the end of the trimester (up to 30 weeks’ gestation). The outcome of one pregnancy with an early-onset polyhydramnios remained unknown. In 4 cases, positive changes were noted in the form of spontaneous disappearance of fluid from the cavities (3) and after intrauterine shunting (1). Two pregnancies were terminated before 22 weeks; intrauterine fetal death occurred in 2 cases; the remaining 7 pregnancies ended in the birth of children of varying degrees of prematurity with signs of non-immune hydrops and early death due to severe respiratory and multiple organ failure.
A total of 271 pregnant women with a prenatal diagnosis of fetal congenital lung disease had a delivery at the Center, and 271 children were born alive. In one case there were twins with lung abnormalities in both children, and in one case there was a sudden intrauterine death of a full-term fetus before the onset of labor (according to pathological studies, due to acute hypoxia caused by umbilical cord knot (1/272 (0.4%)).
Signs of moderate and severe intrapartum asphyxia in the early neonatal period were observed in 26/271 (9.6%) children, while resuscitation with tracheal intubation and mechanical ventilation at birth was required only in 13 cases, namely in 8 children with CDH, in 4 children with non-immune hydrops and 1 child with a large cyst of the lung. In the overwhelming majority of deliveries, no signs of intrapartum asphyxia were observed (245/271 (90.4%)).
All children with a prenatally diagnosed congenital lung disease had a standard set of lung examinations (frontal X-ray, ultrasound of the pleural cavities and retroperitoneal space, chest and abdominal CT or MRI). In children who died in the early neonatal period and a stillborn child, the diagnosis was confirmed by postmortem examination. The spectrum of identified malformations of the chest organs is presented in Table 1.
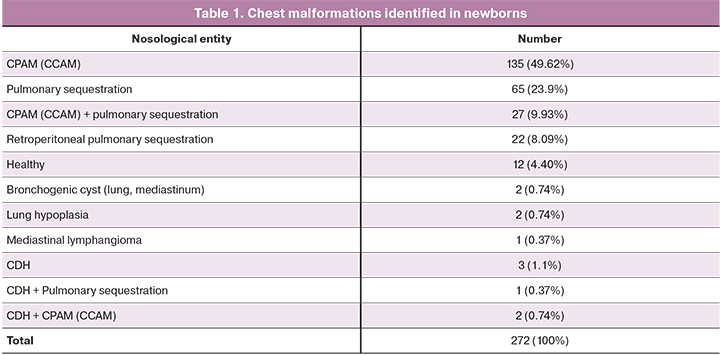
Therefore, prenatally diagnosed congenital lung malformation was confirmed after birth in 252/272 (92.6%) newborns; intrauterine pathology resolved spontaneously in 12/272 (4.4%) cases and 9/272 (3.3%) newborns had it. Other chest malformations were revealed in 3 cases in children with a congenital diaphragmatic hernia, but there were no signs of lung tissue damage; hypoplasia of one lung was detected in combination with vicarious hypertrophy of the contralateral one in 2 cases; bronchogenic cysts of different locations were diagnosed in 2 cases, small-sized mediastinal lymphangioma was revealed in 1 case. Neither lung defect nor congenital diaphragmatic hernia was confirmed in 1 case. The child had a slight partial relaxation of the left diaphragm.
As noted above, echographic signs of lung pathology were absent in 39/318 (12.3%) cases till the end of pregnancy; 35 children from this group were born and examined at the Center. The process resolved and it was confirmed in 4 children; in other cases, previously identified signs of lung tissue defect disappeared.
The analysis of the accuracy of prenatal nosological diagnosis of lung defects in newborns is presented in Table 2.
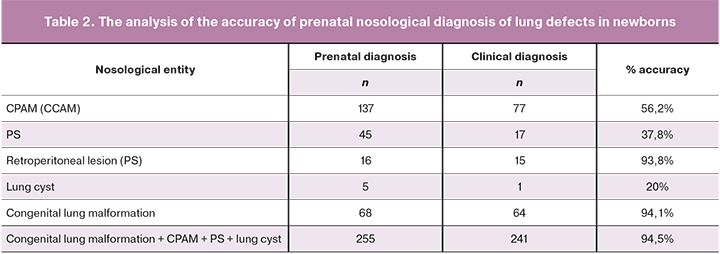
The accuracy of nosological prenatal diagnosis is low, except for the retroperitoneal PS and congenital lung malformations as a whole. Perhaps this is due to the diversity of the ultrasound images of CPAM (CCAM) and PS at the prenatal stage, the absence of their pathognomonic signs and the frequent occurrence of hybrid defects, which may cause diagnostic errors.
In the neonatal period, surgical treatment for congenital lung malformations was performed in 214/252 (84.9%) children. In 32 cases, a conservative strategy was chosen due to the small size of lesions and the absence of respiratory failure (32/252; 12.7%). In 5 cases, surgical intervention was not performed due to the early death of 3/252 (1.2%) children with non-immune fetal hydrops and 2/252 (0.8%) children with congenital diaphragmatic hernia. One child was stillborn (1/252 (0.4%)). Surgical interventions were performed in 199/214 (93%) cases with a minimally invasive thoracoscopic approach, which usually reduces surgical trauma, shortens the postoperative period, and has a good cosmetic result. Since 2012, all surgeries for congenital lung malformations have been performed using video endoscopic technology. A morphological study of all surgical specimens was carried out; the diagnoses of congenital lung tissue defects were confirmed.
Surgical treatment was effective in 213/214 (99.5%) children. They were discharged home, and their growth and development corresponded to age according to follow-up observations lasting from 1 month to 10 years. Postoperative mortality was 0.5%: 1/214 babies was preterm and had immaturity of the lung tissue of conventionally healthy lobes of the lung. Despite the successful removal of the pathological lobe of the lung, death occurred due to increasing severe respiratory failure.
On the basis of the outcomes of 318 fetuses and children with congenital lung malformations (272 children were born at the Center and 46 babies were born in other medical institutions), the total mortality was 15/318 (4.7%) taking into account intrauterine and postnatal losses; it included deaths which occurred preoperatively and postoperatively, 14/318 (4.4%) and 1/318 (0.3%), respectively. We observed a positive result of pregnancy and treatment of newborns in 303/318 (95.3%) cases.
Discussion
CPAM (CCAM) and PS are the most common congenital lung malformations. Prenatal ultrasound diagnostic accuracy of pulmonary malformation is relatively high. Over ten years, we observed 363 fetuses with this pathology. After birth, intrauterine diagnosis accuracy was 92.6%. In the postnatal period, pathology was not detected in 4.4% cases; diagnostic errors were made in 3.3% cases.
Concomitant pathology in pulmonary malformation developed in rare cases, 7.2% in our observations and 3–18% according to the literature [1, 8, 11, 12, 15]. Most often, CDH, cardiovascular and urinary system pathology were noted.
Lung defects were diagnosed at 20–22 weeks’ gestation in 96.7% of cases, the obtained results were consistent with the literature [1, 2, 9, 11, 16].
The ultrasound examination revealed the main signs of the disease, namely, an increase in the lung tissue echogenicity, enlarged pathological lung, the presence of cysts and mediastinal shift to the contralateral side. The signs of fluid accumulation in the cavities (hydrothorax, ascites, hydropericardium) and soft tissues edema of the fetus with the development of non-immune hydrops suggested a complicated course of the disease. The study of the blood flow of the pathological focus was not always successful, despite the precision of modern equipment.
By the end of pregnancy, there was a decrease in the pathological area in the lungs in 77.7% of the fetuses, and the complete disappearance of the echographic signs of this pathology was noted in 12.3% of cases. These results are consistent with the ones presented in the literature, where this phenomenon was observed in 32–76% and 15–50% of cases, respectively [1, 4, 6, 8, 9, 11, 12, 27]. The established pattern is associated with a decrease in the size of the lesion relative to the mass of healthy lung tissue by the end of pregnancy and a change in the echogenicity of both healthy and pathological lung tissue, especially in the minor cystic variant.
There is an opinion that the leading cause of adverse outcomes in pulmonary pathology is the development of fetal hydrops, which is observed in 5–9% of cases [8, 12]. In our study, this complication occurred in 4.4% of fetuses. Due to the many reasons for fetal hydrops, it is often difficult to identify the leading one among them. Both infectious factors and an increase in heart failure due to the compression of the mediastinum play a significant role. According to the literature, non-immune fetal hydrops occurs in 1 case per 3000–4000 newborns [20–22], which is much more frequent than lung defects.
We observed a favorable outcome of pregnancy and treatment of newborns in 303/318 (95.3%) with known outcomes. Taking into account intrauterine and postnatal losses, total fetal and child mortality from this pathology was 4.7% (15/138 of cases), including 4.4% (14) of deaths which occurred preoperatively and 0.3% (1) postoperative deaths. According to the literature, the survival rate of fetuses and newborns is 88–97%. The reasons for the adverse outcome are preterm birth, intrauterine or neonatal death, termination of pregnancy are not related to pulmonary pathology and viral pneumonia after delivery [8, 11, 12].
A total of 271 children were born at the Center. Signs of intrapartum asphyxia were not noted in 90.1% of newborns. Resuscitation and mechanical ventilation were required for 4.8% of newborns with CDH and non-immune fetal hydrops.
Most specialists consider MRI and CT imaging to be the gold standard for diagnosing lung pathology after birth and recommend performing them in all newborns with a prenatally established diagnosis, including with the cases when echographic signs of this pathology disappear completely at the end of pregnancy [6, 10, 11].
In our study, echographic signs of the pulmonary pathology were not detected in 39 fetuses at the end of pregnancy. However, according to MRI and CT performed after birth, they were diagnosed again in 31 newborns (88.6%). According to the literature, the pathology that disappeared during pregnancy was detected again after birth in 60-100% of newborns [6, 11, 12].
Surgical treatment after birth was performed in 214 newborns (84.9%). Postoperative survival rate was 99.5% (213 newborns); mortality was 0.5% (1 preterm newborn).
Analysis of the accuracy of detecting prenatal lung defects showed the high efficiency of echography in diagnosing retroperitoneal PS (93.8%) and lung malformations in general (94.5%).
However, it should be noted that making an accurate diagnosis of the lung malformation at the prenatal stage does not affect the strategy of further surveillance during pregnancy, the choice of delivery mode, and the range of examinations of the newborn. Therefore, we consider it helpful to combine the obtained prenatal ultrasound data into the collective concept of “Congenital lung malformation,” excluding retroperitoneal lesions as they present their own peculiarities and difficulties in diagnosis. Establishing a prenatal diagnosis of congenital lung malformation completely reflects the essence of the process; it allows clinicians to provide future parents with timely explanations, to trace the development of the process to delivery, to examine the newborn and to decide on the subsequent strategy of examination and treatment.
Conclusions
1. In the overwhelming majority of cases, primary diagnosis of congenital lung malformations occurs in the second trimester. At this gestational age, changes in the ultrasound lung image are most typical and informative.
2. In most cases, there are significant difficulties in ultrasound diagnosis of the exact type of the malformation in the prenatal period. However, the collective concept “Congenital lung malformation” completely reflects the essence of the pathological process in the lung tissue; there is no need for further clarification, and it allows for predicting during pregnancy.
3. The absence of previously identified signs of lung defect during dynamic studies in the third trimester of pregnancy is not evidence of complete recovery of the fetus; newborn babies must be examined after birth to confirm or exclude lung tissue anomaly.
4. A severe complication of pregnancy in congenital lung anomalies is non-immune fetal hydrops, which causes prenatal and postnatal mortality due to prematurity and immaturity of the lungs. At the same time, its detection rate does not increase that in the population and it is not a consequence of only pulmonary pathology.
5. Congenital lung malformations are prognostically favorable, which may be confirmed by the patterns of their changes during the intrauterine period, the rarity of concomitant anomalies of other organs and systems, and the results of observation and examination and surgical treatment of newborns.
References
- Laje P., Liechty K.W. Postnatal management and outcome of prenatally diagnosed lung lesions. Prenat. Diagn. 2008; 28(7): 612-8. https//dx.doi.org/10.1002/pd.1966.
- Медведев М.В. Врожденные пороки органов грудной клетки. В кн.: Медведев М.В., ред. Пренатальная эхография. Дифференциальный диагноз и прогноз. М.: Реал Тайм; 2009: 108-36. [Medvedev M.V. Congenital malformations of the chest organs. In: Medvedev M.V., ed. Prenatal echography. Differential diagnosis and prognosis. M.: Real Time. 2009: 108-36 (in Russian)].
- Gallardo M., Alvarez de la Rosa M., De Luis J., Mendoza R.L., Padilla P.A.I., Troyano L.J. Antenatal ultrasound diagnosis and neonatal results of the congenital cystic adenomatoid malformation of the lung. Rev. Chil. Pediatr. 2018; 89(2): 224-30. https//dx.doi.org/10.4067/S0370-41062018000200224.
- Tocchioni F., Lombardi E., Ghionzoli M., Ciardini E., Noccioli B., Messineo A. Long-term lung function in children following lobectomy for congenital lung malformation. J. Pediatr. Surg. 2017: 52(12): 1891-7. https//dx.doi.org/10.1016/j.jpedsurg.2017.08.059.
- Юдина Е.В. Легкие. В кн.: Медведев М.В., ред. Пренатальная эхография. М.: Реальное Время; 2005: 341-71. [Yudina E.V. Lungs. In: Medvedev M.V., ed. Prenatal echography. M.: Real Time. 2005: 341-71 (in Russian)].
- Kunisaki S.M., Ehrenberg-Buchner S., Dillman J.R., Smith E.A., Mychaliska G.B., Treadwell M.C. Vanishing fetal lung malformations: Prenatal sonographic characteristics and postnatal outcomes. J. Pediatr. Surg. 2015; 50(6): 978-82. https//dx.doi.org/10.1016/j.jpedsurg.2015.03.025.
- Stanton M. The argument for a non-operative approach to asymptomatic lung lesions. Semin. Pediatr. Surg. 2015; 24(4): 183-6. https//dx.doi.org/10.1053/j.sempedsurg.2015.01.014.
- Walker L., Cohen K., Rankin J., Crabbe D. Outcome of prenatally diagnosed congenital lung anomalies in the North of England: a review of 228 cases to aid in prenatal counseling. Prenat. Diagn. 2017; 37(10): 1001-7. https//dx.doi.org/10.1002/pd.5134.
- Lima J.S., Camargos P.A.M., Aguiar R.A.L.P., Campos A.S., Aguiar M.J. Pre and perinatal aspects of congenital cystic adenomatoid malformation of the lung. J. Matern. Fetal Neonatal Med. 2014; 27(3): 228-32. https//dx.doi.org/10.3109/14767058.2013.807236.
- Fuchs J. Congenital lung malformations. In: Parikh D., Rajesh P.B., eds. Tips and tricks in thoracic surgery. London: Springer-Verlag; 2018: 3-16. https//dx.doi.org/10.1007/978-1-4471-7355-7.
- Di Prima F.A., Bellia A., Inclimona G., Grasso F., Teresa M., Cassaro M.N. Antenatally diagnosed congenital cystic adenomatoid malformations (CCAM): Research Review. J. Prenat. Med. 2012: 6(2): 22-30.
- Cavoretto P., Molina F., Poggi S., Davenport M., Nicolaides K.H. Prenatal diagnosis and outcome of echogenic fetal lung lesions. al. Ultrasound Obstet. Gynecol. 2008; 32(6): 769-83. https//dx.doi.org/10.1002/uog.6218.
- Дубова Е.А., Павлов К.А., Щеголев А.И., Кучеров Ю.И., Жиркова Ю.В., Курашвили Ю.Б. Забрюшинная секвестрация легкого у новорожденного. Акушерство и гинекология. 2011; 7-2: 83-6. [Dubova E.A., Pavlov K.A., Schegolev A.I., Kucherov U.I., Zhirkova Y.V., Kurashvili Y.B. Retroperitoneal sequestration of the lung in the newborn. Obstetrics and Gynecology. 2011; 7(2): 83-6. (in Russian)].
- Дубова Е.А., Павлов К.А., Кучеров Ю.И., Жиркова Ю.В., Кулабухова Е.А., Щеголев А.И. Внелегочная секвестрация легкого. Российский вестник детской хирургии, анестезиологии и реанимации. 2011; 2: 53-9. [Dubova E.A., Pavlov K.A., Kucherov U.I., Zhirkova Y.V., Kulabuhova E.A., Schegolev A.I. Extrapulmonary sequestration of the lung. Russian bulletin of pediatric surgery, anesthesiology and reanimation. 2011; 2: 53-9. (in Russian)].
- Дорофеева Е.И., Подуровская Ю.Л., Зубков В.В., Пыков М.И., Филиппова Е.А., Кулабухова Е.А. Редкие комбинации врожденных пороков развития легких у новорожденных: диагностика и хирургическое лечение. Доктор.Ру. 2017; 3(132): 26-30. [Dorofeeva E.I., Podurovskaya J.L., Zubkov V.V., Pykov M.I., Filippova E.A., Kulabukhova E.A. Rare combined congenital lung anomaliesin newborns: diagnostics and surgical treatment. Doctor.ru. 2017; 3(132): 26-30. (in Russuan)].
- Туманова У.Н., Дорофеева Е.И., Подуровская Ю.Л., Щеголев А.И., Дегтярев Д.Н. Секвестрация легкого: классификация, диагностика, лечение. Педиатрия. Журнал им. Г.Н. Сперанского. 2018; 97(2): 163-71. [Tumanova U.N., Dorofeeva E.I., Podurovskaya J.L., Schegolev A.I., Degtyarev D.N. Lung sequestration: classification, diagnostics, treatment. Pediatrics. Journal named after G.N. Speranskyi. 2018; 97(2): 163-71. (in Russian)].
- Ehrenberg-Buchner S., Stapf A.M., Berman D.R., Drongowski R.A., Mychaliska G.B., Treadwell M.C., Kunisaki S.M. Fetal lung lesions: can we start to breathe easier? Am. J. Obstet. Gynecol. 2013; 208(2): 151. e1-7. https//dx.doi.org/10.1016/j.ajog.2012.11.012.
- Delacourt C., Bertille N., Salomon L.J., Benachi A., Henry E., Massardier J. et al. Natural prenatal history of congenital pulmonary malformations: the MALFPULM population-based cohort study. Ultrasound Obstet. Gynecol. 2019; 54(3): 381-8. https//dx.doi.org/10.1002/uog.20130.
- Durell J., Thakkar H., Gould S., Fowler D., Lakhoo K. Pathology of asymptomatic, prenatally diagnosed cystic lung malformations. J. Pediatr. Surg. 2016: 51(2): 231-5. https//dx.doi.org/10.1016/j.jpedsurg.2015.10.061.
- Bellini C., Donarini G., Paladini D., Calevo M.G., Bellini T., Ramenghi L.A., Hennekam R.C. Etiology of non-immune hydrops fetalis: An update. Am. J. Genet. A. 2015; 167A(5): 1082-8. https//dx.doi.org/10.1002/ajmg.a.36988.
- Kumar M., Jha V., Singh A. Nonimmune hydrops fetalis: Factors which predict outcome. J. Obstet. Gynaecol. India. 2018; 68(3): 197-203. https//dx.doi.org/10.1007/s13224-017-1011-6.
- Steurer M.A., Peyvandi Sh., Baer R.J., MacKenzie T., Li B.C., Norton M.E. et al. Epidemiology of live born infants with nonimmune hydrops fetalis - insights from a population-based dataset. J. Pediatr. 2017; 187: 182-8. e3. https//dx.doi.org/10.1016/j.jpeds.2017.04.025.
- Macardle C.A., Ehrenberg-Buchner S., Smith E.A., Dillman J.R., Mychaliska G.B., Treadwell M.C., Kunisaki S.M. Surveillance of fetal lung lesions using the congenital pulmonary airway malformation volume ratio: natural history and outcome. Prenat. Diagn. 2016; 36(3): 282-9. https//dx.doi.org/10.1002/pd.4761.
- Chon A.H., Korst L.M., Abdel-Sattar M., Llanes A., Ouzounian J.G., Chmait R.H. Type II and III congenital pulmonary airway malformation with hydrops treated in utero with percutaneous sclerotherapy. Prenat. Diagn. 2018; 38(7): 493-8. https//dx.doi.org/10.1002/pd.5266.
- Ng C., Stanwell J., Burge D.M. Conservative management of antenatally diagnosed cystic lung malformations. Arch. Dis. Child. 2014; 99(5): 432-7. https//dx.doi.org/10.1136/archdischild-2013-304048.
- Kunisaki S. M., Powelson I.A., Haydar B., Bowshier B.C., Jarboe M.D., Mychaliska G.B. et al. Thorascopic vs open lobectomy in infants and young children with congenital lung malformations. J. Am. Coll. Surg. 2014; 218(2): 261-70. https//dx.doi.org/10.1016/j.jamcollsurg.2013.10.010.
- Style C.C., Cass D.L., Verla M.A. Early vs late resection of asymptomatic congenital lung malformations. J. Pediatr. Surg. 2019: 54(1): 70-4. https//dx.doi.org/10.1016/j.jpedsurg.2018.10.035.
- Shamas A.G., Bohara K. Congenital cystic adenomatoid malformation of the lung (CCAM), a retrospective clinical audit and literature review in a tertiary centre in Scotland over a period of 14 years. J. Obstet. Gynaecol. 2017; 37(1): 19-24. https//dx.doi.org/10.1080/01443615.2016.1196480.
Received 08.04.2021
Accepted 30.04.2021
About the Authors
Natalya V. Mashinets, Ph.D., Senior Researcher at the Department of Functional Diagnostics, Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P,Ministry of Health of Russia, +7(906)795-66-47, natashamashinets@yandex.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Vladimir N. Demidov, Dr. Med. Sci., Professor at the Department of Functional Diagnostics, Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, +7(910)451-25-68, demydow@yandex.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Alexander I. Gus, Dr. Med. Sci., Professor, Head of Department of Ultrasound and Functional Diagnostics, Radiology Division, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, +7(985)231-97-44, aleksandr_gus@mail.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Elena I. Dorofeeva, Ph.D., Pediatric Surgeon, Clinical Care Supervisor at the Department of Neonatal Surgery, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)114-21-18, dorofey_i@mail.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Yulia L. Podurovskaya, Ph.D., Department of Neonatal Surgery, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)107-13-88, podurovskaya@yandex.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Artem A. Burov, Ph.D., Head of clinical work, anesthesiologist-resuscitator of the Department of surgery, reanimation and intensive care of newborns, V.I. Kulakov NMRC
for OG&P, Ministry of Health of Russia, +7(495)531-44-44, burovmd@gmail.com, 117997, Russia, Moscow, Ac. Oparin str., 4.
Elena A. Filippova, Ph.D., Head of the Department of Ultrasound Diagnostics in Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
+7(495)531-44-44, fla77@mail.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Alina V. Kozlova, Physician at the Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)531-44-44, av_kozlova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Elena A. Kulabukhova, Ph.D., Physician at the Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)531-44-44,
e_kulabuhova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Authors' contributions: Mashinets N.V., Dorofeeva E.I. – concept and design of the study, manuscript drafting; Mashinets N.V., Dorofeeva E.I., Podurovskaya Yu.L., Burov A.A., Filippova E.A., Kozlova A.V., Kulabukhova E.A. – data collection and analysis;
Demidov V.N., Gus A.I. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients provided informed consent for the publication of their data and associated images.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Mashinets N.V., Demidov V.N., Dorofeeva E.I., Podurovskaya Yu.L., Burov A.A., Filippova E.A., Kozlova A.V., Kulabukhova E.A., Gus A.I. Prenatal ultrasound diagnosis and outcomes of congenital lung malformations: the Center’s 10-year experience with 363 cases.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 9: 72-80 (in Russian)
https://dx.doi.org/10.18565/aig.2021.9.72-80



