Ultrasound characteristics of fetal brain structures in Arnold–Chiari malformation type II
Objective: To evaluate the echographic characteristics of brain structures in fetuses with Arnold–Chiari type II malformation.Chugunova L.A., Shmakov R.G., Gladkova K.A., Kostyukov K.V.
Materials and methods: We analyzed 25 cases of pregnancy with Arnold–Chiari type II malformations diagnosed between 12 and 21 weeks. All patients received clinical consultation at the V.I. Kulakov NMRC for OG&P from January 2019 to February 2022. Fetal evaluation included expert anatomical examination, extended two-dimensional echocardiography, and detailed evaluation of CNS structures. At 23.1–25.5 weeks’ gestation, 20 fetuses underwent successful intrauterine fetal surgery for Spina Bifida. In the remaining 5 cases, the pregnancy was terminated at the place of residence.
Results: At 12–14 weeks of gestation, all 5 fetuses had an abnormal amount of cerebrospinal fluid in the lateral ventricles, stenotic or not visible by ultrasound cerebral aqueduct with displacement of its posterior contour toward the occipital bone, and a «banana»-shaped cerebellum. At 19–21 weeks’ gestation, additional cerebral signs were identified, including corpus callosum abnormalities, dorsal cystic enlargement of ventricle III, interhemispheric holoprosencephaly, beak-like appearance of the tectum, sharpened occipital horns of the lateral ventricles, and delayed sulcation.
Conclusion: Alterations in supra- and infratentorial brain structures are characteristic of Arnold–Chiari malformation type II. Early echographic detection of cerebral signs of Arnold–Chiari type II allows timely decision making on fetal intrauterine surgery for Spina Bifida in the case of prolonged pregnancy.
Keywords
Diagnosis and treatment of congenital malformations of the central nervous system (CNS) continues to be a significant medical and social issue despite the medical advances of recent decades. Diseases of the nervous system are the leading cause of disability in children, with anomalies associated with neural tube defects being the most common, second only to infantile cerebral palsy. The incidence of neural tube defects averages 1 case per 1000 newborns [1]. The combination of early prenatal diagnosis using serum markers and fetal echographic imaging and prevention by prenatal folic acid oral supplementation used in recent years has contributed to a 20–50% reduction in the number of children born with neural tube anomalies [2–4]. However, these malformations have not been completely eradicated; this highlights the fact that not all forms of neural tube defects are folate preventable and that the etiology is probably multifactorial [4–6].
Congenital neural tube defects (NTDs) include a broad group of nosological forms, most of which can be identified early in intrauterine development and, in case of an adverse outcome, terminated. It is more difficult to deal with malformations that are non-lethal, but lead to severe disability due to severe neurological disorders. Such defects include spinal hernias (Spina Bifida), a defect in the development of the spine as a result of neurulation disorders in the third and fourth weeks of fetal development. The main manifestation of this pathology is a decrease in motor activity and sensitivity below the level of the injury, manifested as paresis and paralysis of the lower extremities and pelvic organ dysfunction. The most common malformations are myelomeningocele (MMC) and rachischisis (RS), which are part of the Arnold–Chiari type II malformation (hereafter, Chiari II). This malformation is characterized by protrusion of the dura and substance of the spinal cord through a defect of the vertebral processes, most commonly identified in the lumbosacral region. A characteristic feature is the wedging of the cerebellum and brain stem into the greater occipital foramen with subsequent development of hydrocephalus due to impaired cerebrospinal fluid outflow from the ventricular systems. According to some studies, this defect is associated with such structural abnormalities of the CNS as impaired neuronal migration, periventricular heterotopia, cerebellar and corpus callosum dysplasia, which increases the risk of cognitive deficit and mental disorders [7–15]. This malformation is associated with a high neonatal mortality rate that reaches 50% due to pronounced brain stem dysfunction [2].
Early prenatal diagnosis is crucial in the management of neural tube abnormalities. Clinical practice guidelines developed by internationally recognized medical organizations such as ISUOG, FMF, AIUM, and DEGUM have significantly improved the diagnosis of many chromosomal anomalies and malformations, including brain and spinal cord anomalies, by prenatal screening in the first trimester of pregnancy [16–18]. The search for new opportunities for early detection of congenital anomalies has led many specialists to realize that the fetus can receive exactly the same echographic sections of the anatomical structures of the brain as in neurosonography in children during the first year of life. Therefore, the concept of fetal neurosonography and the first results of detecting spinal dysraphism based on changes in the structure and position of the posterior fetal brain regions already in the first trimester of pregnancy have become part of world practice. For many years, the treatment of Chiari II was to close the defect after birth. In 80–90% of children, due to the progression of hydrocephalus, a ventriculoperitoneal shunt was required during the first year of life. The widespread introduction of intrauterine spinal hernia into world practice since the 2010s has reduced the severity of hydrocephalus and the frequency of shunting [19, 20].
A detailed morphometric evaluation of brain structures in Chiari II will improve the diagnosis of this malformation to identify the category of patients for potentially successful intrauterine repair of Spina Bifida.
The present study aimed to evaluate the echographic characteristics of brain structures in fetuses with Arnold–Chiari type II malformation.
Materials and methods
The study included 25 pregnant women diagnosed with Arnold–Chiari Type II malformations. All patients received a perinatal consultation at the Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia from January 2019 to February 2022. Gestational age was determined by the measurement of fetal crown-rump length during first-trimester ultrasound screening. Fetuses underwent expert anatomical examination, extended two-dimensional echocardiography, and Doppler ultrasound. Evaluation of fetal cerebral structures in the first trimester of pregnancy included the following: in the midsagittal plane – ratio of structures: trunk – IV ventricle – Grand Cistern; in the axial plane – the fact that the posterior margin of the Sylvian aqueduct was displaced to the contour of the occipital bone and absence of visualization of the IV ventricle. When the distance between the posterior contour of the trunk and the occipital bone decreased, a diagnostic search was performed for the level of spinal dysraphism. A detailed assessment of CNS structures from the second trimester of pregnancy included the study of the following parameters (according to the normative tables of Timor-Tritsch I. et al. (2012): corpus callosal length (distance between the points of its most proximal and distal poles measured in the mid-sagittal section), its shape, contours, and thickness in the genu, body, and splenium projection [12, 13, 17]. The length of the tectum (the distance between the maximal distant points of the upper edge – the junction point with the posterior wall of the III ventricle and the lower edge – the junction point with the upper surface of the worm) and its position relative to the brain stem (Fig. 1).
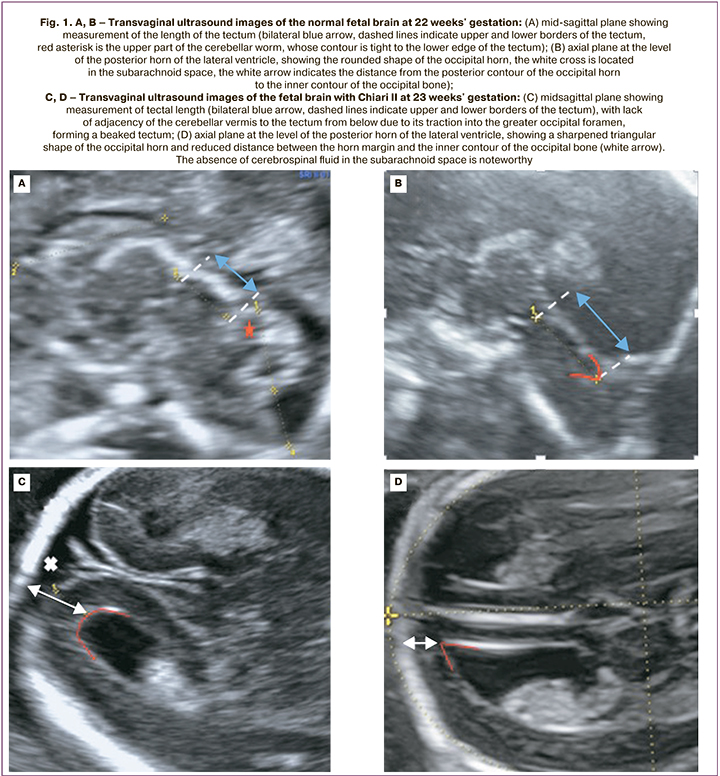
The combination of hypo and hyperplasia of the corpus callosum was considered its dysgenesis when the thickness of an anatomical segment was in the range above the 95th percentile and the thickness of the other segment was below the 5th percentile, regardless of the total length of the corpus callosum. Changes in the configuration of the posterior horn of the lateral ventricle, namely its sharpening and the reduction of the distance between its contour and the occipital bone, were considered as a "sharpened" posterior horn [7, 10]. Fissures were identified according to the 2021 ISUOG Practice Guidelines from the 19th week of pregnancy: parieto-occipital and Sylvian fissures in the axial transventricular and transcerebellar planes, respectively; calcarine fissures in coronal transcerebellar plane [16]. A further dynamic evaluation of fissure development, including the insular lobe formation, was performed pre- and postnatally in three orthogonal planes: axial, coronary, and sagittal [15]. Ultrasound was performed using a 3.5 MHz two-dimensional convex sensor and a 5–9 MHz convex 3D sensor on Voluson E8 (General Electric, Austria) and Samsung Medison WS80 (Samsung Medison, Republic of Korea. In 5 cases of Chiari II diagnosed before 14 weeks, the pregnancy was terminated at the place of residence for medical reasons and parental refusal of intrauterine surgery. In the remaining 20 cases, at 23.1–25.5 weeks of gestation, at the request of the parents, an intrauterine repair of Spina Bifida was successfully performed at the Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology. The 20 patients provided informed consent for fetal intrauterine surgery developed by Center specialists.
Quantitative parameters were presented as median, 25th–75th percentiles, minimum, and maximum values.
Results
Chiari II malformation was diagnosed in 5/25 fetuses at 12-14 weeks and in 20/25 fetuses at 19–21 weeks. At 12–14 weeks' gestation, the following cerebral features were identified in all 5 fetuses: abnormal amount of cerebrospinal fluid in the lateral ventricles (in 2 cases, "dry brain"; in 3 cases, vascular plexus occupied less than 50% of the lateral ventricular lumen); narrowing/absence of visualization of the cerebral aqueduct with its posterior contour shifted toward the occipital bone; the banana shaped cerebellum (Fig. 2). The initial level of Spina Bifida in relation to the spine was visualized from the L2–L3 vertebrae; a pronounced curvature of the spine (90° angle) was detected in 1/5 fetuses (Fig. 3).
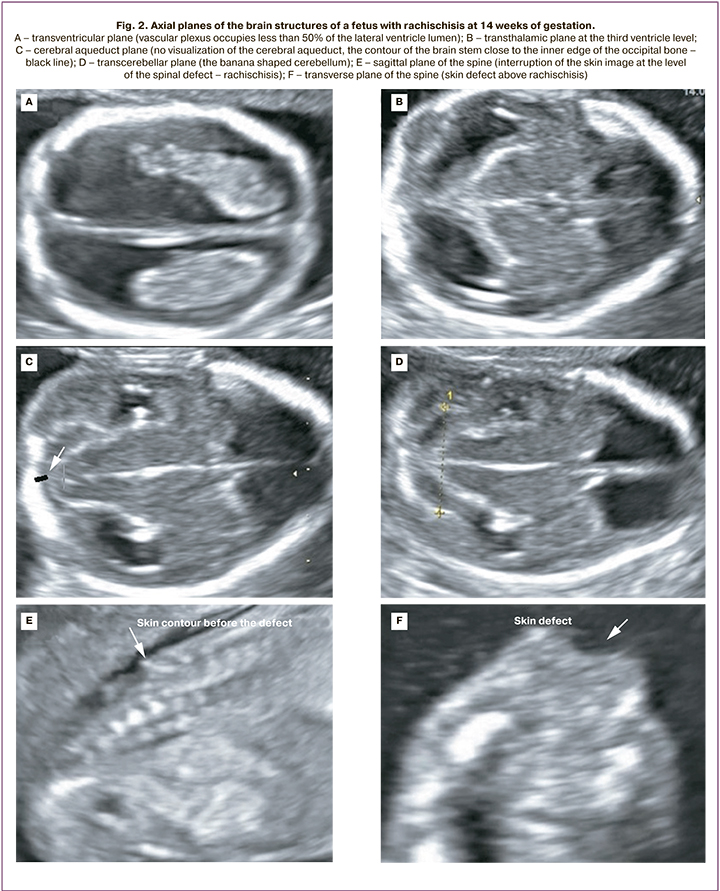
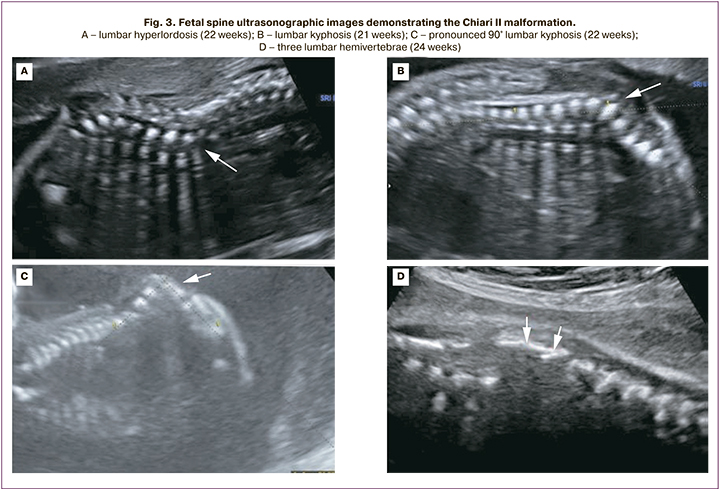
In the second trimester of pregnancy, common echographic signs of Chiari II were identified in all 20 cases, including occlusion of the cerebellum to the greater occipital foramen with the sign of its deformation (banana shape), changes in the configuration of the skull bones (lemon shape), ventriculomegaly, and the presence of spinal hernia. MMC and RSH were determined in 15/20 and 5/20 fetuses, respectively. The initial level of Spina Bifida in relation to the spine was determined in 2 fetuses from vertebra T10, in 14 fetuses from L2–L3, and in 4 fetuses from L4–L5 (Fig. 3).
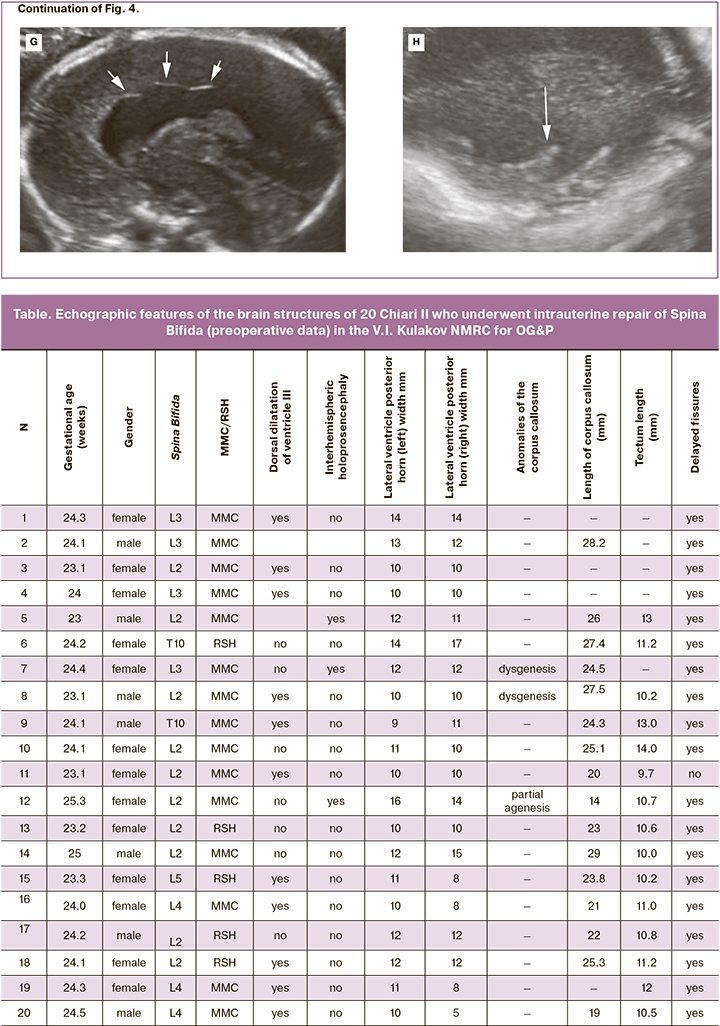
The mean gestational age at detailed fetal cerebral morphometry was 23.9 (23.0–25.3) weeks. The altered characteristic of 20 fetuses included ventriculomegaly (10–17 mm regardless of the level of the spinal cord injury), a beaked tectum (brain roof), "pointed" occipital horns of the lateral ventricles and delayed fissure formation (Fig. 4, Table). The mean width of the posterior horns of the lateral ventricles was 11.5 (9–16) mm on the left and 11 (5–17) mm on the right. Dysgenesis of the corpus callosum was detected in 3/20 patients. The remaining 17 cases were characterized by insignificantly shortened corpus callosum. The mean corpus callosum length was 23.8 (14–29) mm (Table). Half of the fetuses (55%) had dorsal cystic enlargement of ventricle III. Interhemispheric holoprosencephaly was diagnosed in 3 of 20 fetuses with Spina Bifida L2–L3. When evaluating the tectum, we found no significant differences in its length compared to normal values, but in all fetuses, its shape and location were altered. The mean tectum length was 11.1 (9.5–14) mm. Only 5/20 (25%) fetuses had tectum length greater than 11 mm, which corresponds to more than 2 standard deviations for gestational age 24–25 weeks according to Leibovitz Z. et al. [12]. In 80% (16/20) of the patients, there was abnormal formation of an insular lobe fissure with a typical fissure formation in the rest of the brain hemisphere.
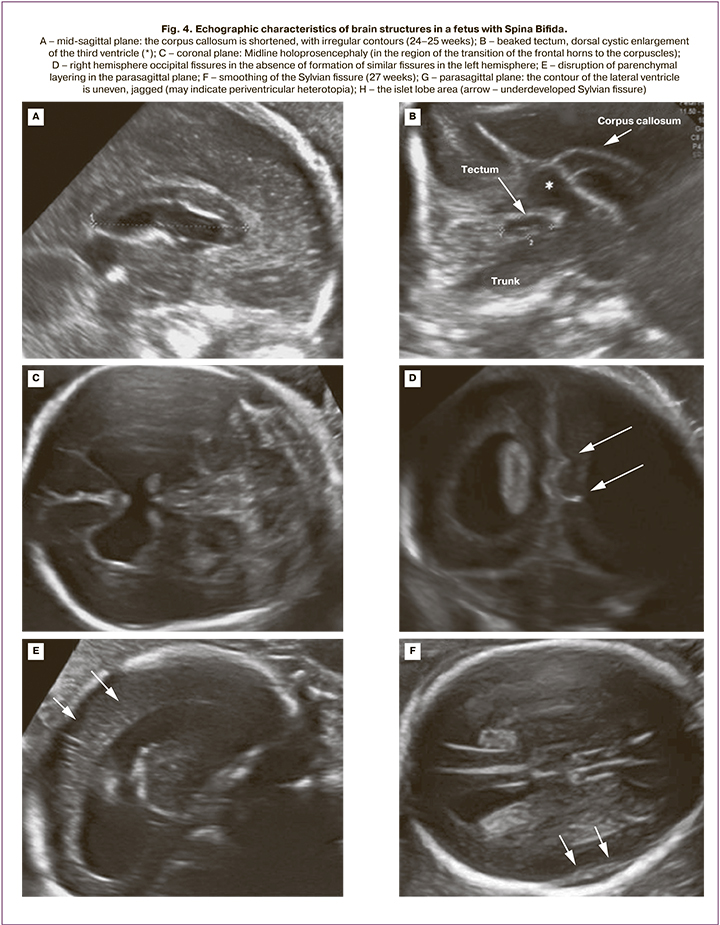
Additional echographic findings in fetuses with Chiari II included clubfoot (in 4/20 fetuses with MMC at the L2 level), uterine duplication (1/20), and pelvic dystopia of the kidney (1/20).
Discussion
The feasibility of intrauterine interventions for Spina Bifida in Arnold-Chiari malformation type II has led to the introduction of advanced fetal neurosonography in prenatal ultrasonography and to the development of new morphometric parameters. Assessment of brain structures in the sagittal and coronary planes, in addition to the axial plane, allows the diagnosis of CNS malformations already in the first trimester of pregnancy. Over the past decades, several cerebral echographic markers of Spina Bifida in the first trimester of pregnancy have been developed. For example, as early as 2011, a group of Australian scientists – Finn M. et al. reported that visualization of the posterior edge of the Sylvian aqueduct displacement to the contour of the occipital bone is a reliable sign of the occipital wedging of the posterior part of the brain into the greater occipital foramen with spinal hernia [11]. Other studies have shown that alterations in the ratio of the structures: trunk – IV ventricle – Grand Cistern, visualized in the mid-sagittal plane, namely, a decrease in the distance between the posterior contour of the trunk and the occipital bone, can also indicate the presence of neural tube malformation [17]. In our study, the detection of these markers allowed the detection of Chiari II in 5 patients in the first trimester of pregnancy.
Given the fact that the majority of Chiari II cases are diagnosed in the second trimester of pregnancy, the use of an expanded neurosonography protocol allows for a reliable and accurate determination of all morphometric features of infra- and supratentorial structures, including for the selection of fetuses for intrauterine repair of Spina Bifida. In our protocol, we have concentrated the diagnostic techniques and markers of this malformation described in the world literature that can be reproduced in each case. Thus, a number of echographic features of the brain structures were identified, including the corpus callosum anomaly, dorsal cystic enlargement of ventricle III, beaked tectum, pointed occipital horn of the lateral ventricle, interhemispheric holoprosencephaly, delayed/anomaly of fissure formation.
The role of these structural abnormalities in the cognitive development of Chiari II patients is poorly understood. These patients often have complex neuropsychological developmental abnormalities, behavioral and learning changes, which are often attributed to hydrocephalus due to impaired cerebrospinal fluid dynamics [21]. However, current magnetic resonance imaging studies demonstrate structural abnormalities that cannot be explained by hydrocephalus alone. In addition, neuropsychological studies have shown that some cognitive abnormalities are independent of hydrocephalus and the use of ventriculoperitoneal shunting, which demonstrates the dysplastic nature of the structural changes [22]. On the other hand, several studies indicate that some cranial Chiari anomaly is secondary to mechanical influences [23, 24].
According to our study, all fetuses showed specific alterations in corpus callosum manifested by dysgenesis, partial agenesis, and, in most cases, slight shortening of its length. Pointed posterior horns of the lateral ventricles with a decreased distance between their contour and occipital bone, as well as interhemispheric cysts (in 50% of cases), which are the posterior and upper continuation of the third ventricle, confirm the theory of white matter deficiency of the posterior departments compared with the anterior departments. Echographic confirmation of the embryological origin of dysplastic brain malformations in Chiari II is also supported by our finding of interhemispheric holoprosencephaly in 15% (3/20) of fetuses.
Cortical anomalies in Chiari II predominates in the posterior medial part of the hemisphere, where the white matter defect is most prominent. As early as 1986, Gilbert et al. in a pathological study of 25 deceased children with Chiari II found cortical malformations in 92% of cases: immature development in 24%, parenchymal layering disorder in 24%, heterotopia, true neuronal migration disorder in 44%, and polymicrogyria in 40% of patients [25]. The most common abnormality is described in the literature as stenogyria (which means "narrow gyri", from the Greek stenos: narrow), characterized by the presence of multiple small compacted gyri separated by shallow sulci, mostly in the underdeveloped posterior medial part of the hemispheres. The signs described above are consistent with the results of our study, in which delayed sulci formation was detected in 100% of cases. Furthermore, abnormal sulcation of the islet lobes, confirmed postnatally, was detected in 80% of fetuses, which has not been previously described in the literature.
Another characteristic feature of brain organization in Chiari II is the altered configuration of the "roof" of the brain, the tectum, which is responsible for visual and auditory reflexes and which, according to some reports, correlates with the severity of oculomotor disorders in Spina Bifida [8]. In 2014. Leibovitz Z. et al. applied normograms of the midbrain and hindbrain developed by them in fetuses with posterior cranial fossa anomalies, including Chiari II, and revealed several features, including elongation of the tectum with formation of a "beak" and reduction of the midbrain anteroposterior diameter by more than 2 standard deviations [12, 13]. In our study, a beaked tectum was detected in all fetuses with Chiari II. However, in most cases, the size of this structure did not exceed the normative values. Only in a quarter of cases, the length of the tectum was greater than 12 mm, which was beyond two standard deviations according to the normative scale developed by Leibovitz Z. et al.
Conclusion
Echographic features of fetal brain structures associated with Chiari II malformation identified in our study are important in the selection of candidates for intrauterine intervention and the interpretation of the results of postoperative fetal brain examination. The CNS abnormalities associated with Chiari II are not exclusion criteria for the intrauterine repair of Spina Bifida.
References
- Демикова Н.С., Подольная М.А., Лапина А.С. Частота и временны́е тренды дефектов нервной трубки в регионах Российской Федерации. Российский вестник перинатологии и педиатрии. 2019; 64(6): 30-8. https://dx.doi.org/10.21508/1027-4065-2019-64-6-30-38. [Demikova N.S., Podolnaya M.A., Lapina A.S. Prevalence and time trends of neural tube defects in regions of the Russian Federation. Rossiyskiy Vestnik Perinatologii i Pediatrii /Russian Bulletin of Perinatology and Pediatrics. 2019; 64(6): 30-8. (in Russian)].https://dx.doi.org/10.21508/1027-4065-2019-64-6-30-38.
- Özek M.M,, Cinalli G., Maixner W., eds. Spina bifida: management and outcome. Springer; 2008.
- Демикова Н.С., Лапина А.С., Подольная М.А., Путинцев А.Н. Значение генетических исследований в изучении природы врожденных пороков развития. Российский вестник перинатологии и педиатрии. 2020; 65(5): 7-11. [Demikova N.S., Lapina A.S., Podolnaya M.A., Putintsev A.N. The value of genetic analysis in the study of the nature of congenital malformations. Rossiyskiy Vestnik Perinatologii i Pediatrii /Russian Bulletin of Perinatology and Pediatrics. 2020; 65(5): 7-11. (in Russian)].
- Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338(8760): 131-7.
- Greene N.D., Copp A.J. Models of neural tube defects: investigating preventive mechanisms. Am. J. Med. Genet. C Semin. Med. Genet. 2005; 135 C(1): 31-41. https://dx.doi.org/10.1002/ajmg.c.30051.
- Galea G.L., Maniou E., Edwards T.J., Marshall A.R., Ampartzidis I., Greene N.D.E., Copp A.J. Cell non-autonomy amplifies disruption of neurulation by mosaic Vangl2 deletion in mice. Nat. Commun. 2021; 12(1): 1159.https://dx.doi.org/10.1038/s41467-021-21372-4.
- Callen A.L., Filly R.A. Supratentorial abnormalities in the chiari II malformation, I: the ventricular "point". J. Ultrasound Med. 2008; 27(1): 33-8.https://dx.doi.org/10.7863/jum.2008.27.1.33.
- Callen A.L., Stengel J.W., Filly R.A. Supratentorial abnormalities in the Chiari II malformation, II: tectal morphologic changes. J. Ultrasound Med. 2009; 28(1): 29-35. https://dx.doi.org/10.7863/jum.2008.27.1.33.
- Wong S.K., Barkovich J.А., Сallen A.L., Filly R.A. Supratentorial abnormalities in the chiari II malformation, III: the interhemispheric cyst. J. Ultrasound Med. 2009; 28(8): 999-1006. https://dx.doi.org/10.7863/jum.2009.28.8.999.
- Filly M.R., Filly R.A., Barkovich A.J., Goldstein R.B. Supratentorial abnormalities in the chiari II malformation, IV: the too-far-back ventricle. J. Ultrasound Med. 2010; 29(2): 243-8. https://dx.doi.org/10.7863/jum.2010.29.2.243.
- Finn M., Sutton D., Atkinson S., Ransome K., Sujenthiran P., Ditcham V. et al. The aqueduct of Sylvius: a sonographic landmark for neural tube defects in the first trimester. Ultrasound Obstet. Gynecol. 2011; 38(6): 640-5.https://dx.doi.org/10.1002/uog.10088.
- Leibovitz Z., Shkolnik C., Krajden Haratz K., Malinger G., Shapiro I., Lerman-Sagie T. Assessment of fetal midbrain and hindbrain in mid-sagittal cranial plane by three-dimensional multiplanar sonography. Part 1: comparison of new and established nomograms. Ultrasound Obstet. Gynecol. 2014; 44(5): 575-80. https://dx.doi.org/10.1002/uog.13308.
- Leibovitz Z., Shkolnik C., Krajden Haratz K., Malinger G., Shapiro I., Lerman-Sagie T. Assessment of fetal midbrain and hindbrain in mid-sagittal cranial plane by three-dimensional multiplanar sonography. Part 2: application of nomograms to fetuses with posterior fossa malformations. Ultrasound Obstet. Gynecol. 2014; 44(5): 581-7. https://dx.doi.org/10.1002/uog.13312.
- Pugash D., Hendson G., Dunham C.P., Dewar K., Money D.M., Prayer D. Sonographic assessment of normal and abnormal patterns of fetal cerebral lamination. Ultrasound Obstet. Gynecol. 2012; 40(6): 642-51.https://dx.doi.org/10.1002/uog.11164.
- Чугунова Л.А., Нароган М.В., Рюмина И.И., Киртбая А.Р., Гус А.И. Возможности 3D-нейросонографии в оценке постнатального формирования коры головного мозга у глубоко недоношенных детей. Акушерство и гинекология. 2017; 7: 120-8. https://dx.doi.org/10.18565/aig.2017.7.120-8. [Chugunova L.A., Narogan M.V., Ryumina I.I., Kirtbaya A.R., Gus A.I. The capabilities of 3D neurosonography in assessing the postnatal formation of the cerebral cortex in very preterm infants. Obstetrics and Gynecology. 2017; 7: 120-8. (in Russian)].
- Paladini D., Malinger G., Birnbaum R., Monteagudo A., Pilu G., Salomon L.J., Timor-Tritsch I.E. ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 2: performance of targeted neurosonography. Ultrasound Obstet. Gynecol. 2021; 57(4): 661-71.https://dx.doi.org/10.1002/uog.23616.
- Timor-Tritsch I., Monteagudo A., Pilu G., Gustavo M. Ultrasonography of the prenatal brain. 3rd ed. 2012. 512p.
- Kozlowski P., Burkhardt T., Gembruch U., Gonser M., Kähler C., Kagan K.O. et al. DEGUM, ÖGUM, SGUM and FMF Germany recommendations for the implementation of first-trimester screening, detailed ultrasound, cell-free DNA screening and diagnostic procedures. Ultraschall Med. 2019; 40(2): 176-93. https://dx.doi.org/10.1055/a-0631-8898.
- Tulipan N., Wellons J.C. 3rd, Thom E.A., Gupta N., Sutton L.N., Burrows P.K.; MOMS Investigators. Prenatal surgery for myelomeningocele and the need for cerebrospinal fluid shunt placement. J. Neurosurg. Pediatr. 2015; 16(6): 613-20. https://dx.doi.org/10.3171/2015.7.PEDS15336.
- Houtrow A.J., Burrows P.K., Thom E.A. Comparing neurodevelopmental outcomes at 30 months by presence of hydrocephalus and shunt status among children enrolled in the MOMS trial. J. Pediatr. Rehabil. Med. 2018; 11(4): 227-35. https://dx.doi.org/10.3233/PRM-170481.
- Miller E., Widjaja E., Blaser S., Dennis M., Raybaud C. The old and the new: supratentorial MR findings in Chiari II malformation. Child's Nerv. Syst. 2008; 24(5): 563-75. https://dx.doi.org/10.1007/s00381-007-0528-x.
- Dennis M., Jewell D., Edelstein K., Brandt M.E., Hetherington R., Blaser S.E., Fletcher J.M. Motor learning in children with spina bifida: intact learning and performance on a ballistic task. J. Int. Neuropsychol. Soc. 2006; 12(5): 598-608. https://dx.doi.org/10.1017/S1355617706060772.
- Tulipan N., Bruner J.P., Hernanz-Schulman M., Lowe L.H., Walsh W.F., Nickolaus D., Oakes W.J. Effect of intrauterine myelomeningocele repair on central nervous system structure and function. Pediatr. Neurosurg. 1999; 31(4): 183-8. https://dx.doi.org/10.1159/000028859.
- Tulipan N., Hernanz-Schulman M., Lowe L.H., Bruner J.P. Intrauterine myelomeningocele repair reverses preexisting hindbrain herniation. Pediatr. Neurosurg. 1999; 31(3): 137-42. https://dx.doi.org/10.1159/000028849.
- Gilbert J.N., Jones K.L., Rorke L.B., Chernoff G.F., James H.E. Central nervous system anomalies associated with meningomyelocele, hydrocephalus, and the Arnold-Chiari malformation: reappraisal of theories regarding the pathogenesis of posterior neural tube closure defects. Neurosurgery. 1986; 18(5): 559-64. https://dx.doi.org/10.1227/00006123-198605000-00008.
Received 26.07.2022
Accepted 07.09.2022
About the Authors
Liliyana A. Chugunova, PhD, Senior Researcher, Department of Ultrasound and Functional Diagnosis, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,l_chugunova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Roman G. Shmakov, Dr. Med. Sci, Professor of the Russian Academy of Sciences, Director of the Institute of Obstetrics, V.I. Kulakov NMRC for OG&P, Ministry of Health
of Russia, r_shmakov@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Kristina A. Gladkova, PhD, Head of the 1st Obstetric Department of Pregnancy Pathology, Senior Researcher at the Fetal Medicine Unit, Institute of Obstetrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, k_gladkova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Kirill V. Kostyukov, Dr. Med. Sci., Head of the Department of the Ultrasound and Functional Diagnosis, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
k_kostyukov@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Authors’ contributions. Chugunova L.A. – conception and design of the study; Chugunova L.A., Shmakov R.G., Gladkova K.A., Kostyukov K.V. – material collection and processing; Chugunova L.A., Shmakov R.G., Kostyukov K.V. – manuscript drafting.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Chugunova L.A., Shmakov R.G., Gladkova K.A., Kostyukov K.V. Ultrasound characteristics of fetal brain structures in Arnold–Chiari malformation type II.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 11: 99-108 (in Russian)
https://dx.doi.org/10.18565/aig.2022.11.99-108



