Possibilities of correction of the endometrial receptor profile in chronic endometritis
Tolibova G.Kh., Tral T.G., Kakhiani M.I.
Background: A broad spectrum of endometrial pathology is linked to chronic endometritis, including infertility, pregnancy failure, and placenta-associated pathology. These conditions result in impaired morphogenesis across the entire spectrum of endometrial transformation. There is a lot of evidence about the treatment of chronic endometritis, but it is not always possible to restore its structural and functional characteristics. In recent years, the effect of a complex of exogenous natural antimicrobial peptides and cytokines (Superlymph) in the treatment of chronic endometritis has been the subject of intensive study.
Objective: To evaluate the endometrial receptor profile (estrogen receptors (ER) and progesterone receptors (PR)) in patients with chronic endometritis treated with exogenous natural antimicrobial peptides and cytokines (Superlymph).
Materials and methods: The study included patients aged 25–38 years with uterine factor infertility and severe chronic endometritis. All patients received antibacterial therapy in combination with exogenous natural antimicrobial peptides and cytokines (Superlymph). Histological and immunohistochemical studies of receptor profile and severity of chronic endometritis were performed.
Results: A statistically significant decrease in morphological manifestations and severity of chronic endometritis was noted in patients who underwent antibacterial therapy in combination with Superlymph. A statistically significant increase in ER and PR expression in the stromal component of the endometrium was verified (p<0.001). ER expression was shown to be 1.53 [1.33;1.66] times higher in the stromal component and PR expression was shown to be 1.25 [1.15 1.53] times higher (p<0.001). There was an inverse correlation between the expression of ER and PR in the stroma before treatment and the extent of their increase after treatment, rs=-0.91 and rs=-0.92 respectively (p<0.001).
Conclusion: In severe chronic endometritis, adequate timely antibacterial therapy in combination with exogenous natural antimicrobial peptides and cytokines (Superlymph) leads to a decrease in the severity of pathology and restoration of the endometrial receptor profile.
Authors’ contributions: Tolibova G.Kh., Tral T.G. – developing the concept and design of the study, writing the text; Kakhiani M.I., Tral T.G. – collecting and processing the material, statistical processing; Tolibova G.Kh. – editing the text.
Conflicts of interest: Authors declare lack of the possible conflicts of interest.
Funding: The study was conducted without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of the D.O. Ott Research Institute of Obstetrics, Gynecology and Reproduction.
Patient Consent for Publication: The patients provided an informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Tolibova G.Kh., Tral T.G., Kakhiani M.I. Possibilities of
correction of the endometrial receptor profile in chronic endometritis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (10): 121-128 (in Russian)
https://dx.doi.org/10.18565/aig.2024.244
Keywords
A wide range of endometrial pathologies is associated with chronic endometritis in terms of reproductive function (infertility, pregnancy failure, placenta-associated pathology) [1–4].
Despite the proven importance of chronic endometritis in the genesis of reproductive failures, there are still debates regarding endometritis itself, its influence on the links of morphogenesis (receptor, vascular, immunological) and the consequences of long-term inflammatory process in the uterine cavity.
This may be related to the historical refusal to consider chronic endometritis as a nosology, due to the monthly desquamation of the endometrium and the presumed sterility of the uterine cavity. Moreover, many Russian scientists use only data from foreign studies, which do not always accurately and fully reflect diagnostic approaches and they do not rely on the achievements of numerous Russian studies on the problem of verification and methods of treatment of chronic endometritis.
It should be noted that there are disagreements not only about the relative influence of chronic endometritis on the possibility of achieving conception, but also about the treatment of this pathology, despite the large number of studies with a high level of evidence [5–7]. This is likely to contribute to late treatment of the disease and the formation of secondary organic damage in the endometrium, resulting in persistent impairment of its implantation characteristics [8, 9].
The main problem in case of chronic endometritis is not only the presence of the pathological agent in the endometrium, but also the duration of the disease course, the degree of imbalance of the local immune system, dysregulation of the vascular channel and receptor profile of the endometrium [10–13].
The receptor profile of the endometrium, namely estrogen receptors (ER) and progesterone receptors (PR), is fully verified during the ovulatory menstrual cycle and in infertility of different genesis. It is characterized by the dynamic and constant expression patterns: dynamic pattern is characteristic of ER and PR in the glandular component, and constant pattern is characteristic of PR expression in the stroma during all phases of the menstrual cycle. Chronic endometritis impairs all links of morphogenesis in cyclic transformation of the endometrium and primarily ER and PR expression; it alters the cellular response to steroids, which contributes to endometrial dysfunctional states and affects the success of implantation, in assisted reproductive technology (ART) programs as well. Significant changes in ER and PR expression in both stromal and glandular components are verified in patients with chronic endometritis compared to healthy women [8, 14–16].
Despite the widespread use of hormonal preparations (in the preconception period, ART protocols, pregnancy maintaining and gynecological diseases) that directly affect ER and PR, there is not enough attention paid to the importance of the endometrial receptor profile in reproductive pathology associated with uterine factor in clinical practice.
The sex steroid hormones play a key role in endometrial morphogenesis, indirectly or directly affecting all links of endometrial transformation. Cyclic endometrial transformation is based on the full function and balance of receptor interaction and is coordinated by 17β-estradiol (E2) and progesterone (P4). E2 is a predictor of endometrial growth, optimal proliferative activity of epithelial and stromal cells during the follicular phase and P4 is responsible for the formation of full secretory transformation [17–19].
Estradiol (E2) interacts with estrogen and IL-1β receptors via NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and increases the expression of a set of genes involved in subsequent implantation. In addition to direct genomic pathways, estrogen can also exert its regulatory effects through non-genomic pathways by activating the GPER1 receptor located on the cell membrane [20–22].
Progesterone modifies the expression of many genes involved in endometrial transformation by genomic mechanisms. Progesterone, like estrogen, interacts with tyrosine kinases through non-genomic pathways and regulates expression through the membrane receptors mPRα, mPRβ and mPRγ [23].
Currently, the data have been accumulated on the etiotropic therapy of chronic endometritis. Furthermore, standard therapies for unidentified etiological factors are widely used. However, it is not possible to confirm the high efficacy of therapeutic measures for chronic endometritis and restoration of morphological and functional characteristics of the endometrium without verification of the degree of severity of the inflammatory process, assessment of the receptor profile, vascular component before and after therapy.
The aim of the study was to evaluate the endometrial receptor (ER) profile in patients with chronic endometritis treated with exogenous natural antimicrobial peptides and cytokines (Superlymph).
Materials and methods
This was the study of endometrial receptor profile in 130 patients with uterine factor infertility and severe chronic endometritis. Histological and immunohistochemical examinations of receptor profile and severity of chronic endometritis were performed. The study was conducted in the Pathomorphology Department of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproduction, St. Petersburg, Russia from 2021 to 2024. Informed consent of the patients was obtained.
There were the following inclusion criteria: age 25–38 years; uterine factor of infertility; normal ovarian and pituitary hormone levels; normoponic menstrual cycle (28 days); middle stage of secretion phase; histologically and immunohistochemically verified severe chronic endometritis.
There were the following exclusion criteria: proliferative phase endometrium; endometrial hyperplasia; genital endometriosis regardless of the degree; uterine myoma regardless of the form; pregnancy during the study; SARS-CoV-2 3 months before biopsy; taking any hormonal medications 2-3 months before inclusion in the study.
Methods of treatment. All patients received antibacterial therapy (doxycycline monohydrate 100 mg twice a day for 10 days) in combination with immunomodulatory therapy containing a complex of exogenous natural antimicrobial peptides and cytokines (Superlymph, Altpharm LLC, Russia). Suppositories of 25 units were administered during three menstrual cycles from day 5 to day 25 once a day vaginally in the evening. Two months after therapy, a repeat pipelle biopsy of the endometrium was performed on the 19th–22nd day of the cycle.
The study was approved by the Ethical Review Board of the D.O. Ott Research Institute of Obstetrics, Gynecology and Reproduction.
The histological examination of endometrial biopsy specimens was performed according to the standard technique with material fixation in 10% neutral formalin (pH 7.2) and histological wiring in a Histo-Tek VP1 histoprocessor (Sakura, Japan); the material was embedded in paraffin and 3–4 μm thick sections were made from the obtained blocks with subsequent staining with hematoxylin and eosin (BioVitrum, Russia). Light microscopy assessed the compliance of endometrial structure with the day of menstrual cycle, the condition of glands, stroma and vascular component of the endometrium, the presence of histological signs of inflammatory and pathological changes. The study was performed using an Olympus CX31 microscope (Japan) with magnification 100×, 200×, 400×.
Immunohistochemical study was performed on paraffin sections using standard technique with a one-step protocol for antigen demasking. The immunohistochemical method of the study included quantitative and qualitative assessment of estrogen and progesterone (ERα and PR) expression in endometrial biopsy specimens using antibodies to ERα receptor (clone 1D5) and PR receptor (clone PR 636) in a standard dilution of 1:50 produced by Dako Cytomation (Denmark). The diagnosis of chronic endometritis was made using antibodies, namely CD8+, CD20+, CD4+, and CD138+, in a standard dilution manufactured by Dako Cytomation (Denmark). Chronic endometritis was verified by a complex method based on histological findings (infiltration of endometrium with lymphocytes, monocytes, plasmocytes, stromal fibrosis, sclerosis of vascular bed) and immunohistochemical method via the calculation of the number of immunopositive cells with magnification ×400 per high power field of the endometrium. The degree of chronic endometritis severity was determined according to the classification of G. Tolibova [3]. Estrogen and progesterone receptor expression was assessed on the basis of Histochemical Score (H-Score) = ΣP(i) × I, where I is the intensity of staining expressed as a score from 0 to 3; P(i) is the percentage of cells stained with different intensity. The degree of staining is classified as follows: 0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining. The maximum count value is equivalent to 300 units. The receptor expression distribution in the material was considered, taking into account whether it was uniform or non-uniform. The expression of sex hormone receptors (estrogen and progesterone) below 70 points was considered as a decrease [3].
Statistical analysis
Statistical analysis of the study results was performed using STATISTICA 10.0 software programmes (StatSoft Inc.). Qualitative variables are presented as absolute (number) and relative (frequency in %). The data were tested for normality of distribution using the Shapiro–Wilk test. The McNemar test was used to compare dependent variables that were measured on a nominal scale; treatment effectiveness was assessed by calculating the odds ratio (OR) with 95% confidence interval (95% CI). The comparison of quantitative continuous signs was performed using the non-parametric Wilcoxon test for dependent samples and the Mann–Whitney U test for independent samples. The results are presented as medians (Me) and interquartile range [Q1; Q3]. The correlation analysis was performed using Spearman’s rank correlation coefficient (rs). The critical level of reliability of the null statistical hypothesis (the absence of significant differences or factor influences) was considered to be 0.05.
Results and Discussion
The subjects were aged between 25 and 38 years. Pregnancy history was reported by 101/130 (77.7%) patients, surgical termination of pregnancy was reported by 60/130 (46.2%) women, missed miscarriage and spontaneous abortion in the first trimester was reported by 41/130 (31.5%) patients. The history of benign cervical diseases was noted in 91/130 (70.0%) women, urogenital infection was reported in 61/130 (46.9%) women; 29/130 (22.3%) women were carriers of high-risk papillomavirus and 8/130 (6.2%) women were carriers of genital herpes.
The results of the histological study, as presented in Table, demonstrated a statistically significant reduction in the morphological signs of chronic endometritis in patients with uterine factor of infertility and severe chronic endometritis after therapy. This was observed in follicle-like infiltrates (p<0.001), perivascular fibrosis (p<0.001), fibroplastic changes of the stromal component (p<0.001), and sclerosis of the vascular wall of spiral arteries (p<0.001).
The obtained results proving that signs of chronic endometritis were controlled after therapy with Superlymph were consistent with the findings of other scientists [24–26].
Immunohistochemical findings after treatment of chronic endometritis also demonstrate a significant decrease in the severity of the disease. Mild (88/130 (67.7%)) and moderate chronic endometritis (36/130 (27.7%)) prevailed in the patients, while severe chronic endometritis after therapy was verified in only 6/130 (4.6%) women.
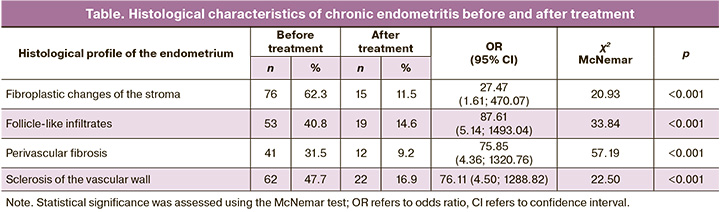
In endometrial biopsy specimens obtained prior to the treatment of chronic endometritis, an uneven distribution and multifocal upregulation of receptor expression were confirmed when ER expression was evaluated in the glandular component of the mid-stage endometrium during the secretion phase of the suspected implantation window. After therapy, there was a statistically significant decrease in ER expression in the glandular component (p<0.001). A statistically significant increase in ER was verified in the stromal component (p<0.001). The data are presented in Figure 1.
The analysis of PR expression after treatment verified a statistically significant decrease (p<0.001) in the glandular component and a statistically significant increase (p<0.001) in the endometrial stromal component (Figure 2).
It should be noted that there was a 2.7-fold increase in the expression of progesterone receptors in the endometrial stroma after similar therapy demonstrated in the study by Dobrokhotova Yu.E. et al [26].
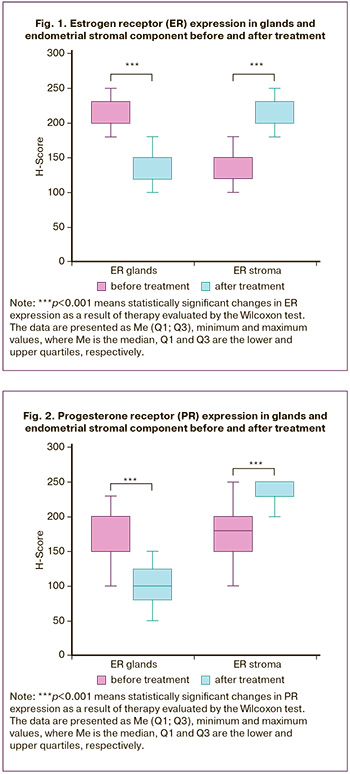
Figures 3 and 4 show the morphological profile of the endometrium before and after treatment of chronic endometritis.
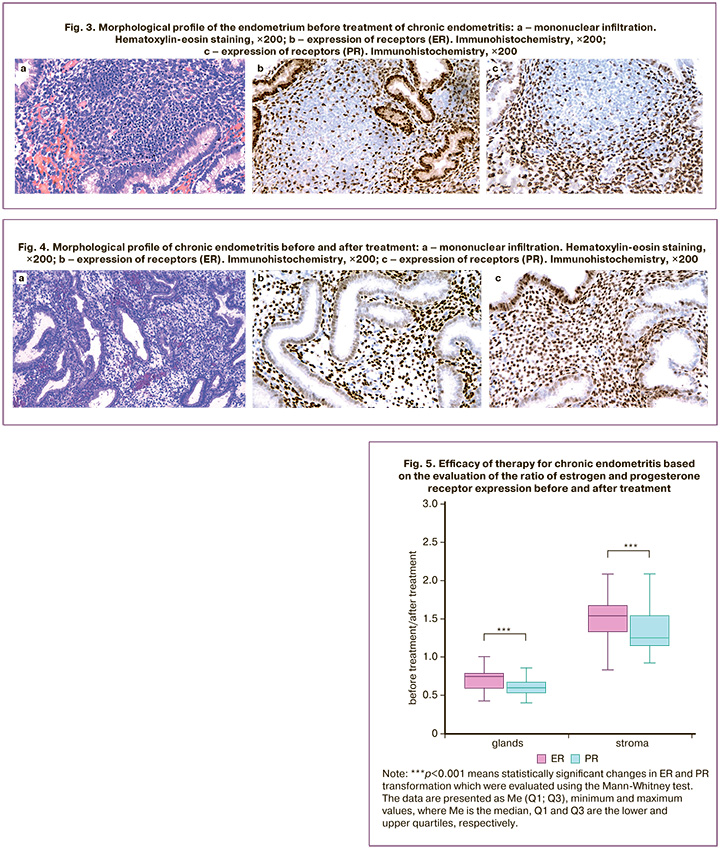
The obtained results showed that restoration of the endometrial receptor profile with an even distribution of ER and PR in the endometrial stromal component was diagnosed after treatment of chronic endometritis. The relative efficacy of therapy for chronic endometritis was evaluated based on the ratio of ER and PR expression (Figure 5).
The results demonstrated that after antibiotic therapy in combination with Superlymph®, there was an increase in ER expression in the stroma by 1.53 [1.33;1.66] times, which was higher compared to the increase in PR, whose expression in the stroma after treatment increased by 1.25 [1.15;1.53] times (p<0.001).
Moreover, after therapy there is a greater decrease in PR expression in the glands by 1.67 [1.50;1.88] times, compared to a decrease in ER by 1.33 [1.28;1.67] times (p<0.001). In addition, the relationship between ER and PR expression in the glandular and stromal component before treatment and the extent of their change after treatment (ratio of post-treatment expression to pre-treatment expression) was demonstrated. There was an inverse correlation between the expression of ER and PR in the stroma before treatment and the extent of its increase after treatment, rs=-0.91 and rs=-0.92, respectively (p<0.001).
Therefore, there was a more significant increase in ER and PR expression after treatment with a more dramatic decrease in their expression in the stroma before treatment. Higher ER and PR expression values in endometrial glands before treatment are more effectively reduced after treatment, rs=-0.50 and rs=-0.71, respectively (p<0.001).
ER expression normally reaches a maximum in the early proliferative phase with a slight decrease in the late proliferative and early secretory phases, both in the glands and in the stroma of the functional layer of the endometrium. Then, ER expression decreases significantly in the late secretory phase more in glandular cells than in stromal cells. PR expression increases progressively during the proliferative phase and early secretion phase in endometrial glands, reaching zero values in the middle and late stages of the secretion phase. This pattern is consistent with the dynamics of sex hormone secretion and endometrial response to their effects [14, 15, 27, 28].
A number of foreign studies confirmed increased expression of ER and PR in glands accompanied by decreased expression of ER and PR in the stromal component of the endometrium on day 19–22 of the cycle in patients with chronic endometritis [15, 16]. Impaired ratio of ER and PR expression in endometrial glands and stroma leads to imbalance of endometrial secretory transformation and poor preparation for blastocyst implantation. PR expression dependent on ER expression is accordingly suboptimal for implantation of the fertilized oocyte. Therefore, increased ER expression with concomitant decreased PR expression in endometrial cells during the early secretory phase may impair the ability of the endometrium to implant a fertilized egg and lead to infertility. In chronic endometritis, the shift of the maximum peak of ER and PR expression in the stroma towards the proliferative phase is characterized by an earlier depletion of the receptor profile with a significant decrease in ER in the early secretory phase.
The decrease in ER and PR expression in glandular cells and the increase in expression in the stroma after treatment with Superlymph® compared to the initial data suggests that the receptor profile of the endometrium could be restored in accordance with the cellular processes occurring in the secretory phase of the cycle, from an initially ‘uneven’ profile to a ‘uniform’ profile after treatment. Moreover, the positive changes in ER and PR receptor expression correlated with the degree of chronic endometritis severity; the mildest form of severity demonstrated the most significant benefit. Given that there were 124/130 (95.4%) cases of mildly and moderately severe chronic endometritis after treatment in the study, it can be stated that restoration of the endometrial receptor profile due to the use of a complex of antimicrobial peptides and cytokines increases the chances of successful implantation and reproductive outcomes.
Pregnancy is a reliable indicator of the effectiveness of the therapy, and the criteria of live birth indicate the restoration of not only morphological and functional characteristics of the endometrium, but also all links of morphogenesis of conception transformation, including the receptor profile of the endometrium.
Previous clinical studies demonstrated that the use of the Superlymph in combination with standard therapy leads to a 3-fold increase in the pregnancy rate in patients with infertility for more than 5 years. The rate of viable fetal birth increased by an average of 20%, and pregnancy loss in the first trimester decreased by 6 times. In addition, combination therapy has been shown to reduce the incidence of preterm labor by 2.5 times and obstetric complications of pregnancy (pre-eclampsia, antenatal hypoxia and fetal growth restriction) twice [29]. The absence of plasma cells in endometrial biopsy specimens was detected in 79.3% of the patients. There was a 1.5-fold increase in the pregnancy rate in ART programs, with a live birth rate of 45.3%. Particularly significant are the data on the increase in the pregnancy rate among patients with a duration of infertility of 5 years or more (1.4-fold) and the rate of live births (2.7-fold) [25]. Considering that in a prospective, randomised, placebo-controlled, blinded trial of comprehensive treatment of chronic endometritis in infertile patients with Superlymph, there was a smaller reduction in the proportion of severe chronic endometritis in the group of patients who received only antibiotics [24], it is likely that abnormalities in the receptive profile persisted more frequently than in the main group, which could also lead to poorer infertility outcomes.
Conclusion
The results of the conducted study provide evidence to support the efficacy of exogenous natural antimicrobial peptides and cytokines in the complex treatment of chronic endometritis. The use of these peptides and cytokines has been shown to have a significant positive effect on the immunological component of the morphogenesis of endometrial transformation, resulting in a reduction in the number of proinflammatory cells and the prevention of the cascade of complex pathogenetic pathways of organic damage to the endometrium. It is the reduction of chronic endometritis severity that leads to the restoration of the receptor profile of the endometrium in patients with uterine reproductive pathology.
References
- Ищенко А.И., Унанян А.Л., Коган Е.А., Демура Т.А., Коссович Ю.М. Клинико анамнестические, иммунологические, эхографические и гистероскопические особенности хронического эндометрита, ассоциированного с нарушением репродуктивной функции. Вестник РАМН. 2018; 73(1): 5 15. [Ishchenko A.I., Unanyan A.L., Kogan E.A., Demura T.A., Kossovich Yu.M. Clinical anamnestic, immunological, echographic and hysteroscopic features of chronic endometritis associated with reproductive dysfunction. Bulletin of the Russian Academy of Medical Sciences. 2018; 73(1): 5-15. (in Russian)]. https://dx.doi.org/10.15690/vramn927.
- Мальцева Л.И., Шарипова Р.И. Хронический эндометрит – новое время, новые подходы к лечению. Практическая медицина. 2019; 17(4): 15-9. [Maltseva L.I., Sharipova R.I. Chronic endometritis – a new time, new approaches to treatment. Practical Medicine. 2019; 17(4): 15-9. (in Russian)]. https://dx.doi.org/10.32000/2072-1757-2019-4-15-19.
- Толибова Г.Х., Траль Т.Г., Клещев М.А. Эндометриальная дисфункция: алгоритм клинико-морфологического исследования. Санкт-Петербург: ВВМ; 2016. 44 с. [Tolibova G.Kh, Tral T.G., Kleschev M.A. Endometrial dysfunction: clinical and morphological study algorithm. St. Petersburg; 2016. 44 p. (in Russian)].
- Puente E., Alonso L., Laganà A.S., Ghezzi F., Casarin J., Carugno J. Chronic endometritis: old problem, novel insights and future challenges. Int. J. Fertil. Steril. 2020; 13(4): 250-6. https://dx.doi.org/10.22074/ijfs.2020.5779.
- Vitagliano A., Laganà A.S., De Ziegler D., Cicinelli R, Santarsiero C.M., Buzzaccarini G. et al. Chronic endometritis in infertile women: impact of untreated disease, plasma cell count and antibiotic therapy on IVF outcome - a systematic review and meta-analysis. Diagnostics (Basel). 2022; (12)9: 2250. https://dx.doi.org/10.3390/diagnostics12092250.
- Margulies S.L., Flores V., Parkash V., Pal L. Chronic endometritis: A prevalent yet poorly understood entity. Int. J. Gynaecol. Obstet. 2022; 158(1): 194-200. https://dx.doi.org/10.1002/ijgo.13962.
- Klimaszyk K., Svarre Nielsen H., Wender-Ozegowska E., Kedzia M. Chronic endometritis - is it time to clarify diagnostic criteria? Ginekol. Pol. 2023; 94(2): 152-7. https://dx.doi.org/10.5603/GP.a2022.0147.
- Толибова Г.Х., Траль Т.Г. Коган И.Ю., Олина А.А. Эндометрий. Атлас. М.: StatusPraesens; 2022. 184 с. [Tolibova G.Kh., Tral T.G. Kogan I.Yu., Olina A.A. Endometrium. Atlas. Moscow: StatusPraesens; 2022. 184 p. (in Russian)].
- Břečka K., Sehnal B., Maxová K., Halaška M.J., Keprtová K., Hruda M. et al. Chronic endometritis - a constantly discussed issue in infertile women. Ceska Gynekol. 2024; 89(3): 230-6. (in English). https://dx.doi.org/10.48095/cccg2024230.
- Толибова Г.Х., Траль Т.Г. Хронический эндометрит – затянувшаяся дискуссия. Уральский медицинский журнал. 2023; 22(2): 142-52.
- [Tolibova G.Kh., Tral T.G. Chronic endometritis – a protracted discussion. Ural Medical Journal. 2023; 22(2): 142-52. (in Russian)]. https://dx.doi.org/10.52420/2071-5943-2023-22-2-142-152.
- Kimura F., Takebayashi A., Ishida M., Nakamura A., Kitazawa J., Morimune A. et al. Review: Chronic endometritis and its effect on reproduction. J. Obstet. Gynaecol. Res. 2019; 45(5): 951-60. https://dx.doi.org/10.1111/jog.13937.
- Li Y., Yu S., Huang C., Lian R., Chen C., Liu S. et al. Evaluation of peripheral and uterine immune status of chronic endometritis in patients with recurrent reproductive failure. Fertil. Steril. 2020; 113(1): 187-96. https://dx.doi.org/10.1016/j. fertnstert.2019.09.001.
- Kaltsas A., Zikopoulos A., Moustakli E., Zachariou A., Tsirka G., Tsiampali C. et al. The silent threat to women's fertility: uncovering the devastating effects of oxidative stress. Antioxidants (Basel). 2023; 12(8): 1490. https://dx.doi.org/ 10.3390/antiox12081490.
- Айламазян Э.К., Толибова Г.Х., Траль Т.Г., Петросян М.А., Клейменова Т.С., Коган И.Ю. и др. Конфокальная лазерная сканирующая микроскопия. Верификация экспрессии рецепторов эстрогена и прогестерона в течение менструального цикла. Молекулярная медицина. 2017; 3: 27-31. [Aylamazyan E.K., Tolibova G.Kh., Tral T.G., Petrosyan M.A., Kleimenova T.S., Kogan I.Yu. et al. Confocal laser scanning microscopy. Verification of the expression of estrogen and progesterone receptors during the menstrual cycle. Molecular Medicine. 2017; (3): 27-31. (in Russian)].
- Mishra K., Wadhwa N., Guleria K., Agarwal S. ER, PR and Ki-67 expression status in granulomatous and chronic non-specific endometritis. J. Obstet. Gynaecol. Res. 2008; 34(3): 371-8. https://dx.doi.org/10.1111/j.1447-0756.2007.00700.x.
- Wu D., Kimura F., Zheng L., Ishida M., Niwa Y., Hirata K. et al. Chronic endometritis modifies decidualization in human endometrial stromal cells. Reprod. Biol. Endocrinol. 2017; 15(1): 16. https://dx.doi.org/10.1186/s12958-017-0233-x.
- Довжикова И.В., Андриевская И.А. Рецепторы эстрогенов (обзор литературы). Ч. 2. Бюллетень физиологии и патологии дыхания. 2019; 73: 125-33. [Dovzhikova I.V., Andrievskaya I.A. Estrogen receptors (literature review). Part 2. Bulletin of physiology and pathology of respiration. 2019; (73): 125-33. (in Russian)]. https://dx.doi.org/10.36604/1998-5029-2019-73-125-133.
- Bhurke A.S., Bagchi I.C., Bagchi M.K. Progesterone-regulated endometrial factors controlling implantation. Am. J. Reprod. Immunol. 2016; 75(3): 237-45. https://dx.doi.org/ 10.1111/aji.12473.
- Wang W., Vilella F., Alama P., Moreno I., Mignardi M., Isakova A. et al. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med. 2020; 26(10): 1644-53. https://dx.doi.org/10.1038/s41591-020-1040-z.
- Stefkovich M.L., Arao Y., Hamilton K.J., Korach K.S. Experimental models for evaluating non-genomic estrogen signaling. Steroids. 2018; 133: 34-7. https://dx.doi.org/ 10.1016/j.steroids.2017.11.001.
- Blanco-Breindel M.F., Singh M., Kahn J. Endometrial receptivity. 2023 Jun 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–.
- Dias Da Silva I., Wuidar V., Zielonka M., Pequeux C. Unraveling the dynamics of estrogen and progesterone signaling in the endometrium: an overview. Cells. 2024; 13(15): 1236. https://dx.doi.org/ 10.3390/cells13151236.
- Garg D., Ng S.S.M., Baig K.M., Driggers P., Segars J. Progesterone-mediated non-classical signaling. Trends Endocrinol. Metab. 2017; 28(9): 656-68. https://dx.doi.org/ 10.1016/j.tem.2017.05.006.
- Тапильская Н.И., Толибова Г.Х., Савичева А.М., Копылова А.А., Глушаков Р.И., Будиловская О.В., Крысанова А.А., Горский А.Г., Гзгзян А.М., Коган И.Ю. Эффективность локальной цитокинотерапии хронического эндометрита пациенток с бесплодием. Акушерство и гинекология. 2022; 2: 91-100. [Tapilskaya N.I., Tolibova G.Kh., Savicheva A.M., Kopylova A.A., Glushakov R.I., Budilovskaya O.V., Krysanova A.A., Gorskii A.G., Gzgzyan A.M., Kogan I.Yu. The effectiveness of local cytokine therapy for chronic endometritis in patients with infertility. Obstetrics and Gynecology. 2022; (2): 91-100. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.2.91-100.
- Суханов А.А., Дикке Г.Б., Остроменский В.В., Кукарская И.И., Шилова Н.В. Течение и исходы беременности, наступившей в результате экстракорпорального оплодотворения, у пациенток с хроническим эндометритом, получавших комплексное лечение с использованием препарата «Суперлимф» на прегравидарном этапе (рандомизированное контролируемое испытание «ТЮЛЬПАН 2»). Акушерство и гинекология. 2023; 8: 123-34. [Sukhanov A.A., Dikke G.B., Ostromensky V.V., Kukarskaya I.I., Shilova N.V. Course and outcomes of pregnancy following IVF in patients with chronic endometritis receiving complex treatment with the Superlymph medication at the preconception stage (TULIP 2 randomized controlled trial). Obstetrics and Gynecology. 2023; (8): 123-34. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.190.
- Доброхотова Ю.Э., Ганковская Л.В., Боровкова Е.И., Нугуманова О.Р. Экзогенная цитокинотерапия в лечении пациенток с хроническим эндометритом. Акушерство и гинекология. 2021; 2: 119 26. [Dobrokhotova Yu.E., Gankovskaya L.V., Borovkova E.I., Nugumanova O.R. Exogenous cytokine therapy in the treatment of patients with chronic endometritis. Obstetrics and Gynecology. 2021; (2): 119-26. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.2.119-126.
- Mertens H.J., Heineman M.J., Theunissen P.H., de Jong F.H., Evers J.L. Androgen, estrogen and progesterone receptor expression in the human uterus during the menstrual cycle. Eur. J. Obstet. Gynecol. 2001; 98(1): 58-65. https://dx.doi.org/10.1016/s0301-2115(00)00554-6.
- Fung H.Y., Wong Y.L., Wong F.W., Rogers M.S. Study of oestrogen and progesterone receptors in normal human endometrium during the menstrual cycle by immunocytochemical analysis. Gynecol. Obstet. Invest. 1994; 38(3): 186-90. https://dx.doi.org/10.1159/000292476.
- Дикке Г.Б., Суханов А.А., Остроменский В.В., Кукарская И.И. Течение и исходы беременности у пациенток с хроническим эндометритом и нарушением репродуктивной функции, получавших комплексное лечение с использованием препарата «Суперлимф» (рандомизированное контролируемое испытание в параллельных группах «ТЮЛЬПАН»). Акушерство и гинекология. 2023; 4: 132 44. [Dikke G.B., Sukhanov A.A., Ostromensky V.V., Kukarskaya I.I. Course and outcomes of pregnancy in patients with chronic endometritis and impaired reproductive function after receiving complex treatment with drug Superlymph: randomized control trial in parallel groups “TULIP”. Obstetrics and Gynecology. 2023; (4): 132-44. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.74.
Received 07.10.2024
Accepted 21.10.2024
About the Authors
Gulrukhsor Kh. Tolibova, Dr. Med. Sci., Head of the Pathomorphology Department, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,3 Mendeleevskaya line, St. Petersburg, Russia, 199034; Professor, S.N. Davydov Department of Obstetrics and Gynecology, I.I. Mechnikov North-Western State Medical University, Ministry of Health of Russia, +7(981)777-85-20, gulyatolibova@yandex.ru, https://orcid.org/0000-0002-6216-6220
Tatyana G. Tral, Dr. Med. Sci., Head of the Immunohistochemistry Laboratory of the Pathology Department, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 3 Mendeleevskaya line, St. Petersburg, Russia, 199034; Professor, Department of Pathological Anatomy with course of Forensic Medicine, St. Petersburg State Pediatric Medical University, Ministry of Health of Russia, +7(911)760-15-09, ttg.tral@yandex.ru, https://orcid.org/0000-0001-8948-4811
Maka I. Kakhiani, PhD, obstetrician-gynecologist, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 3 Mendeleevskaya line, St. Petersburg, Russia, 199034; Associate Professor, S.N. Davydov Department of Obstetrics and Gynecology, I.I. Mechnikov North-Western State Medical University, Ministry of Health of Russia, +7(911)937-42-29, kakhiani74@mail.ru, https://orcid.org/0000-0001-6702-6350
Corresponding author: Tatiana G. Tral, ttg.tral@yandex.ru



