Association of clinical, laboratory, and morphological characteristics of the ovaries in girls with Turner syndrome and spontaneous puberty
Turchinets A.I., Badlaeva A.S., Uvarova E.V., Asaturova A.V., Kamaletdinov N.S., Kiseleva I.A., Kumykova Z.Kh., Gavisova A.A.
Objective: To present the characteristics of the ovarian reserve in girls with Turner syndrome based on morphological, clinical, laboratory, and instrumental parameters.
Materials and methods: A comparative morphological, morphometric, and immunohistochemical study of the ovarian cortex fragments was conducted in 20 girls. This group included 10 patients with Turner syndrome and spontaneous puberty, who exhibited no clinical or laboratory signs of premature ovarian insufficiency, and 10 girls with paraovarian cysts. The study examined the relationship between the morphological characteristics of the obtained samples and the stage of sexual development according to Tanner, ultrasound parameters of the ovaries, results of cytogenetic studies of blood lymphocytes, and hormonal status of girls with Turner syndrome.
Results: All girls with paraovarian cysts and 9 of 10 patients with Turner syndrome had follicles in the ovarian cortex, and abnormal morphology was observed in all girls with Turner syndrome and 80% in the control group. All follicles from patients with Turner syndrome expressed the oocyte-specific immunohistochemical markers ZP2, GDF9, BMP15, and CD117. Follicle density in Turner syndrome was 6.8 times lower than that in the control group and did not correlate with age, stage of puberty according to the Tanner scale, serum levels of FSH, LH, estradiol, AMH, inhibin B, testosterone, antral follicle count, or ovarian volume. However, this was associated with the presence of a 46,XX, or 47,XXX cell clone in the karyotype. Healthy follicles in the ovarian cortex were not detected in girls with Turner syndrome who had FSH and AMH levels > 15 IU/l and < 0.307 ng/ml, respectively. Among patients with X-chromosome mosaicism without structural anomalies, a positive correlation was found between follicle density and inhibin B level in the blood.
Conclusion: The prospects for performing the ovarian cortex cryopreservation procedure in girls with Turner syndrome may be determined by the combined presence of X-chromosome mosaicism without structural abnormalities, normal serum FSH levels for the corresponding age, inhibin B levels appropriate for the stage of sexual development according to Tanner, and detectable AMH levels.
Authors' contributions: Uvarova E.V., Asaturova A.V. – conception and design of the study; Badlaeva A.S., Turchinets A.I., Kamaletdinov N.S., Kiseleva I.A., Gavisova A.A. – collection and processing of material; Turchinets A.I., Badlaeva A.S. – data analysis; Badlaeva A.S., Turchinets A.I. – drafting of the manuscript; Uvarova E.V., Asaturova A.V., Kumykova Z.Kh. – editing
of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov
NMRC for OG&P on October 22, 2022.
Patient Consent for Publication: The parents (legal representatives) of patients provided informed consent for the publication
of data and associated images.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Turchinets A.I., Badlaeva A.S., Uvarova E.V., Asaturova A.V., Kamaletdinov N.S., Kiseleva I.A.,
Kumykova Z.Kh., Gavisova A.A. Association of clinical, laboratory, and morphological characteristics of
the ovaries in girls with Turner syndrome and spontaneous puberty.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (5): 97-109 (in Russian)
https://dx.doi.org/10.18565/aig.2025.42
Keywords
Turner syndrome (TS) is recognized as one of the most common causes of premature ovarian insufficiency (POI) and the leading cause of chromosomal abnormalities associated with accelerated depletion of the ovarian reserve (OR) owing to the absence or structural abnormality of the X chromosome [1, 2]. The literature indicates that the onset of POI in girls and women with TS is linked to their karyotypes. Specifically, in mosaic variants of TS that include a 46,XX cell clone, spontaneous puberty is more frequently observed, and the age at which POI is diagnosed is generally higher compared to girls with a monosomic karyotype of 45,X [3, 4].
Research on gonadal biopsies has shown that during embryogenesis, the migration of primary germ cells in fetuses with the 45,X karyotype is unaffected. Up to the 12th week of intrauterine development, oogonia and first-order oocytes were identified in the ovarian tissue; however, after this period, there was a noted increase in accelerated apoptosis [5, 6]. One proposed reason for the loss of first-order oocytes in TS is the disruption of chromosome pairing during the prophase of meiosis [7]. Additionally, it has been reported that the apoptosis of these oocytes in TS may stem from impaired connections between oocytes and granulosa cells, which disrupts bidirectional signaling essential for primordial follicle development [8].
Numerous studies on X-chromosome breakpoints have identified two distinct loci on the long arm of the X chromosome (Xq13-q21 and Xq23-q27) and a region on the short arm (Xp22.1-p11.2) that are significantly associated with ovarian function [9, 10]. PGRMC1, located on Xq22-q24, encodes a membrane receptor that binds progesterone and mediates the anti-apoptotic effect of hormones on granulosa cells. On the proximal part of the short arm (Xp11.2), BMP15 is a member of the transforming growth factor (TGF)-β superfamily, which is expressed in oocytes throughout folliculogenesis and regulates granulosa cell function [11, 12].
Consequently, disorders of chromosome pairing and haploinsufficiency of specific genes due to X-chromosome aneuploidy and/or structural abnormalities in TS significantly affect folliculogenesis, leading to reduced follicle density (FD) in girls with TS at birth or complete loss of oocytes with the formation of streak gonads [11, 13]. However, existing studies on the detection of growing follicles in ovarian tissue and the possibility of spontaneous pregnancies in patients with TS, including those without X-chromosome mosaicism, suggest that some oocyte maturation processes remain unaffected [14–17].
Given the high risk of POI in this patient cohort, it is currently recommended that counseling regarding rapid OR depletion and the consideration of fertility preservation be initiated at the earliest possible age [18]. Therefore, studying the relationship between clinical and laboratory-instrumental characteristics and morphological features of ovarian tissue in girls with TS who show no signs of POI is of particular interest for predicting their reproductive potential.
This study aimed to present the characteristics of ovarian reserve in girls with Turner syndrome based on morphological, clinical, laboratory, and instrumental parameters.
Materials and methods
The study included 10 girls aged 2 months to 17 years with confirmed TS and no Y-chromosome karyotype, based on cytogenetic analysis. The participants had serum follicle-stimulating hormone (FSH) levels of less than 25 IU/l and underwent cryopreservation of the ovarian cortex to preserve future reproductive potential. The procedure followed a standard slow-freezing protocol and was approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Ovarian tissue fragments, measuring no larger than 2.5×2.0×1.0 mm, underwent morphometric, morphological, and immunohistochemical studies. For comparative analysis, ovarian cortex fragments from 10 girls aged 3–17 years, obtained during the removal of paraovarian cysts intimately fused with the ovary (control group), were subjected to similar studies. The exclusion criteria for both groups included a history of ovarian surgery, hormonal or gonadotoxic therapy, and the absence of signed informed consent from the patient or their legal representative to participate in the study.
Preparation for histological examination
The samples were fixed in 10% neutral buffered formalin, dehydrated with alcohol, treated with xylene, and prepared as paraffin blocks. Afterward, the sections were stained with hematoxylin and eosin.
Morphometric study
An Aperio AT2 scanning microscope (Leica Biosystems, Germany) was used to obtain digital images of micropreparations. To count the number of primordial follicles in the samples and determine the section area, every fifth section with a thickness of 10 μm was assessed using the QuPath 0.3.2 program. FD was estimated using the method presented by Schmidt K.L. et al. in 2003 [19]. A modified version of the correction factor, α, was employed to avoid multiple counts of the same follicle. The total number of follicles, volume of the ovarian fragment, and FD in 1 mm³ were calculated using the correction factor γ, accounting for missed areas as described by Hassan et al. (2023) [20].
Morphological examination
In the stained sections, the quality of the follicles was assessed in accordance with the accepted characteristics: healthy follicles, with a non-pyknotic, non-shrunken oocyte and non-pyknotic granulosa cells; degenerating follicles, having one of the above-mentioned features; and atretic follicles-with karyopyknosis, both in the oocyte and in the granulosa cells [21]. Additionally, the presence of the following features was assessed: an incomplete granulosa cell layer, pale nuclear material with unclear contours, wrinkling of the oocyte cytoplasm, empty follicles, partial absence of oocyte connection with the basement membrane, and diocyte follicles.
Immunohistochemistry
For immunohistochemistry, 4 μm paraffin sections were mounted on positively charged glass slides. The slides were stained using a BenchMark XT immunostainer (Ventana, Roche) using standard protocols (incubation temperature 37°C, incubation time from 16 to 24 min), DAB Universal ultraView detection panel (Ventana, Roche), primary rabbit antibodies to CD117 (clone EP10, Ventana), ZP2 (polyclonal, Cusabio, dilution 1/200), GDF9 (polyclonal, Cusabio, dilution 1/400), and BMP15 (polyclonal, Cusabio, dilution 1/25). Numerous studies in animals and human models have demonstrated the importance of the expression of zona pellucida proteins (ZP), growth factors GDF-9, BMP-15, and CD117 ligands, which are regulated by the main oocyte-specific transcription factors FIGLA and NOBOX, in preantral folliculogenesis and oocyte maturation; therefore, they were chosen in our study to assess the qualitative characteristics of follicles [22–27]. To study the intensity of immunohistochemical staining for markers, the semi-quantitative I-score (immunoreactive score according to Remmele and Stegner) system was used: IRS=A×B, where A is the percentage of positively stained cells, expressed in points, and B is the staining intensity, expressed in points. The I-score results were interpreted as follows: 0–2 points – negative reaction (0), 3–4 points – weak expression (+), 5–8 – moderate expression (++), 9–12 – pronounced expression (+++) [28].
In the second stage of the study, we examined the relationship between the morphological characteristics of cortical layer fragments in girls with TS and their stage of sexual development, according to Tanner. We also analyzed ultrasound parameters, including ovarian volume and the number of antral follicles (AFC), the results of cytogenetic studies of blood lymphocytes, and preoperative levels of hormones such as FSH, luteinizing hormone (LH), estradiol, testosterone, inhibin B, and anti-Müllerian hormone (AMH). Ultrasound examinations were conducted using a MyLabClass C device (Esaote, Italy) with linear and convex sensors operating at a frequency of 1.8–6.0 MHz. Hormonal status was assessed using electrochemiluminescence on a Cobas e411 automatic analyzer (Roche, Switzerland).
Statistical analysis
Statistical analysis was conducted using GraphPad Prism 9.3.1 (Dotmatics, USA) and Jamovi 2.5.7.0 (Australia). The assumption of normality was evaluated using the Shapiro-Wilk test. Variables that did not meet normality assumptions were reported as median (Me) and interquartile range [Q1–Q3]. Categorical data were summarized as counts and percentages. Group comparisons for continuous variables were performed using the Mann–Whitney U test, and in cases of unequal variance, the Brunner–Munzel test was applied. Correlation analysis was conducted by calculating the Spearman's rank correlation coefficients. Comparisons of percentages in multi-way contingency tables were performed using Fisher's exact test for small sample sizes. Statistical significance was set at p<0.05.
Results
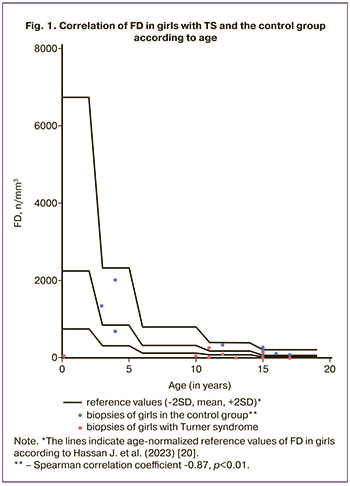
The median age of the girls with TS was 12.50 [10.75-17.00] years and did not differ from that of the control group (15.00 [4.00–16.00] years; p=0.53). On morphological examination, follicles were found in all girls in the control group and in 9 (90%) patients with TS. Furthermore, the FD in the cortical fragments was 6.8 times lower in the TS group than in the control group (37.8 [21.75–112.0] and 262.0 [101–1005] mm3, respectively; p=0.02). However, as shown in Figure 1, in contrast to the control group, in which FD was negatively correlated with age (Spearman correlation coefficient -0.87, p<0.01), no such dependence was found in the group of patients with TS. Abnormal follicular morphology was observed in all ovarian cortex fragments in patients with TS and in eight (80%) of the control group (Table 1).

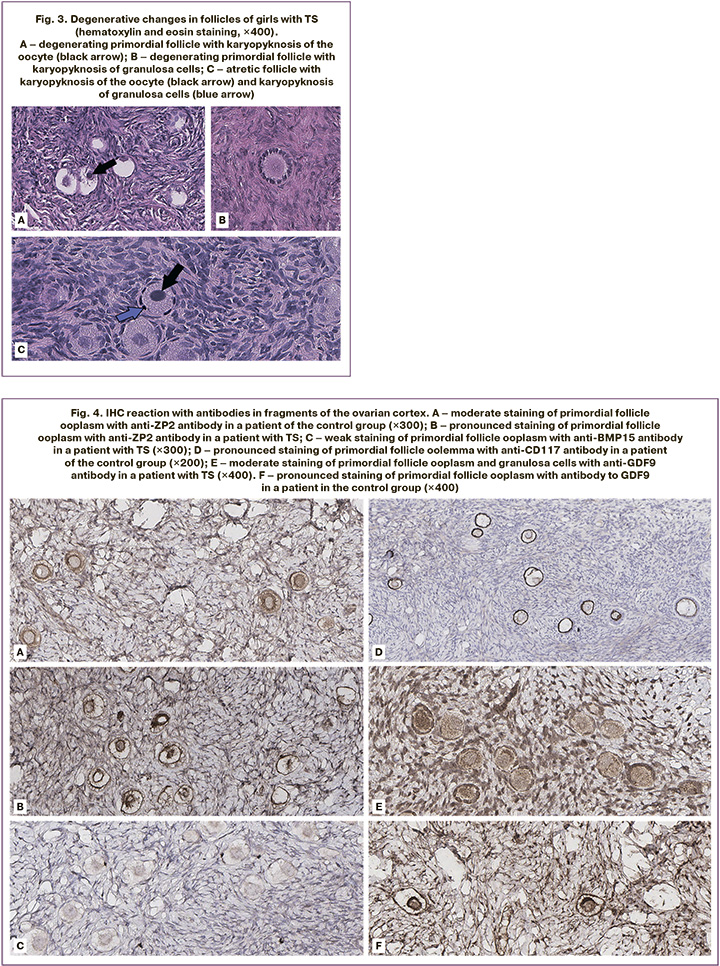
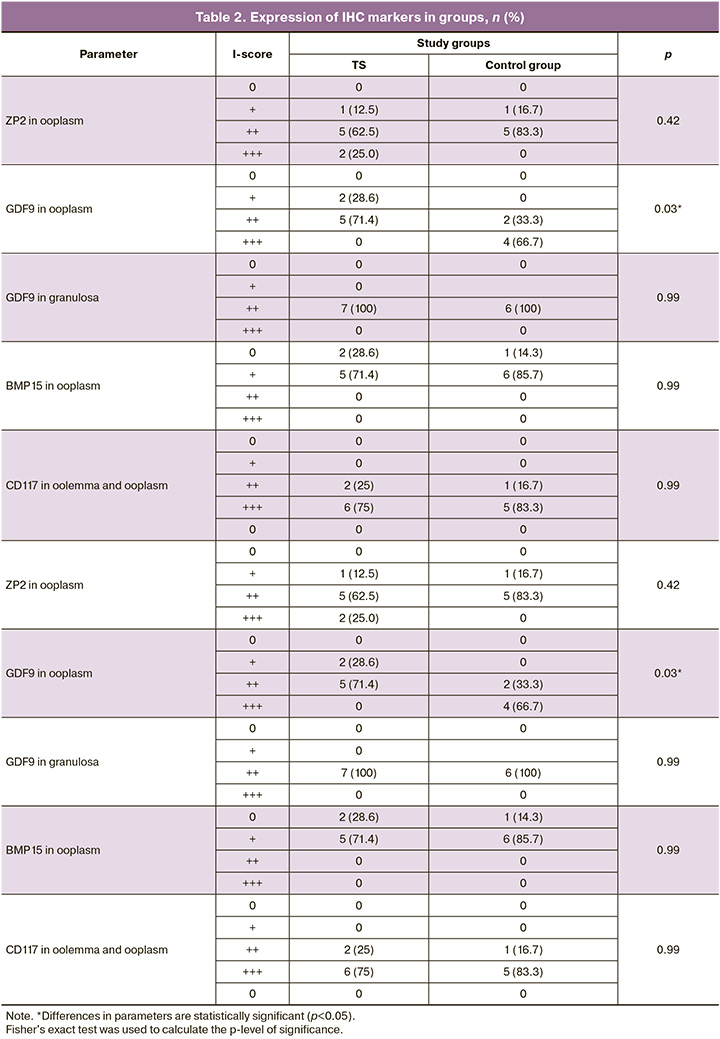
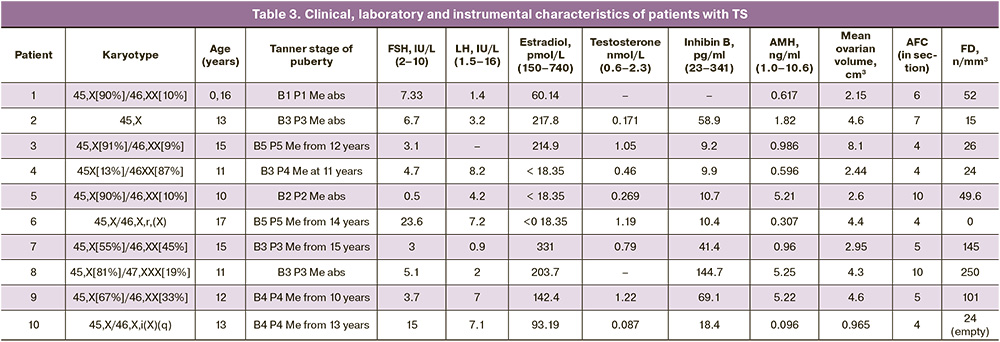
The study revealed no statistically significant differences in the frequency of detection of oocytes with an incomplete granulosa cell layer (Fig. 2A), and diocyte follicles (Fig. 2D) between the two groups (p=0.07). The presence of pale nuclear material with blurred contours (Fig. 2B), indicating the absence of the germinal vesicle membrane, was significantly more frequent in the TS group than in the control group (p=0.01). Empty follicles (Fig. 2B) was almost 4 times more frequent in the TS group (p=0.02). At the same time, a partial lack of connection between the oocyte and the basement membrane was observed only in the control group (n=1) and not in the TS group (Fig. 2G). There were no significant differences in the frequency of detection or proportion of degenerating follicles between the groups (p=0.15). Oocyte karyotyping (Fig. 3A) was detected in 50% of the samples from patients in the control group and only in 37.5% of the samples from patients with TS (p=0.66). However, in five (62.5%) girls with TS, oocyte cytoplasm shrinkage was observed (Fig. 2B), and 2 (22.2%) karyopyknosis of granulosa cells (Fig. 3B), including in combination with oocyte pyknosis (Fig. 3D), whereas ooplasm and granulosa pyknosis were not observed in the control group (p<0.01, p=0.21). As shown in Table 2, when evaluating the results of immunohistochemical staining, no differences in the expression of ZP2, CD117, or BMP15 were found in either group (p=0.42, p=0.99, p=0.99). In most follicles in both groups, moderate staining of the ooplasm with ZP2, pronounced expression of CD117 in the oolemma and ooplasm, and a weak reaction to the BMP15 antibody in the cytoplasm of oocytes was detected (Fig. 4A, B, D). At the same time, only 2 girls with TS (25%) showed pronounced staining with the antibody to ZP2 in the ooplasm (Fig. 4B). The expression of GDF9 (Fig. 4D, E) in granulosa cells, moderate staining was detected in all cases, both in patients with TS and in the control group. However, the protein expression in the ooplasm of girls in the control group was significantly higher (p=0.03). The clinical and laboratory-instrumental characteristics of the patients with TS are presented in Table 3. Despite the absence of a relationship between the age of girls with TS and FD, a relationship was found with the determination of morphologically normal follicles in the cortex: age over 12 years was associated with the absence of healthy follicles in the examined fragment of the cortex (p<0.05). When studying the correlation of FD with the clinical parameters of patients with TS, no dependence on the stage of sexual development according to the Tanner scale and gynecological age after menarche was found (p=0.46). Nine out of 10 girls had spontaneous puberty, of which six had menstruations with a periodicity of 1 to 2 months, and in one infant girl, the study coincided with the period of mini-puberty. Menarche was not associated with the detection of healthy, degenerating, or atretic follicles; however, karyopyknosis in oocytes or cumulus cells was detected only in the cortex of girls, either prepubertal or in the year of menarche (p>0.99).
In addition, in girls with TS, no correlation was found between FD and the levels of all studied hormone parameters and ovarian ultrasound data. In particular, no significant difference in FD was found between girls with AMH levels above or below 0.5 ng/ml and 1.1 ng/ml (p=0.11, p=0.44). Moreover, there were no statistically significant differences in AMH levels between girls with FD >25/mm3, which corresponds to the -2SD indicator for healthy late adolescent girls, according to Hassan et al. (2023) (p=0.07) [20].
At the same time, FD was associated with the presence of a 46,XX or 47,XXX cell clone in the karyotype (p=0.03). In girls with disomy or trisomy X in the karyotype, the median FD was 52 [26–145] per mm3, while in two girls with mosaicism and structural X-chromosome abnormalities, follicles in the ovary were either not detected or were empty, and only the patient with X monosomy had the lowest FD of all the studied cortical fragments (15.00/mm3). However, the percentage of X-monosomal cells in the mosaic karyotype without structural X-chromosome abnormalities, according to the cytogenetic study of lymphocytes, showed no correlation with the FD value (p=0.8). It is also noteworthy that the highest FD among all girls with TS was detected in a patient with 45,X/47,XXX mosaicism. As shown in Figure 5, in seven girls with 45,X/46,XX and 45,X/47,XXX mosaicism, a correlation was found between FD and the level of inhibin B (Spearman’s correlation coefficient 0.89, p=0.03) in the absence of a significant relationship with the level of AMH and other hormones (p=0.17).
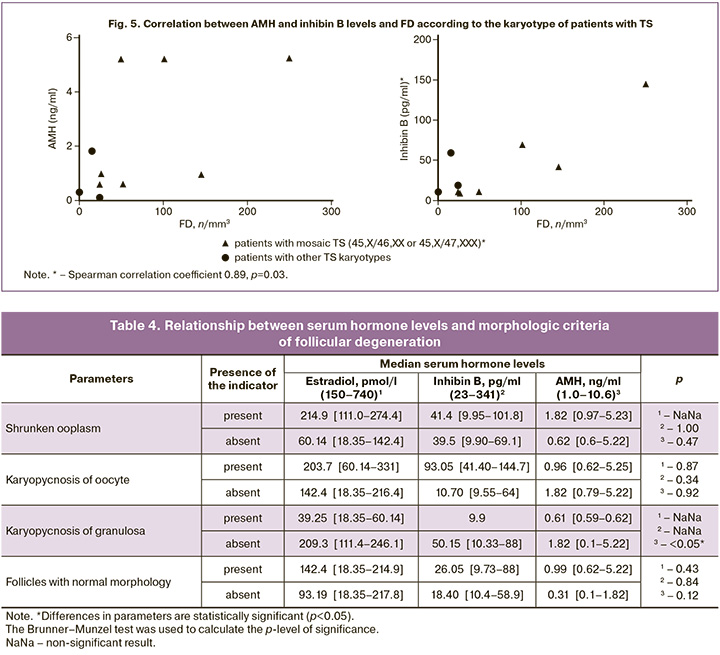
When studying the relationship between hormones synthesized by the ovaries and morphological criteria of follicular degeneration, it was found that girls with TS and karyopyknosis granulosa had a decrease in the concentration of serum AMH by an average of three times compared with girls without signs of cumulus cell degeneration (p<0.05). At the same time, no statistically significant difference was observed between the levels of estradiol, AMH, inhibin B, and the presence of other criteria for degenerative changes in follicles as well as morphologically unchanged follicles (Table 4).
A high degree of ZP2 staining in primordial follicles detected in two girls with TS, according to the literature, may indicate an early stage of oocyte atresia. At the same time, during morphological examination, atretic follicles were not detected in the fragments of the ovarian cortex of these patients, and the expression of other oocyte-specific markers did not differ from that in the control group, which indicates the possible functionality of the remaining follicles.
Discussion
Based on the results from studying ovarian cortex fragments, follicles were detected in 9 of 10 patients with TS who experienced spontaneous puberty and showed no clinical or laboratory signs of POI. However, only 3 girls with 45X/46,XX and 45,X/47,XXX mosaicism had FD within the 95% confidence interval of the normal reference values for healthy girls of the corresponding age [20]. Notably, follicles were absent in the cortex of the oldest patient, a 17-year-old with karyotype 45,X/46,X,r,(X), despite normal menstruation.
The most common morphological findings in TS included empty follicles, follicles with pale nuclear material and fuzzy edges, and a shrunken ooplasm. These features may indicate early oocyte atresia occurring alongside ongoing follicular development [29]. Only follicles with abnormal morphology were identified in girls with 45,X monosomy, 45,X/46,X,i(X)(q) mosaic karyotype, and 45,X/46,XX mosaicism. In contrast, six (66.7%) patients exhibited morphologically normal follicles, suggesting that fertility preservation procedures may be feasible in cases of TS with X-chromosome mosaicism lacking structural abnormalities.
Several studies have reported abnormal primordial follicle morphology, characterized by an incomplete layer of granulosa cells and empty follicles, in patients with monosomy 45,X [15, 16, 30]. Nadesapillai S. et al. (2021) noted the presence of morphologically complete primordial follicles in a girl with a monosomic karyotype, although the growing follicles exhibited altered morphology. In 2023, they found that abnormal follicle morphology was most frequently observed in cases with structural abnormalities of the X chromosome in the karyotype [14, 31]. Furthermore, FD in X-monosomy showed significant variability, with a median of 46 [5.5–111.5] mm³ [14–16, 30]. In a study by Mamsen L.S. et al. (2019), three out of six girls with structural abnormalities of the X chromosome and no signs of POI had no follicles in ovarian tissue fragments, while the remaining patients exhibited very low follicle densities, ranging from 3/mm³ to 20/mm³ [16].
Interestingly, our study found no differences in the detection of degenerating and atretic follicles between patients with TS and the control group. Consistent with findings from Hreinsson et al. (2002) and Mamsen L.S. et al. (2019), we did not observe a clear correlation between FD and age in girls with TS, even when considering the presence of mosaicism on the X chromosome [15, 16]. It is also noteworthy that there was no difference in FD between patients aged 2 months (52/mm³) and 10 years (49.6/mm³), who had the same proportion of 45,X cells in the mosaic karyotype 45,X/46,XX. This observation aligns with the view that most follicles in TS undergo atresia prenatally, leading to gradual depletion of the ovarian reserve (OR) [11]. Additionally, we found that girls with TS possessing fully developed follicles in the ovarian cortex were, on average, three years younger than those with only abnormal follicle morphology, supporting the notion that cryopreservation of the cortex is best performed at a younger age in this cohort [32].
All follicles of patients with TS showed the expression of oocyte-specific immunohistochemical markers. Weak GDF9 staining in the oocyte cytoplasm is characteristic of resting primordial follicles, as this factor is involved in granulosa cell proliferation and theca development [33, 34]. However, given the pronounced staining with the GDF9 antibody in the comparison group in our study, its low expression in girls with TS may indicate aberrant follicle development and delayed oocyte maturation [35]. Moreover, the expression of GDF9 in granulosa cells, which is necessary for bidirectional communication between the oocyte and cumulus cells, as well as the oocyte growth factor BMP15 and the ligand CD117, the production of which is influenced by GDF9, was not impaired in patients in this cohort [34, 35]. Moreover, given the normal staining with antibodies to ZP2 and CD117 in combination with the absence of morphological signs of degeneration, one can assume intact growth and development potential of some follicles in girls with TS. A different opinion regarding the results of the study was presented by Hreinsson J.G. et al. in 2002, who observed a correlation between FSH and FD levels. However, their study included girls with serum FSH levels exceeding 25 IU/l, which was an exclusion criterion in our study [15]. Mamsen L.S. et al. in 2019 reported that the presence of follicles was associated with detectable serum AMH levels and normal FSH levels [16]. In 2023, Nadesapillai S. et al. also reported a correlation between the presence of follicles in the ovaries of girls with TS and the levels of AMH≥0.1 ng/mL, FSH≤10 IU/L, and inhibin B≥10 ng/L [31]. And despite the fact that in our study, healthy follicles in the cortex were not detected in two girls with elevated FSH levels>15 IU/L and minimum AMH values<0.307 ng/ml, we did not determine a statistical relationship between FD, as well as the presence of morphologically normal follicles and the follicle FSH, LH, estradiol, AMH, inhibin B, testosterone, AFC and ovarian volume. At the same time, inhibin B levels corresponding to the laboratory reference values were detected in our study in three girls with normal FD for the corresponding age. It is known from the literature that the serum concentration of inhibin B varies significantly depending on the stage of sexual development according to Tanner and the phase of the menstrual cycle; therefore, its role as an OR marker is currently under investigation [36, 37]. However, it is noteworthy that the level of inhibin B in girls with normal FD in our study corresponded to or exceeded the median values of healthy girls with the corresponding stage of puberty, according to Borelli-Kjær A. et al. (2024). In patients with low FD, hormone concentration was two or more times lower than the average values described by the authors [38]. According to the established correlation between FD and inhibin B level in girls with X-chromosome mosaicism without structural abnormalities, it can be assumed that the detection of an inhibin B level corresponding to average or high values within the reference range for the corresponding stage of thelarche, in combination with low FSH and detectable AMH, can be a predictor of unchanged OR in patients with karyotype 45,X/46,XX or 45,X/47,XXX. In addition, low AMH levels showed a correlation only with the presence of degenerative changes in granulosa cells, which may indicate a reduced number of growing follicles due to impaired interaction of some oocytes with the cumulus.
Given the small sample size and the heterogeneity of the karyotype among the patients in this study, it is challenging to establish clear criteria for recommending OR preservation methods for individuals with TS. Nevertheless, based on our findings and the existing literature, cryopreservation of the ovarian cortex in girls with an X-monosomal karyotype or structural anomalies of the X chromosome appears less promising due to a higher prevalence of abnormal follicle morphology and lower FD compared to girls with 45,X/46,XX mosaicism, regardless of the degree of mosaicism.
Conclusion
Considering the presence of morphologically normal primordial follicles in ovarian fragments from girls with TS and the known risk of POI, ovarian cortex cryopreservation in this patient group may be a promising option.
The absence of clinical and laboratory signs of POI should not be regarded as the sole criterion justifying fertility preservation programs in patients with TS, particularly in those with a monosomic karyotype or structural anomalies of the X chromosome. The combined presence of X-chromosome mosaicism without structural anomalies, normal serum FSH levels for the corresponding age, and inhibin B levels appropriate for the stage of sexual development according to Tanner, along with detectable AMH levels, can serve as predictors of unchanged FD and folliculogenesis potential. Given the lack of large-scale studies establishing criteria for the presence of healthy follicles in the ovaries of girls with TS, as well as clear correlations between known OR and FD markers and long-term outcomes of ovarian cortex cryopreservation procedures in this patient cohort, it is essential to communicate the risks of obtaining non-functional material during counseling regarding the preservation of reproductive potential.
References
- Dowlut-McElroy T., Shankar R.K. The care of adolescents and young adults with Turner syndrome: a pediatric and adolescent gynecology perspective. J. Pediatr. Adolesc. Gynecol. 2022; 35(4): 429-34. https://dx.doi.org/10.1016/j.jpag.2022.02.002
- Webber L., Davies M., Anderson R., Bartlett J., Braat D., Cartwright B. et al.; European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum. Reprod. 2016; 31(5): 926-37. https://dx.doi.org/10.1093/humrep/dew027
- Lunding S.A., Aksglaede L., Anderson R.A., Main K.M., Juul A., Hagen C.P. et al. AMH as predictor of premature ovarian insufficiency: a longitudinal study of 120 Turner syndrome patients. J. Clin. Endocrinol. Metab. 2015; 100(7): E1030-8. https://dx.doi.org/10.1210/jc.2015-1621
- Fitz V.W., Law J.R., Peavey M. Characterizing ovarian function by karyotype in a cohort of women with Turner’s syndrome. Fertil. Steril. 2019; 111(4): e27-e28. https://dx.doi.org/10.1016/j.fertnstert.2019.02.074
- Reynaud K., Cortvrindt R., Verlinde F., De Schepper J., Bourgain C., Smitz J. Number of ovarian follicles in human fetuses with the 45,X karyotype. Fertil. Steril. 2004; 81(4): 1112-9. https://dx.doi.org/10.1016/j.fertnstert.2003.12.011
- Hook E.B., Warburton D. Turner syndrome revisited: review of new data supports the hypothesis that all viable 45,X cases are cryptic mosaics with a rescue cell line, implying an origin by mitotic loss. Hum. Genet. 2014; 133(4): 417-24. https://dx.doi.org/10.1007/s00439-014-1420-x
- Burgoyne P.S., Baker T.G. Perinatal oocyte loss in XO mice and its implications for the aetiology of gonadal dysgenesis in XO women. J. Reprod. Fertil. 1985; 75(2): 633-45. https://dx.doi.org/10.1530/jrf.0.0750633
- Modi D.N., Sane S., Bhartiya D. Accelerated germ cell apoptosis in sex chromosome aneuploid fetal human gonads. Mol. Hum. Reprod. 2003; 9(4): 219-25. https://dx.doi.org/10.1093/molehr/gag031
- Mercer C.L., Lachlan K., Karcanias A., Affara N., Huang S., Jacobs P.A. et al. Detailed clinical and molecular study of 20 females with Xq deletions with special reference to menstruation and fertility. Eur. J. Med. Genet. 2013; 56(1): 1-6. https://dx.doi.org/10.1016/j.ejmg.2012.08.012
- Persani L., Rossetti R., Cacciatore C., Bonomi M. Primary ovarian insufficiency: X chromosome defects and autoimmunity. J. Autoimmun. 2009; 33(1): 35-41. https://dx.doi.org/10.1016/j.jaut.2009.03.004
- Fukami M. Ovarian dysfunction in women with Turner syndrome. Front. Endocrinol. (Lausanne). 2023; 14: 1160258. https://dx.doi.org/10.3389/fendo.2023.1160258
- Persani L., Rossetti R., Di Pasquale E., Cacciatore C., Fabre S. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum. Reprod. Update. 2014; 20(6): 869-83. https://dx.doi.org/10.1093/humupd/dmu036
- San Roman A.K., Page D.C. A strategic research alliance: Turner syndrome and sex differences. Am. J. Med. Genet. C Semin. Med. Genet. 2019; 181(1): 59-67. https://dx.doi.org/10.1002/ajmg.c.31677
- Nadesapillai S., van der Velden J., Smeets D., van de Zande G., Braat D., Fleischer K. et al. Why are some patients with 45,X Turner syndrome fertile? A young girl with classical 45,X Turner syndrome and a cryptic mosaicism in the ovary. Fertil. Steril. 2021; 115(5): 1280-7. https://dx.doi.org/10.1016/j.fertnstert.2020.11.006
- Hreinsson J.G., Otala M., Fridström M., Borgström B., Rasmussen C., Lundqvist M. et al. Follicles are found in the ovaries of adolescent girls with Turner syndrome. J. Clin. Endocrinol. Metab. 2002; 87(8): 3618-23. https://dx.doi.org/10.1210/jcem.87.8.8753
- Mamsen L.S., Charkiewicz K., Anderson R.A., Telfer E.E., McLaughlin M., Kelsey T.W. et al. Characterization of follicles in girls and young women with Turner syndrome who underwent ovarian tissue cryopreservation. Fertil. Steril. 2019; 111(6): 1217-1225.e3. https://dx.doi.org/10.1016/j.fertnstert.2019.02.003
- Bernard V., Donadille B., Zenaty D., Courtillot C., Salenave S., Brac de la Perrière A. et al. CMERC Center for Rare Disease. Spontaneous fertility and pregnancy outcomes amongst 480 women with Turner syndrome. Hum. Reprod. 2016; 31(4): 782-8. https://dx.doi.org/10.1093/humrep/dew012
- Gravholt C.H., Andersen N.H., Christin-Maitre S., Davis S.M., Duijnhouwer A., Gawlik A. et al.; International Turner Syndrome Consensus Group; Backeljauw P.F. Clinical practice guidelines for the care of girls and women with Turner syndrome. Eur. J. Endocrinol. 2024; 190(6): G53-G151. https://dx.doi.org/10.1093/ejendo/lvae050
- Schmidt K.L., Byskov A.G., Nyboe Andersen A., Müller J., Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum. Reprod. 2003; 18(6): 1158-64. https://dx.doi.org/10.1093/humrep/deg246
- Hassan J., Knuus K., Lahtinen A., Rooda I., Otala M., Tuuri T. et al. Reference standards for follicular density in ovarian cortex from birth to sexual maturity. Reprod. Biomed. Online. 2023; 47(4): 103287. https://dx.doi.org/10.1016/j.rbmo.2023.103287
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr. Rev. 1996; 17(2): 121-55. https://dx.doi.org/10.1210/edrv-17-2-121
- Pangas S.A., Choi Y., Ballow D.J., Zhao Y., Westphal H., Matzuk M.M. et al. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc. Natl. Acad. Sci. U. S. A. 2006; 103(21): 8090-5. https://dx.doi.org/10.1073/pnas.0601083103
- Gook D.A., Edgar D.H., Borg J., Martic M. Detection of zona pellucida proteins during human folliculogenesis. Hum. Reprod. 2008; 23(2): 394-402. https://dx.doi.org/10.1093/humrep/dem373
- Rankin T.L., O'Brien M., Lee E., Wigglesworth K., Eppig J., Dean J. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development. 2001; 128(7): 1119-26. https://dx.doi.org/10.1242/dev.128.7.1119
- Park M.J., Ahn J.W., Kim K.H., Bang J., Kim S.C., Jeong J.Y. et al. Prediction of ovarian aging using ovarian expression of BMP15, GDF9, and C-KIT. Exp. Biol. Med. (Maywood). 2020; 245(8): 711-9. https://dx.doi.org/10.1177/1535370220915826
- Belli M., Shimasaki S. Molecular aspects and clinical relevance of GDF9 and BMP15 in ovarian function. Vitam. Horm. 2018; 107: 317-48. https://dx.doi.org/10.1016/bs.vh.2017.12.003
- Driancourt M.A., Reynaud K., Cortvrindt R., Smitz J. Roles of KIT and KIT LIGAND in ovarian function. Rev. Reprod. 2000; 5(3): 143-52. https://dx.doi.org/10.1530/ror.0.0050143
- Remmele W., Stegner H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987; 8: 138-40.
- Dolmans M.M., Donnez J., Camboni A., Demylle D., Amorim C., Van Langendonckt A. et al. IVF outcome in patients with orthotopically transplanted ovarian tissue. Hum Reprod. 2009; 24(11): 2778-87. https://dx.doi.org/10.1093/humrep/dep289
- Schleedoorn M.J., Fleischer K., Braat D., Oerlemans A., van der Velden A., Peek R. Why Turner patients with 45, X monosomy should not be excluded from fertility preservation services. Reprod. Biol. Endocrinol. 2022; 20(1): 143. https://dx.doi.org/10.1186/s12958-022-01015-z
- Nadesapillai S., van der Velden J., van der Coelen S., Schleedoorn M., Sedney A., Spath M. et al. TurnerFertility trial: fertility preservation in young girls with Turner syndrome by freezing ovarian cortex tissue-a prospective intervention study. Fertil. Steril. 2023; 120(5): 1048-60. https://dx.doi.org/10.1016/j.fertnstert.2023.08.004
- Jeve Y.B., Gelbaya T., Fatum M. Time to consider ovarian tissue cryopreservation for girls with Turner's syndrome: an opinion paper. Hum. Reprod. Open. 2019; 2019(3): hoz016. https://dx.doi.org/10.1093/hropen/hoz016
- Bayne R.A., Kinnell H.L., Coutts S.M., He J., Childs A.J., Anderson R.A. GDF9 is transiently expressed in oocytes before follicle formation in the human fetal ovary and is regulated by a novel NOBOX transcript. PLoS One. 2015; 10(3): e0119819. https://dx.doi.org/10.1371/journal.pone.0119819
- Gilchrist R.B., Lane M., Thompson J.G. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update. 2008; 14(2): 159-77. https://dx.doi.org/10.1093/humupd/dmm040
- Wei L.N., Huang R., Li L.L., Fang C., Li Y., Liang X.Y. Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2014; 31(11): 1483-90. https://dx.doi.org/10.1007/s10815-014-0319-8
- Sehested A., Juul A.A., Andersson A.M., Petersen J.H., Jensen T.K., Müller J. et al. Serum inhibin A and inhibin B in healthy prepubertal, pubertal, and adolescent girls and adult women: relation to age, stage of puberty, menstrual cycle, follicle-stimulating hormone, luteinizing hormone, and estradiol levels. J. Clin. Endocrinol. Metab. 2000; 85(4): 1634-40. https://dx.doi.org/10.1210/jcem.85.4.6512
- Wen J., Huang K., Du X., Zhang H., Ding T., Zhang C. et al. Can inhibin B reflect ovarian reserve of healthy reproductive age women effectively? Front. Endocrinol. (Lausanne). 2021; 12: 626534. https://dx.doi.org/10.3389/fendo.2021.626534
- Borelli-Kjær A., Aksglaede L., Jensen R.B., Hagen C.P., Ljubicic M.L., Busch A.S. et al. Serum concentrations of inhibin B in healthy females and males throughout life. J. Clin. Endocrinol. Metab. 2024; 110(1): 70-7. https://dx.doi.org/10.1210/clinem/dgae439
Received 20.02.2025
Accepted 30.04.2025
About the Authors
Anna I. Turchinets, PhD student, Physician at the Department of Pediatric and Adolescent Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,117997, Russia, Moscow, Ac. Oparin str., 4, +7(926)522-87-39, Ponomarevaanna28@gmail.com, https://orcid.org/0000-0002-4478-9133
Alina S. Badlaeva, PhD, Senior Researcher at the 1st Pathology Department, Physician at the 1st Pathology Department, V.I. Kulakov NMRC for OG&P, Ministry of Health
of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, alinamagnaeva03@gmail.com, https://orcid.org/0000-0001-5223-9767
Elena V. Uvarovа, Corresponding Member of the Russian Academy of Sciences, Dr. Med. Sci., Professor at the Department of Obstetrics, Gynecology and Perinatology,
I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University); Head of the Department of Pediatric and Adolescent Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, elena-uvarova@yandex.ru, https://orcid.org/0000-0002-3105-5640
Aleksandra V. Asaturova, Dr. Med. Sci., Head of the 1st Pathology Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow,
Ac. Oparin str., 4, a_asaturova@oparina4.ru, https://orcid.org/0000-0001-8739-5209
Nail S. Kamaletdinov, Embryologist at the 1st Gynecological Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow,
Ac. Oparin str., 4, sunsh86@mail.ru
Irina A. Kiseleva, PhD, Clinical Care Supervisor at the Department of Pediatric and Adolescent Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, kiseleva_i@oparina4.ru
Zaira Kh. Kumykova, PhD, Senior Researcher at the Department of Pediatric and Adolescent Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, zai-kumykova@yandex.ru, https://orcid.org/0000-0001-7511-1432
Alla A. Gavisova, Dr. Med. Sci., Head of the 1st Gynecological Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow,
Ac. Oparin str., 4, gavialla@yandex.ru
Corresponding author: Anna I. Turchinets, Ponomarevaanna28@gmail.com



