Assessment of embryological outcomes in IVF programs for patients of different age groups based on AMH levels
Nazarenko T.A., Khachatryan N.A., Krylova E.I., Biryukova A.M.
Objective: To evaluate embryological outcomes of IVF programs by examining the number of high-quality blastocysts in relation to AMH levels across different patient age groups.
Materials and methods: A retrospective analysis was conducted on the clinical and laboratory characteristics and results of IVF programs involving 900 patients treated between 2023 and 2024 at the V.I. Kulakov NMRC for OG&P. The mean age of women in the first group was 29.06 (2.85) years, in the second group was 39.01 (1.94) years, and in the third group was 43.03 (1.17) years.
Results: A low number of oocytes (0–5) was consistently observed in women of an advanced reproductive age. A count of 6–10 oocytes was found with equal frequency in both the older and middle-aged groups, while it was less common among younger women, who had a greater number of oocytes (p<0.001). When analyzing the number of MII oocytes, a similar quantity was noted in both the first and second groups, regardless of the total number of oocytes. The most significant finding was the quality of the blastocysts obtained. In the first and second groups, most blastocysts were of excellent or good quality, whereas in the third group, most were of low quality. The analysis of IVF outcomes in subgroups of patients with decreased ovarian reserve revealed that 67% of younger patients and 52% of middle-aged patients produced high-quality blastocysts, whereas no high-quality embryos were obtained from women of advanced reproductive age.
Conclusion: Even with comparable AMH levels in young and older patients, the proportion of high-quality blastocysts significantly declines with age, approaching zero in women over 40. This decline is likely to be associated with oocyte quality, embryo aneuploidy, and impaired development in older patients. In contrast, younger patients can still achieve high-quality embryos despite indicators of decreased ovarian reserve. These findings support the feasibility of conducting IVF programs to achieve pregnancy in younger patients, even when ovarian reserve is reduced.
Authors' contributions: Nazarenko T.A. – conception and design of the study, editing and final approval of the manuscript; Khachatryan N.A., Krylova E.I., Biryukova A.M. – collection of material, statistical analysis, drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients participating in the study signed an informed consent form. They were informed that the study data would be published without their names being used in written reports or publications.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Nazarenko T.A., Khachatryan N.A., Krylova E.I., Biryukova A.M. Assessment of
embryological outcomes in IVF programs for patients of different age groups based on AMH levels.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (6): 124-130 (in Russian)
https://dx.doi.org/10.18565/aig.2025.41
Keywords
While acknowledging the undeniable success of assisted reproductive technologies (ART) in enabling childbirth for infertile couples, it is difficult to overlook a noticeable stagnation in the outcomes of in vitro fertilization (IVF) along with a decline in pregnancy rates per embryo transfer. Similar data have been presented in domestic and international documents [1–4].
The primary reason for this stagnation is likely the trend of postponing childbirth to later reproductive ages, resulting in an increased number of older patients seeking IVF programs [5].
The mean age of a mother at the birth of her first child, calculated for conditional generations, steadily decreased from the 1960s to the 1980s and the first half of the 1990s, reaching a minimum of 22.5 years in 1994. However, in subsequent years, the mean age began to rise, with the rate of increase significantly surpassing the rate of decrease in the previous period. In 1995, the mean age was 22.67 years; by 2000, it rose to 23.54 years; in 2005, it was 24.11 years. By 2008, the mean age exceeded the 1960 level, reaching 24.44 years, and by 2010, it had reached 24.90 years. In 2013, it surpassed the 25-year mark, hitting 25.14 years. In 2015, the mean age was 25.46 years, in 2018 it was 25.91 years, and in 2019 it was 25.94 years [6]. A similar, although more pronounced, pattern was observed in patients seeking pregnancy through IVF. According to RAHR data, the proportion of women over 39 years of age seeking ART treatment for infertility in 2022 increased by 2.5 times, reflecting the trend toward delayed childbearing observed in many countries worldwide. However, an increase in the number of ART cycles has not been accompanied by an increase in the number of pregnancies; in fact, a decrease has been noted. The pregnancy rate per cycle was 28.6% in 2021, 28.9% in 2020, 32.3% in 2019, and 34.8% in 2016 [6, 7].
Undoubtedly, a decrease in pregnancy frequency also affects the rate of live births, leading to an increase in reproductive losses among older women. The live birth rate is 41.5% for women under 35 years, 31.9% for women aged 35–37 years, 22.1% for women aged 38–40 years, 12.4% for women aged 41–42 years, 5% for women aged 43–44 years, and 1% for women over 44 [8–10].
According to regulatory documents from the Ministry of Health of the Russian Federation, limitations for conducting IVF programs include reduced ovarian reserve indicators: an anti-Müllerian hormone (AMH) level below 1.2 ng/ml and a total antral follicle count of fewer than five [11].
The AMH level indirectly reflects the antral follicle count but does not indicate the quality of the obtained oocytes or their ability to fertilize [12, 13]. Consequently, AMH levels cannot be used to predict embryo quality or pregnancy frequency. Furthermore, it is well-established that the outcomes of IVF programs are directly related to a woman's age, which is influenced not only by the decline in antral follicle count and AMH levels but also by oocyte quality, as a significant portion of oocytes may be aneuploid and unable to result in the formation of a high-quality embryo [14–16].
This study aimed to evaluate the embryological outcomes of IVF programs by examining the number of high-quality blastocysts in relation to AMH levels in different patient age groups.
Materials and methods
A retrospective analysis was conducted on the clinical and laboratory characteristics and outcomes of IVF programs for patients treated in 2023-2024 at the F. Paulsen Research and Educational Center for ART with the Clinical Department of the V.I. Kulakov NMRC for OG&P.
Patients with significant somatic diseases, gynecological conditions such as large uterine fibroids, endometrioid ovarian cysts, history of deep infiltrating endometriosis, uterine developmental anomalies, and severe forms of pathozoospermia in their partners were excluded from the study. Patient medical records were categorized according to patient age: 24–34 years, 35–41 years, and 42–46 years. For each age group, 300 medical documents were selected, resulting in an analysis of IVF programs from 900 women. The data assessed included the nature and duration of infertility, prior treatments, and number and outcomes of previous IVF attempts.
Prior to entering the IVF program, the levels of AMH, antral follicle count, follicle-stimulating hormone (FSH), and partner sperm parameters were evaluated. The following metrics were recorded: the number of days of ovarian stimulation using a gonadotropin-releasing hormone antagonist; the total dose of medications administered during the ovarian stimulation program; ovulation trigger; the number of oocyte-cumulus complexes (OCC) obtained; the number and percentage of mature oocytes; the percentage of blastocyst formation; and the distribution of blastocysts by quality, according to the Gardner classification (ESHRE 2011 modified classification of D. Gardner) [17]. Additionally, a subgroup analysis was performed among patients with diminished ovarian reserves to assess the impact of age on embryological outcomes.
Statistical analysis
Data are presented as mean (M) and standard deviation (SD) – M (SD). The graphs are displayed as bar charts or histograms. The assumption of normal distribution was tested using a statistical package (D'Agostino & Pearson, Anderson–Darling, Shapiro–Wilk, Kolmogorov–Smirnov). For continuous variables, statistical analysis was performed using the nonparametric Kruskal–Wallis test followed by Dunn's test for multiple comparisons. For categorical data, the multiple Mann–Whitney test was employed, applying both versions of multiple comparisons with a P-value threshold based on the Holm-Šídák method and false discovery rate (FDR) using a two-step increase method (Benjamini, Krieger, and Yekutieli).
Results and discussion
The mean age of the women in group 1 was 29.06 (2.85) years, in group 2 – 39.01 (1.94) years in group 3 – 43.03 (1.17) years (Fig. 1A), with significant differences between the groups (p<0.001).
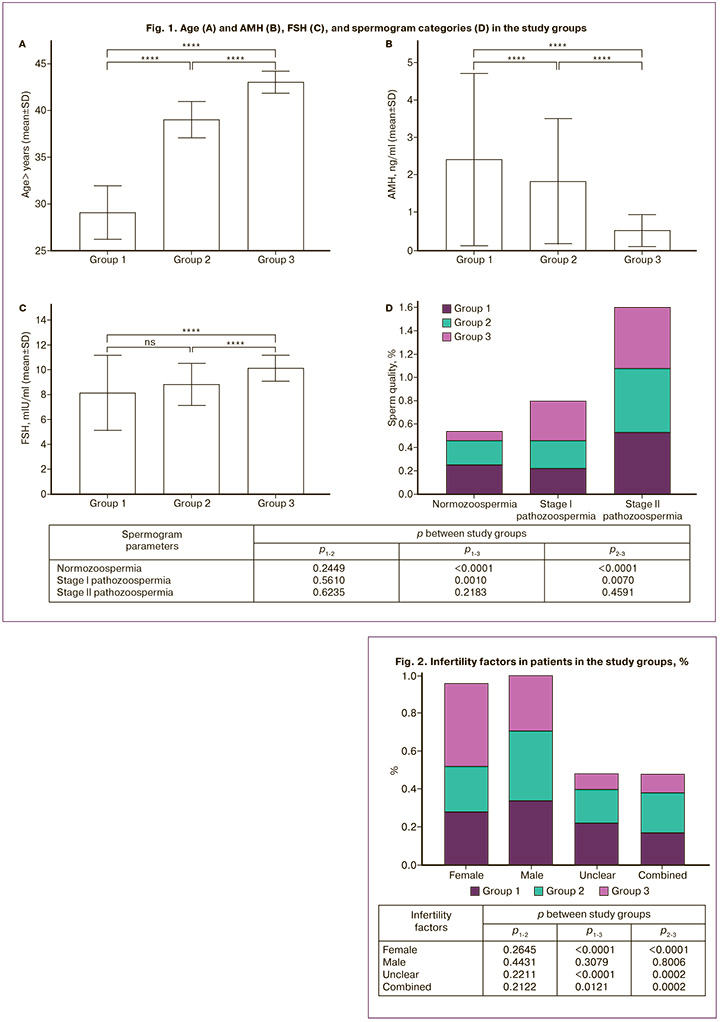
The AMH level was 2.41 (2.31), 1.8 (1.68), and 0.5 (0.43) ng/ml in groups 1, 2, and 3, respectively, with significant differences between the groups (p<0.001). Comparative analysis of AMH values in different age groups showed a significant decrease in hormone levels with age (p<0.001), which was clearly reflected in the scatterplot with 95% lower and upper confidence intervals (Fig. 1B).
It should also be noted that FSH levels ranged from 3.2 to 13.7 mIU/ml with mean values of 8.1 (3.0), 8.8 (1.67) and 10.1 (1.02) mIU/ml in groups 1, 2 and 3, respectively (Fig. 1B). Simultaneously, an increase in FSH levels was noted in older patients, with significant differences between the groups (p<0.001).
When evaluating spermograms, differences in sperm quality (normozoospermia, pathozoospermia I, and II) were found between the groups (Fig. 1G). When analyzing the causes of infertility (Fig. 2), no significant differences were found for any cause-and-effect factors between the 1st and 3rd groups. No differences were found among the three groups for male factor of infertility. At the same time, significant differences were found between the parameters of the 1st and 3rd groups, as well as the 2nd and 3rd groups regarding female, unclear, and combined factors of infertility (p<0.001). Female infertility was more common in the 3rd group compared to in the other groups.
Next, we analyzed the number of OCC obtained in the study groups by distributing the obtained oocytes by their number (Fig. 3A).
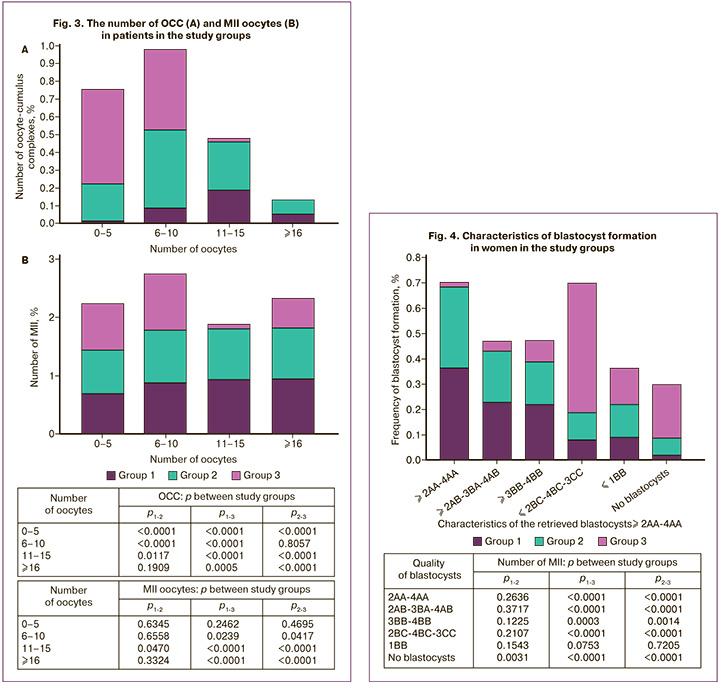
Statistically significant differences were noted between the groups in terms of the number of OCCs obtained. A small number of oocytes (0–5) was naturally obtained from women of late reproductive age. 6–10 oocytes were obtained with equal frequency in the older and middle-aged groups and less frequency in the younger group, from whom a greater number of oocytes were obtained. A large number of oocytes – 11–15 and more than 16 were obtained with equal frequency in women aged 29 to 40 years, whereas among patients aged 40 years and older, the maximum number of oocytes obtained did not exceed 10, leaning towards 5-6 oocytes (p<0.001). Moreover, in group 1, a significantly lower level of a small number (0–5 and 6–10) of oocytes was noted compared to other groups (p<0.001); In the 2nd group we mainly received 6–10 oocytes, in the 3rd group we mainly received 0–5 oocytes.
When analyzing the number of MII oocytes, a consistently identical number of this indicator was noted in the 1st and 2nd groups, regardless of the number of oocytes (Fig. 3B). Moreover, an increase in the number of oocytes retrieved from young patients in the 1st group did not lead to an increase in the percentage of mature oocytes, which allowed us to consider obtaining 11–15 oocytes as the optimal number in IVF programs (p<0.001). Older patients (group 3) had a comparable number of mature oocytes when obtaining up to 10 cells; their number sharply decreased with a smaller number of OCC.
Figure 4 presents the data of patients in the selected groups, assessing the embryological stage of the IVF programs. These data demonstrate an obvious difference in the nature of embryo development between the groups. The most striking demonstration was the quality of blastocysts obtained. Thus, in the 1st and 2nd groups, blastocysts of the 2AA-4AA, 2AB-3BA-4AB, and 3BB-4BB types were predominantly noted, whereas in the 3rd group, blastocysts were mainly of 2BC-4BC-3CC quality, as well as an increased value of the “No blastocysts” indicator (p<0.001) (Fig. 4).
The presented data demonstrate an obvious and well-known dependence of the effectiveness of IVF programs on the state of the patient’s ovarian reserve and the AMH level, which is primarily determined by the age of the woman. However, AMH levels determine the number of follicles capable of responding to gonadotropin stimulation, but not their quality and ability to form a full-fledged embryo. To assess the influence of age on the parameters of early embryogenesis, subgroups with comparable AMH levels, ovarian reserve status, and age were identified in each group. Patient characteristics are presented in Table 1. The patients did not differ significantly in the state of ovarian reserve, which was assessed as reduced in all groups.
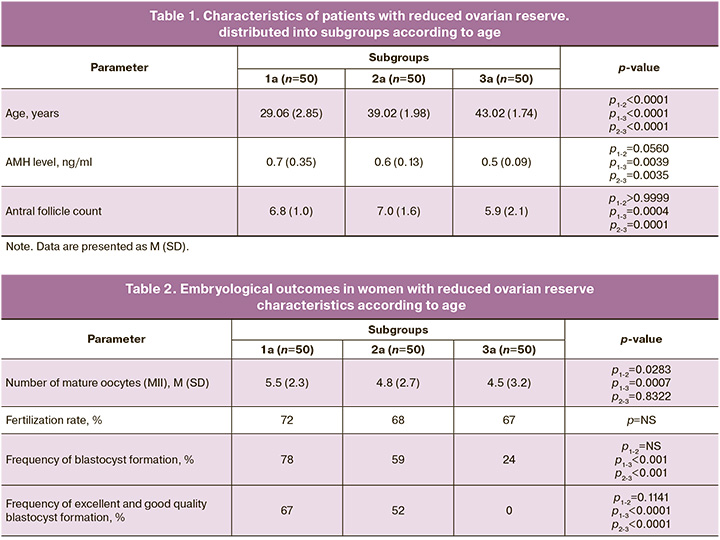
Table 2 presents the embryological stage data of the identified subgroups of women.
According to the study findings, the number of mature oocytes among the groups did not differ significantly (p>0.05), and the fertilization rates were comparable. In subgroup 1a, blastocysts were obtained from 78% of women; of these blastocysts, 67% of the embryos exhibited good or excellent quality (2AA-4AA, 2AB-3BA-4AB, and 3BB-4BB), while 33% were of low quality (2BC-4BC). In subgroup 2a, blastocysts were obtained from 59% of the patients, with good quality recorded in 52% of these cases. In subgroup 3a, only 24% of women were able to obtain blastocysts classified as 2BC-4BC-3CC quality, whereas no blastocysts were formed in the remaining 76%. Thus, despite equal initial conditions among the patients in the selected groups and a similar "poor" response to ovarian stimulation, 67% of young and 52% of middle-aged women achieved good-quality blastocysts, whereas none of the women of late reproductive age produced good-quality embryos.
The data presented indicate that both the state of the ovarian reserve and, more importantly, the patient's age are primary prognostic factors for IVF outcomes. Therefore, it is advisable to consider the woman's age when determining the approach to achieving pregnancy. Young patients have a chance of pregnancy even with reduced ovarian reserve and low AMH levels, whereas after the age of 40, even when ovarian reserve parameters are preserved, the quality of the resulting embryos declines, making it challenging to obtain a blastocyst suitable for transfer.
This study also assessed the spermogram parameters of the partners to determine the effect of pathozoospermia on early embryogenesis. An unexpected finding was that the evaluation of sperm quality (normozoospermia, pathozoospermia I, and II) revealed the best spermogram indices in the partners of patients in the 3rd group, where only normozoospermia and 1st-degree pathozoospermia were observed (p<0.001) (Fig. 1G). Meanwhile, 2nd-degree pathozoospermia was equally detected in the partners of patients in the 1st and 2nd groups. Nevertheless, the spermogram indices did not influence the results of early embryogenesis; in women of late reproductive age, high-quality blastocysts were not obtained, even with normozoospermia in the partner.
Conclusion
Therefore, even when AMH levels in young women and older patients are comparable, the proportion of good-quality blastocysts significantly decreases with age, approaching zero after the age of 40 years. This decline is likely due to the quality of oocytes, aneuploidy of embryos, and developmental disorders in patients of late reproductive ages. In contrast, young patients can still obtain good-quality embryos even with reduced ovarian reserve indicators. Therefore, we suggest implementing IVF programs for younger patients, including those with diminished ovarian reserves, and integrating this approach into state-supported guarantees for the application of ART.
References
- Российская Ассоциация Репродукции Человека. Регистр ВРТ. Отчет за 2022 год. Доступно по: https://www.rahr.ru/d_registr_otchet/RegistrVRT_2022.pdf [Russian Association of Human Reproduction. ART register. 2022 report. Available at: https://www.rahr.ru/d_registr_otchet/RegistrVRT_2022.pdf (in Russian)].
- European IVF Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embryology (ESHRE); Wyns C., De Geyter C., Calhaz-Jorge C., Kupka M.S., Motrenko T., Smeenk J. et al. ART in Europe, 2018: results generated from European registries by ESHRE. Hum. Reprod. Open. 2022; 2022(3): hoac022. https://dx.doi.org/10.1093/hropen/hoac022
- Zegers-Hochschild F., Crosby J.A., Musri C., Souza M.D.C.B., Martinez A.G., Silva A.A. et al., Reproduction LANOA. Assisted reproductive technologies in Latin America: the Latin American Registry, 2019. JBRA Assist. Reprod. 2022; 26(4): 637-58. https://dx.doi.org/10.5935/1518-0557.20220034
- Katagiri Y., Jwa S.C., Kuwahara A., Iwasa T., Ono M., Kato K. et al. Assisted reproductive technology in Japan: A summary report for 2020 by the ethics Committee of the Japan Society of obstetrics and gynecology. Reprod. Med. Biol. 2023; 22(1): e12494. https://dx.doi.org/10.1002/rmb2.12494
- Корнеева И.Е., Назаренко Т.А., Перминова С.Г., Митюрина Е.В., Цыбизова Т.И., Дашиева А.Э. Медико-социальные факторы бесплодия в России. Акушерство и гинекология. 2023; 3: 65-72. [Korneeva I.Е., Nazarenko Т.А., Perminova S.G., Mityurina E.V., Cybizova T.I., Dashieva A.E. Medical and social factors of infertility in Russia. Obstetrics and Gynecology. 2023; (3): 65-72 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.279
- Архангельский В.Н., Калачикова О.Н. Возраст матери при рождении первого ребенка: динамика, региональные различия, детерминация. Экономические и социальные перемены: факты, тенденции, прогноз. 2020; 13(5): 200-17. [Arkhangel’skiy V.N., Kalachikova O.N. Maternal age at first birth: dynamics, regional differences, determination. Economic and Social Changes: Facts, Trends, Forecast. 2020; 13(5): 200-17. (in Russian)]. https://dx.doi.org/10.15838/esc.2020.5.71.12
- Российская Ассоциация Репродукции Человека. Регистр ВРТ. Отчет за 2021 год. Доступно по: https://www.rahr.ru/d_registr_otchet/RegistrVRT_2021.pdf [Russian Association of Human Reproduction. ART register. 2021 report. Available at: https://www.rahr.ru/d_registr_otchet/RegistrVRT_2021.pdf (in Russian)].
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014; 101(3): 633-4. https://dx.doi.org/10.1016/j.fertnstert.2013.12.032.
- Farquhar C.M., Bhattacharya S., Repping S., Mastenbroek S., Kamath M.S., Marjoribanks J. et al. Female subfertility. Nat. Rev. Dis. Primers. 2019; 5(1): 7. https://dx.doi.org/10.1038/s41572-018-0058-8
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: Assessment and Treatment for People with Fertility Problems. London: Royal College of Obstetricians & Gynaecologists;2013 Feb.
- Приказ Министерства здравоохранения Российской Федерации от 31.07.2020 № 803н «О порядке использования вспомогательных репродуктивных технологий, противопоказаниях и ограничениях к их применению» (с 01.01.2021). [Order of the Ministry of Health of the Russian Federation of 31.07.2020 No. 803n "On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use" (since 01.01.2021) (in Russian)].
- Pereira N., Setton R., Petrini A.C., Lekovich J.P., Elias R.T., Spandorfer S.D. Is anti-Müllerian hormone associated with IVF outcomes in young patients with diminished ovarian reserve? Womens Health (Lond). 2016; 12(2): 185-92. https://dx.doi.org/10.2217/whe.15.102
- Li N.J., Yao Q.Y., Yuan X.Q., Huang Y., Li Y.F. Anti-müllerian hormone as a predictor for live birth among women undergoing IVF/ICSI in different age groups: an update of systematic review and meta-analysis. Arch. Gynecol. Obstet. 2023; 308(1): 43-61. https://dx.doi.org/10.1007/s00404-022-06683-1
- Adamyan L., Pivazyan L., Obosyan L., Krylova E., Isaeva S. Preimplantation genetic testing for aneuploidy in patients of different age: a systematic review and meta-analysis. Obstet. Gynecol. Sci. 2024; 67(4): 356-79. https://dx.doi.org/10.5468/ogs.24028
- Ron-El R., Sermon K., Traeger-Synodinos J. Aneuploidy in oocytes from women of advanced maternal age: analysis of the causal meiotic errors and impact on embryo development. Hum. Reprod. 2023; 38(12): 2526-35. https://dx.doi.org//10.1093/humrep/dead201
- Погосян М.Т., Назаренко Т.А., Гайсин Э.А. Анализ клинико-лабораторных характеристик пациенток с остановкой развития эмбрионов в раннем эмбриональном периоде программ экстракорпорального оплодотворения. Акушерство и гинекология. 2024; 2: 89-96. [Pogosyan M.T., Nazarenko T.A., Gaysin E.A. Clinical and laboratory characteristics of patients with embryonic arrest in the early embryonic period of in vitro fertilization programs. Obstetrics and Gynecology. 2024; (2): 89-96 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.271
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum. Reprod. 2011; 26(6): 1270-83. https://dx.doi.org/10.1093/humrep/der037
Received 19.02.2025
Accepted 21.05.2025
About the Authors
Tatyana A. Nazarenko, Dr. Med. Sci., Professor, Head of the Institute of Reproductive Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, t_nazarenko@oparina4.ru, https://orcid.org/0000-0002-5823-1667Nelli A. Khachatryan, PhD, doctor at the F. Paulsen Research and Educational Center for ART with the Clinical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, n_khachatryan@oparina4.ru
Ekaterina I. Krylova, Resident, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, kr.katrin00@gmail.com, https://orcid.org/0000-0002-0220-0474
Almina M. Biryukova, PhD, Clinical Supervisor at the F. Paulsen Research and Educational Center for ART with the Clinical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, a_birukova@oparina4.ru



