Diagnostic value of early echography in pregnant women at risk for placenta accreta
Objective: To study the diagnostic value of ultrasound assessment at 5–10 weeks gestation in women with risk factors for placenta accreta spectrum (PAS). Materials and methods: This was a retrospective analysis of the data of 181 women. Inclusion criterion was the presence of uterine scar. The ultrasound assessment was performed at 5–10 weeks gestation, the women gave birth at Regional Clinical Hospital No. 2, Krasnodar, Russia from 2017 to 2019. We studied the relationship between PAS and potential predictors, including ultrasound signs (low position of the gestational sac in the uterine cavity; implantation of the gestational sac in the scar; hypervascular pattern at the implantation site; bulge of the uterine wall at the implantation site; subchorionic hematoma) and maternal clinical and demographic parameters (age, parity, number of cesarean sections and other surgeries of the uterus). Results: Diagnostic significance for the prediction of PAS in pregnant women was revealed for cesarean section in history (OR=2.30; 95% CI: 1.50–3.53, p<0.001), low position of gestational sac (OR=17.70; 95% CI: 5.87–53.40, p<0.001); increase in the number of ultrasound signs (+1) (OR=7.37; 95% CI: 3.74–14.53, р<0.001). The absence of ultrasound signs had OR=0.102; 95% CI: 0.049–0.21, p<0.001. Parity and hematoma in the uterine cavity had significant differences only for invasive forms of PAS (increta, percreta). According to ultrasound data at 5-10 weeks gestation, a low position of the gestational sac in combination with the presence of a hematoma in the uterine cavity is suggestive of PAS in pregnant patients with ≥1 scar after cesarean section with Se=61.11 (95% CI: 48.89–72.38); Sp=88.07 (95% CI: 80.47–93.49); LR(+)=5.12 (95% CI: 2.98–8.81); LR(-)=0.44 (95% CI: 0.33–0.59); PPV=98.98 (95% CI: 98.26–99.41); NPV=10.65 (95% CI: 8.13–13.83). Conclusion: Ultrasound assessment at an early stage of pregnancy can be used for selective screening of PAS.Makukhina T.B., Penzhoyan G.A., Dontsova M.V., Krivonosova N.V.
Keywords
Early diagnosis of placenta accreta spectrum (PAS) is important for timely referral of a pregnant woman to a large center specialized in this pathology where it is possible to solve such diagnostic and therapeutic issues using a multidisciplinary approach, to clarify the diagnosis, assess the risks of complications, and determine individualized treatment tactics [1–6].
Screening for abnormal placentation includes an obligatory assessment of the placenta location relatively to the uterine scar during the ultrasound assessment in the second trimester of pregnancy [7, 8]. However, PAS signs can be determined using ultrasound data in 90% of pregnant women who have this pathology already in the first trimester [9–11]. The disadvantages of the previous studies are the small sample size, heterogeneity of ultrasound signs and gestational age at the time of the study, inclusion of only confirmed cases of PAS, as well as the lack of information about the specificity of ultrasound assessment.
The aim of the study was to identify the diagnostic value of ultrasound assessment at 5–10 weeks gestation in women with risk factors for PAS.
Materials and methods
This was a retrospective analysis of the data of pregnant women who gave birth at Regional Clinical Hospital No. 2, Krasnodar, Russia from 2017 to 2019.
We analyzed the results of the ultrasound assessment performed at 5–10 weeks gestation (before the combined screening period of the first trimester); the results were taken from the electronic database of the Perinatal Diagnostics Centre of Regional Clinical Hospital No. 2, Krasnodar. The data on the outcome of childbirth were obtained from the records on labor archived in the Perinatal Center. The gestational age at the time of delivery was taken into account; the presence of PAS and its depth were considered after histological examination of the surgical material.
The inclusion criteria are pregnancy, previous history of at least one cesarean section (CS) and/or another uterine surgery, pelvic ultrasound assessment performed at 5–10 weeks gestation, childbirth in Perinatal Centre of Regional Clinical Hospital No. 2, Krasnodar. The exclusion criterion is the absence of ultrasound results available for analysis till 11 weeks gestation.
The data on the presence of ultrasound signs of PAS were introduced into the protocol prospectively during the initial ultrasound assessment. Two specialists in ultrasound diagnostics who did not have the data on the outcome of pregnancy and histological examination of the surgical material during childbirth analyzed the descriptions and images (if they were available) independently. The descriptions and images were classified depending on the ultrasound signs.
The indications for performing ultrasound assessment before 11 weeks gestation were identification of the gestational age in case of irregular menstrual cycle, vaginal blood discharge, including spotting, nagging pains in the lower abdomen, the patient’s desire, etc. Transvaginal echography was performed in all cases. In all cases ultrasound assessment was performed using GE Voluson E6 (GE Healthcare) or echographic equipment with 4.0–9.0 MHz endocavital transducers (Accuvix A30, Samsung Medison). When performing Doppler mapping, the ALARA safety principle was observed, thermal safety index (TIb) < 1.0. All PAS cases were verified according to the FIGO classification (clinically and histologically) [12, 13].
Since the study was retrospective, informed consent of the patients was not necessary; the data were depersonalized and used for scientific purposes.
The STARD (Standards for Reporting of Diagnostic Accuracy Studies) protocol for diagnostic studies was observed [14].
We studied the relationship between PAS and potential predictors, including five ultrasound signs (low position of the gestational sac in the uterine cavity; implantation of the gestational sac in the scar; hypervascular pattern at the implantation site; bulge of the uterine wall at the implantation site; subchorionic hematoma) and four maternal clinical and demographic parameters (age, parity, number of cesarean sections and other surgeries of the uterus) [15].
Statistical analysis
The statistical analysis was performed according to the method of Cali G. et al. (2018) [15]. Initially, a one-dimensional statistical analysis was carried out and the patients were divided into some groups due to the parameters (without PAS, with PAS and by the depth of invasion): chi-square test (χ2) with Yates’ correction or Fisher’s exact test (if there are expected frequencies n<5 in the conjugacy table) for categorical parameters, Student’s t-test (for numerical data with normal distribution) and Mann–Whitney U-test (for numerical data which are not normally distributed). The distribution was estimated using the Shapiro–Wilk test.
The number of CS and the number of ultrasonic signs of PAS were included in the analysis as a separate type calculated for each sign and after dichotomization. The number of CS was divided into three categories using the dichotomization method: 1) none/ one, 2) two, 3) three or more; the number of ultrasound signs was divided into three categories: 1) none, 2) one, 3) two or more.
Further, a gradual logistic regression analysis was carried out to identify potential independent predictors of PAS (an exclusion method based on the calculation of the likelihood ratio was used). The following parameters were used as predictors: age, parity, number of CS, presence of other operations on the uterus. The risk assessment of abnormal placentation based on ultrasound signs was corrected taking into account the influence of the listed predictors. All predictors were tested for inclusion in the final model, where only parameters significant for one-dimensional analysis were saved.
The Hosmer–Lemeshow goodness-of-fit test was used to check the accuracy of the model approximation to the initial data. The hypothesis of model consistency was accepted at p>0.05. Predictive value was calculated using ROC analysis (area under the receiving–operating characteristics curve). There were no missing values in the data, so there was no need to use the missing values replacement method.
The potential significance of each ultrasound sign in the PAS diagnosis was assessed using regression models. We determined sensitivity (Se), specificity (Sp), positive and negative likelihood ratios (LR+ and LR-), positive and negative predictive values (PPV and NPV), as well as odds ratio (OR) when constructing a multiple regression model (adjusted odds ratio, AjOR) with the definition of 95% confidence interval (CI). Statistical difference was considered to be significant at the level of p<0.05. The analysis was performed using SPSS 26.0 (StataCorp, College Station, TX, USA, 2013).
Results
After applying the exclusion criteria, 181 patients with CS or other uterine surgery were included in the analysis (20 pregnant women did not have a previous history of CS) (Figure).
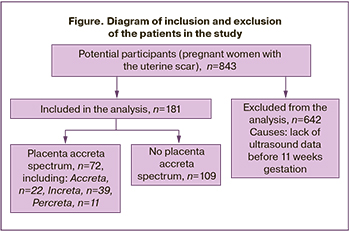
PAS was confirmed in 39.8% of patients (95% CI: 37.2–51.3%), including placenta accreta – in 12.2% (95% CI: 8.0–17.5%), increta – in 21.5% (95% CI: 16.0–28.0%), and percreta – in 6.1% (95% CI: 3.3–10.3%). The comparative analysis of the parameters depending on the presence and depth of PAS is presented in Table 1.
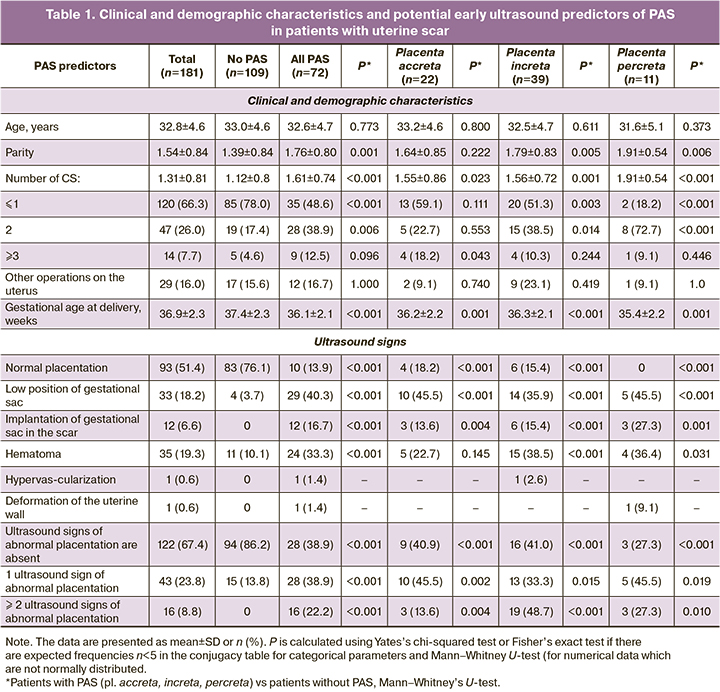
The comparative analysis did not reveal any age differences between patients with PAS and normal placentation (32.6±4.7 vs 33.0±4.6; p=0.773). Pregnant women with PAS had a higher parity (1.76±0.80 vs 1.39±0.84; p=0.001), a greater number of CS in the history (1.61±0.74 vs 1.12±0.80; p<0.001), as well as a lower gestational age at the time of delivery (36.1±2.1 vs 37.4±2.3; p<0.001). The difference remained when the PAS depth was stratified (except for placenta accreta), when parity was compared (p=0.222) and when the number of CS was ≤ 2, which can be due to the small sample size in groups with different depth of PAS. Other uterine operations were not associated with any group (PAS or normal placentation).
Such ultrasound signs as the low position of the gestational sac, implantation of the gestational sac on the scar and subchorionic hematoma were significantly more common in the group of patients with PAS compared with the patients with normal placentation (Table 1).
According to the logistic regression analysis, an increase in the number of births and CS in the history and a combination of abnormal ultrasound signs (>1) were independently associated with the presence of PAS (Table 2). All cases with the combination of ≥2 abnormal ultrasound signs which were included in the sample were associated with PAS. It was not possible to provide a statistical assessment of the PAS risk in comparison with normal placentation with a combination of several ultrasound signs, since there were no cases of such a combination in the group of patients without PAS.
The logistic regression method was used to assess the value of the predictor «≥2 ultrasound signs of abnormal placentation» for early diagnosis of the depth of placental invasion but only in the sample with PAS. The analysis did not reveal any significant differentiation of this predictor depending on the depth of invasion. But it should be noted that there was a small number of observations with such a combination of signs in the sample and it could affect the results (n=16, most of them (n=10) were cases of placenta increta). Subchorionic hematomas were significantly more often described in ultrasound protocols of patients with invasive forms of PAS (placenta increta, p<0.001; placenta percreta, p=0.031) (Table 2). The presence of at least one ultrasound sign of abnormal placentation, especially the low position of the gestational sac (OR=17.70, 95% CI: 5.87-53.40); p<0.001) was of the greatest value for the diagnosis of placenta percreta (Table 2).
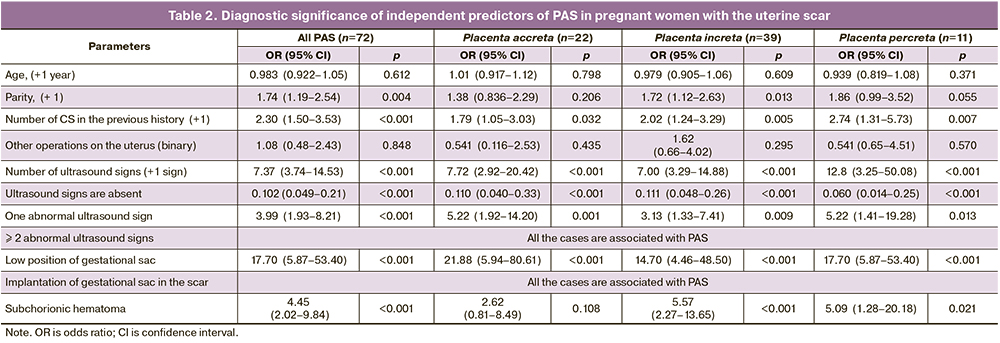
The value of OR for the parameter «one abnormal ultrasound sign» turned out to be less than OR for individual ultrasound signs of abnormal placentation (Table 2) due to the fact that the positive values of this parameter cover all possible ultrasound signs of abnormal placentation, including those that were rarely found in the sample (hypervascularization of the placentation site and deformation of the uterine wall).
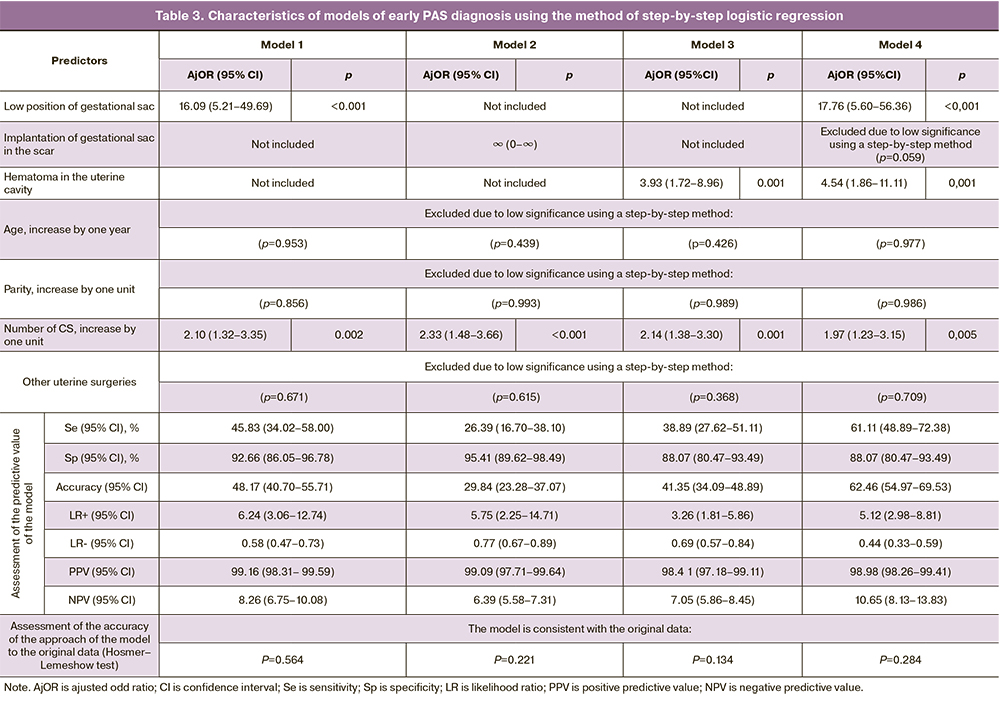
Four experimental models of PAS diagnosis were constructed using multivariate regression modeling. They were based on ultrasound signs of abnormal placentation according to echography data up to 11 weeks gestation depending on the clinical and anamnestic data (Table 3). The comparison of diagnostic parameters showed that the optimal ratio of Se, Sp, PPV and NPV levels for early PAS diagnosis is presented in model 4. This model includes two ultrasound signs, namely «low position of the gestational sac» (AjOR=17.76 (95% CI: 5.60–56.36), p<0.001) and the presence of subchorionic hematoma (AjOR=4.54 (95% CI 1.86–11.11), p=0.001) with compulsory analysis of the influence of the number of CS (Table 3). Inclusion of only one ultrasound sign of abnormal placentation in the diagnostic model reduces sensitivity by 33% when the sign «low position of the gestational sac» is included (model 1), by 2.3 times when the sign «implantation of the gestational sac on the scar» is included (model 2) and by 1.5 times when the sign «subchorionic hematoma» is included (model 3). Such parameters as age, parity, and a history of other operations on the uterus did not have a statistically significant effect on the diagnostic capabilities of the model. All constructed models are consistent with the observed data (Hosmer–Lemeshow test) (Table 3).
Discussion
Numerous studies demonstrate the importance of early ultrasound (up to 8 weeks gestation) for screening and diagnosis of pathological implantation of the gestation sac [11, 16–22], however, most of them are based on retrospective studies [9, 10, 18].
The research conducted by Cali G.et al. (2018) [15] showed significant parameters for the diagnosis of PAS when performing ultrasound at 11–14 weeks gestation in women who had placenta previa in the third trimester. However, it is known that most cases of chorion/placenta previa in the first and second trimesters tend to have a normal position of the placenta by the third trimester [23, 24]. Therefore, this analysis did not include the cases when there could have been the signs of abnormal invasion in the first trimester leading to a false positive diagnosis but excluded during the subsequent assessment of the placenta position in the third trimester.
In addition, the diagnosis of PAS at the end of the first trimester in the presence of the chorionic invasion limits the possibilities of a differentiated approach to the further management of patients. On the one hand, the termination of such a pregnancy is associated with significant technical difficulties in complete removal of the gestational sac and reliable intraoperative hemostasis [20, 22, 25, 26]. On the other hand, it is more difficult for a woman psychologically to give a consent to termination of pregnancy after the end of the period of embryogenesis with a developed fetus.
Early diagnosis is critically important because serious consequences for a woman’s reproductive health can be prevented during the course of pregnancy if a woman is referred to a specialized center for individualized treatment tactics. In a high-risk population, the prognostic value of any screening test will be higher, since the screened condition is more common [27]. The study conducted by Panaiotova J. et al. (2019) [28] showed that 1298 patients at high risk of PAS were examined at 12–16 weeks gestation and among 14 diagnosed cases of PAS there were 13 women whose diagnosis was confirmed during delivery. Thus, the prognostic value of a positive ultrasound test was 92.9%. This result was predetermined by the expert level of the study which was carried out in standardized conditions by a small group of specialists (3 experts).
Risk assessment enables a woman to make an appropriate decision about carrying a pregnancy or early elimination of the gestational sac. Clinical studies demonstrate a significantly lower number of complications with early termination of pregnancy and low implantation [20–22, 25, 26]. This is probably due to the fact that the process of trophoblast invasion with remodeling of large vessels of the myometrium in the early stages of pregnancy has not been fully realized yet. This hypothesis is confirmed in recent studies demonstrating that low implantation of the gestational sac in the early stages (<8 weeks gestation) does not have macroscopic ultrasound signs of invasion of the myometrium, which are present at 11–14 weeks gestation [29].
Our study revealed a high level of agreement among specialists interpreting ultrasound signs. No discrepancies were found in the assessment of the cases between the doctors who performed the ultrasound and the researchers who carried out the repeated analysis of the data of the stored ultrasound images. At the same time, during the repeated analysis, experts noted the presence of additional signs of the pathology in 13 (20.6%) women with PAS.
Comparable results were published in a study by Ali G. et al. (2018) [15]. The authors also found no discrepancies between the interpretation of ultrasound data during the initial study and the repeated examination performed by two researchers. At the same time, the analysis of data in another study [30] showed that repeated assessment of early ultrasound signs of PAS by experts led to a decrease in the number of suspected cases of PAS from 25 (5.4%) to 11 (2.4%), which increased sensitivity from 75% (95% CI: 34.9–96.8%) to 87.5% (95% CI: 47.3–99.7%) and the prognostic value of a positive test from 24.0 (95% CI: 14.8–36.4) to 63.6 (95% CI: 38.9–82.8), respectively. The high level of coincidence in our study can be explained by the fact that the study was performed by doctors of the Perinatal Diagnostic Center specialized in the management and delivery of patients with PAS and these doctors have sufficient clinical experience in performing such studies. Our data prove that the combination of several ultrasound signs increases the diagnostic accuracy of the model. Thus, it is advisable to verify the initial conclusion about the pathological implantation of the gestational sac in the period up to 11 weeks gestation by expert ultrasound assessment and consultation in the shortest possible time to determine further clinical tactics.
Conclusion
The combination of a low position of the gestational sac and the presence of a hematoma in the uterine cavity according to ultrasound data at 5-10 weeks gestation makes it possible to diagnose PAS during the course of pregnancy if likelihood ratio is LR(+)=5.12 (95% CI: 2.98–8.81), LR(-)=0.44 (95% CI: 0.33–0.59), positive predictive value is 98.98 (95% CI: 98.26–99.41); negative predictive value is 10.65 (95% CI: 8.13–13.83) in women with ≥ 1 scar after CS. Thus, ultrasound assessment at an early stage of pregnancy can be used for selective screening of PAS.
The strengths of the study are prospective data as it was possible to include in the analysis patients with a normal placenta position in the third trimester of pregnancy, since in the first trimester of pregnancy it is impossible to accurately predict the placenta previa by the end of pregnancy; objective risk factors for PAS (the presence of a scar on the uterus after CS, or other surgery on the uterus) made it possible to reduce the risk of a mistake in determining the diagnostic capabilities of the test after evaluating the analyzed parameters in the group formed before the test; stratification of the analysis by the degree of invasion of the placenta and evaluation of specificity.
The limitations of the study are retrospective design, exclusion of a large number of patients from the study due to the lack of saved early ultrasound data for retrospective analysis, the lack of data on standardization of Doppler unit settings during the study, the inability to evaluate all ultrasound signs suggestive of PAS, in the absence of saved images. The study did not include the patients whose pregnancy was terminated before the stage of viability (miscarriages, pregnancy in the scar after CS, elective abortion, PAS with bleeding up to 22 weeks gestation). The results are relevant only to women with a previous uterine scar. However, PAS is known to occur even in women without classical risk factors.
The clinical significance of the data of early selective ultrasound screening of PAS in patients with a uterine scar requires validation by large-scale prospective studies.
References
- Баринов С.В., Медянникова И.В., Тирская Ю.И., Безнощенко Г.Б., Кадцына Т.В., Лазарева О.В., Биндюк А.В., Неустроева Т.Н., Степанов С.С. Прогнозирование приращения плаценты при ее предлежании. Акушерство и гинекология. 2021; 1: 61-9. [Barinov S.V., Medyannikova I.V., Tirskaya Yu.I., Beznoshchenko G.B., Kadtsyna T.V., Lazareva O.V., Bindyuk A.V., Neustroeva T.N., Stepanov S.S. Prediction of placenta accreta in case of placenta previa. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2021; 1: 61-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.1.61-69.
- Курцер М.А., Григорьян А.М. Временная баллонная окклюзия при врастании плаценты. Эндоваскулярная хирургия. 2020; 7(2): 113-22. [Kurtser M.A., Grigoryan A.M. Temporary balloon occlusion in the placenta accreta. Endovaskulyarnaya hirurgiya/Endovascular surgery. 2020; 7(2): 113-22. (in Russian)]. https://dx.doi.org/10.24183/2409-4080-2020-7-2-113-122.
- Лисицына О.И., Низяева Н.В., Михеева А.А. Врастание плаценты. Эволюция знаний и умений. Акушерство и гинекология. 2021; 6: 34-40. [Lisitsyna O.I., Nizyaeva N.V., Mikheeva A.A. Placenta accreta. Evolution of knowledge and skills. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2021; 6: 34-40. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.6.34-40.
- Пенжоян Г.А. Служба родовспоможения в крупном городе. Проблемы социальной гигиены, здравоохранения и истории медицины. 2003; 3: 37. [Penzhoyan G.A. Obstetrics service in a large city. Problemy social'noj gigieny, zdravoohraneniya i istorii mediciny/Problems of social hygiene, health care and the history of medicine. 2003; 3: 37 (in Russian)].
- Пенжоян Г.А. Эффективность современных перинатальных технологий. Проблемы социальной гигиены, здравоохранения и истории медицины. 2002; 6: 42. [Penzhoyan G.A. The effectiveness of modern perinatal technologies. Problemy social'noj gigieny, zdravoohraneniya i istorii mediciny/Problems of social hygiene, health care and the history of medicine. 2002; 6: 42 (in Russian)].
- Марченко Р.Н., Кукарекая И.И., Ербактанова Т.А. Эффективность эмболизации маточных артерий у пациенток с приращением плаценты. Consilium Medicum. 2020; 22(6): 25-7. [Marchenko R.N., Kukarekaya I.I., Erbaktanova T.A. The effectiveness of uterine artery embolization in patients with placenta accreta. Consilium Medicum. 2020; 22(6): 25-7. (in Russian)]. https://dx.doi.org/10.26442/20751753.2020.6.200303.
- Министерство здравоохранения Российской Федерации. Приказ «Об утверждении Порядка оказания медицинской помощи по профилю "акушерство и гинекология"» от 20.10.2020 №1130н (зарегистрирован 12.11.2020 №60869). [Ministry of Health of the Russian Federation. Order "On approval of the Procedure for providing medical care in the profile "obstetrics and gynecology"" dated 10/20/2020 No. 1130n (registered on 12.11.2020 No. 60869). (in Russian)].
- Гус А.И., Бойкова Ю.В., Ярыгина Т.А., Яроцкая Е.Л. Современные подходы к пренатальной диагностике и скринингу врастания плаценты (обзор рекомендаций). Акушерство и гинекология. 2020; 10: 5-12. [Gus A.I., Boykova Yu.V., Yarygina T.A., Yarotskaya E.L. Modern approaches to prenatal diagnosis and screening of placenta accreta (review of recommendations). Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2020; 10: 5-12. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.10.5-12.
- Kaelin Agten A., Cali G., Monteagudo A., Oviedo J., Ramos J., Timor-Tritsch I. The clinical outcome of cesarean scar pregnancies implanted "on the scar" versus "in the niche". Am. J. Obstet. Gynecol. 2017; 216(5): 510.e1-510.e6. https://dx.doi.org/10.1016/j.ajog.2017.01.019.
- D'Antonio F., Timor-Tritsch I.E., Palacios-Jaraquemada J., Monteagudo A., Buca D., Forlani F. et al. First-trimester detection of abnormally invasive placenta in high-risk women: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018; 51(2): 176-83. https://dx.doi.org/10.1002/uog.18840.
- Timor-Tritsch I.E., D'Antonio F., Calí G., Palacios-Jaraquemada J., Meyer J., Monteagudo A. Early first-trimester transvaginal ultrasound is indicated in pregnancy after previous Cesarean delivery: should it be mandatory? Ultrasound Obstet. Gynecol. 2019; 54(2): 156-63. https://dx.doi.org/10.1002/uog.20225.
- Jauniaux E., Ayres-de-Campos D.; FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Introduction. Int. J. Gynaecol. Obstet. 2018; 140(3): 261-4. https://dx.doi.org/10.1002/ijgo.12406.
- Jauniaux E., Ayres-de-Campos D., Langhoff-Roos J., Fox K.A., Collins S.; FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int. J. Gynaecol. Obstet. 2019; 146(1): 20-4. https://dx.doi.org/10.1002/ijgo.12761.
- Bossuyt P.M., Reitsma J.B., Bruns D.E., Gatsonis C.A., Glasziou P.P., Irwig L. et al.; STARD Group. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015; 351: h5527. https://dx.doi.org/10.1136/bmj.h5527.
- Cali G., Forlani F., Foti F., Minneci G., Manzoli L., Flacco M.E., Buca D., Liberati M., Scambia G., D'Antonio F. Diagnostic accuracy of first-trimester ultrasound in detecting abnormally invasive placenta in high-risk women with placenta previa. Ultrasound Obstet. Gynecol. 2018; 52(2): 258-64. https://dx.doi.org/10.1002/uog.19045.
- Comstock C.H., Bronsteen R.A. The antenatal diagnosis of placenta accreta. BJOG. 2014; 121(2): 171-81; discussion 181-2. https://dx.doi.org/10.1111/1471-0528.12557.
- Gonzalez N., Tulandi T. Cesarean scar pregnancy: A systematic review. J. Minim. Invasive Gynecol. 2017; 24(5): 731-8. https://dx.doi.org/10.1016/j.jmig.2017.02.020.
- Timor-Tritsch I.E., Monteagudo A., Cali G., El Refaey H., Kaelin Agten A., Arslan A.A. Easy sonographic differential diagnosis between intrauterine pregnancy and cesarean delivery scar pregnancy in the early first trimester. Am. J. Obstet. Gynecol. 2016; 215(2): 225.e1-7. https://dx.doi.org/10.1016/j.ajog.2016.02.028.
- Calì G., Timor-Trisch I.E., Palacios-Jaraquemada J., Monteaugudo A., Forlani F., Minneci G. et al. Changes in ultrasonography indicators of abnormally invasive placenta during pregnancy. Int. J. Gynaecol. Obstet. 2018; 140(3): 319-25. https://dx.doi.org/10.1002/ijgo.12413.
- Цхай В.Б., Яметов П.К., Вергунов Н.А. Беременность в рубце на матке после кесарева сечения. Современное состояние проблемы. Диагностика. Клиника. Врачебная тактика. Акушерство и гинекология. 2017; 3: 5-10. [Tskhay V.B., Yametov P.K., Vergunov N.A. Cesarean scar pregnancy. Current state of the problem. Diagnostics. Clinical symptoms. Medical tactics. Akusherstvo i ginekologiya/ Obstetrics and gynecology. 2017; 3: 5-10 (in Russian)]. https://dx.doi.org/10.18565/aig.2017.3.5-10.
- Makukhina T.B., Makukhina V.V. Ectopic low-lying implantation pregnancy: analysis of outcomes depending on gestation age. Rus. Open Med. J. 2019; 8: e0208. https://dx.doi.org/10.15275/rusomj.2019.0208.
- Пенжоян Г.А, Макухина Т.Б., Мингалева Н.В., Солнцева А.В., Амирханян А.М. Менеджмент пациенток с врастанием плаценты на разных сроках гестации. Акушерство и гинекология: новости мнения, обучение. 2019; 7(1): 79-84. [Penzhoyan G.A., Makukhina T.B., Mingaleva N.V., Solntseva A.V., Amirkhanyan A.M. Management of patients with abnormal invasive placenta in different gestation age. Akusherstvo i ginekologia: novosti, mneniya, obuchenie/Obstetrics and Gynecology: News, Opinions, Training. 2019; 7(1): 79-84. (in Russian)]. https://dx.doi.org/10.24411/2303-9698-2019-11011.
- Демидов В.Н., Гус А.И., Ярыгина Т.А. О возможности высокоточной диагностики врастания плаценты в рубец на матке после кесарева сечения при ультразвуковом исследовании. Пренатальная диагностика. 2020; 19(4): 336-42. [Demidov V.N., Gus A.I., Yarygina T.A. On the possibility of high-precision diagnosis of placenta accreta in the uterine scar after cesarean section by ultrasound. Prenatal'naya diagnostika/Prenatal diagnosis. 2020; 19(4): 336-42 (in Russian)]. https://dx.doi.org/10.21516/2413-1458-2020-19-4-336-342.
- Гаязов Д.Р., Терегулова Л.Е., Терегулов А.Ю., Юсупова А.Ф. Лучевая диагностика и органосохраняющие операции при приращении плаценты. Практическая медицина. 2017; 7: 22-5. [Gayazov D.R., Teregulova L.E., Teregulov A.Yu., Yusupova A.F. Radiation diagnostics and organ-preserving operations in placental accreta. Prakticheskaya medicina/Practical medicine. 2017; 7(108): 22-5 (in Russian)].
- Буянова С.Н., Щукина Н.А., Чечнева М.А., Пучкова Н.В., Земскова Н.Ю., Торобаева М.Т. Беременность в рубце после кесарева сечения: возможности хирургической коррекции. Российский вестник акушера-гинеколога. 2020; 20(6): 65-70. [Buyanova S.N., Shchukina N.A., Chechneva M.A., Puchkova N.V., Zemskova N.Yu., Torobaeva M.T. Pregnancy in the scar after caesarean section: the possibility of surgical correction. Rossijskij vestnik akushera-ginekologa/Russian Bulletin of obstetrician-gynecologist. 2020; 20(6): 65-70. (in Russian)]. https://dx.doi.org/10.17116/rosakush20202006165.
- Сонова М.М., Гашенко В.О., Ласкевич А.В., Торубаров С.Ф. Эктопическая беременность в рубце на матке после кесарева сечения. Проблемы репродукции. 2018; 24(1): 42-7. [Sonova M.M., Gashenko V.O., Laskevich A.V., Torubarov S.F. Ectopic pregnancy in the uterine scar after caesarean section. Problemy reprodukcii/Problems of Reproduction. 2018; 24(1): 42-7. (in Russian)]. https://dx.doi.org/10.17116/repro201824142-47.
- Николаидес К. Ультразвуковое исследование в 11–13+6 недель беременности. Пер. с англ. Михайлова А.В., Некрасовой Е.С. СПб.: ИД «Петрополис»; 2007. 144с. [Nikolaides K. Ultrasound examination at 11–13+6 weeks of pregnancy. Translation from English by Mikhailov A.V., Nekrasova E.S. S-Pb.: Publishing House "Petropolis"; 2007. 144 р. (in Russian)].
- Panaiotova J., Tokunaka M., Krajewska K., Zosmer N., Nicolaides K.H. Screening for morbidly adherent placenta in early pregnancy. Ultrasound Obstet. Gynecol. 2019; 53(1):101-6. https://dx.doi.org/10.1002/uog.20104.
- D'Antonio F., Cali G., Palacios-Jaraquemada J., Khalil A., Timor-Tritsch I.E. Cesarean scar pregnancy is associated with abnormal implantation but not macroscopic myometrial invasion in early first trimester of pregnancy. Ultrasound Obstet. Gynecol. 2022; 59(4): 550-1. https://dx.doi.org/10.1002/uog.24790.
- Doulaveris G., Ryken K., Papathomas D., Trejo F.E., Meislin R., Rotenberg O., Dar P. OC30.04: Performance of transvaginal ultrasound between 11–14 weeks in women with prior Caesarean delivery for early prediction of placenta accreta spectrum. Ultrasound Obstet. Gynecol. 2019; 54(Suppl. 1): 79-80.
Received 12.03.2022
Accepted 06.05.2022
About the Authors
Tatiana B. Makukhina, PhD, Associate Professor, Associate Professor of the Department of Obstetrics, Gynecology and Perinatology, Faculty of Advanced Training and Professional Retraining of Specialists, Kuban State Medical University, Ministry of Healthcare of the Russian Federation, 350063, Russian Federation, Krasnodar, Sedina str., 4; specialist of ultrasound diagnostics at center of perinatal diagnostics of Perinatal Center, Regional Clinical Hospital No. 2, 350012, Russian Federation,Krasnodar, Krasnykh Partisan str., 6/2, soltatiana@mail.ru, https://orcid.org/0000-0003-0536-4500
Grigoriy A. Penzhoyan, Dr. Med. Sci., Professor, Head of the Department of Obstetrics, Gynecology and Perinatology, Faculty of Advanced Training and
Professional Retraining of Specialists, Kuban State Medical University, Ministry of Healthcare of the Russian Federation, 350063, Russian Federation, Krasnodar, Sedina str., 4;
obstetrician-gynecologist at Perinatal Center, Regional Clinical Hospital No. 2, 350012, Russian Federation, Krasnodar, Krasnykh Partisan str., 6/2, pga05@mail.ru,
https://orcid.org/0000-0002-8600-0532
Maria V. Dontsova, PhD (sociological sciences), Associate Professor, Associate Professor at the Department of Sociology, Kuban State University, Ministry of Science and Higher Education of the Russian Federation, 350040, Russian Federation, Stavropolskaya str., 149, dontsova@yandex.ru, https://orcid.org/ 0000-0003-0957-2200
Natalia V. Krivonosova, PhD, Associate Professor, Associate Professor of the Department of Obstetrics, Gynecology and Perinatology Faculty of Advanced Training and Professional Retraining of Specialists, Kuban State Medical University, Ministry of Healthcare of the Russian Federation, 350063, Russian Federation,
Krasnodar, Sedina str., 4, natalja.krivonosova@yandex.ru, https://orcid.org/0000-0002-8222-5670
Corresponding author: Tatiana B. Makukhina, soltatiana@mail.ru
Authors’ contributions: Makukhina T.B., Penzhoyan G.A. – developing the concept and design of the study; Makukhina T.B., Krivonosova N.V. – collecting and processing the material; Dontsova M.V. – statistical data processing; Makukhina T.B. – writing the text; Penzhoyan G.A. – editing the text.
Conflicts of interest: The authors declare that they have no competing interests.
Funding: The study was performed without external funding.
Patient Consent for Publication: The retrospective design of the study did not require patients’ informed consent to statistical processing of depersonalized data for subsequent publication.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Makukhina T.B., Penzhoyan G.A., Dontsova M.V., Krivonosova N.V.
Diagnostic value of early echography in pregnant women at risk for placenta accreta.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 5: 74-82 (in Russian)
https://dx.doi.org/10.18565/aig.2022.5.74-82



