Peripheral blood NK cells in patients with uterine scars and abnormally adherent and invasive placenta
Ponikarova N.Yu., Davydova A.A., Shelepova E.S., Osipova N.A., Sokolov D.I., Mikhailova V.A., Oganyan K. A., Selkov S.A., Zazerskaya I.E.
Objective: The aim of the study was to evaluate cytotoxic activity of peripheral blood NK cells against trophoblast cells in pregnant women with uterine scar and with or without abnormally adherent and invasive placenta (AAIP).
Materials and methods: The study included 18 pregnant women who were divided into 3 groups. The main group (n=6) included women with uterine scar after cesarean section and AAIP. The comparison group (n=6) included women with uterine scar and without AAIP. The control group (n=6) included pregnant women without uterine scar. The women were enrolled in the study at 28–32 weeks of pregnancy. Cytotoxic activity of peripheral blood NK cells against trophoblast cells of the JEG-3 line was evaluated in all patients.
Results: There were no differences in cytotoxic activity of NK cells between the group of women with AAIP and the comparison and control groups. However, the cytotoxic activity of pNK was lower in the group of pregnant women who underwent cesarean section in history versus women with normal pregnancy (p= p=0.022).
Conclusion: The data obtained in this study suggest that reduced cytotoxic activity of pNK cells in women with uterine scar and without AAIP is due to the mechanism that prevents adherence of placenta in a number of patients in risk group.
Authors’ contributions: Ponikarova N.Yu. – data collection and analysis, manuscript drafting; Davydova A.A. – cell culture work and and processing of biological material; Shelepova E.S., Osipova N.A. – data collection and analysis; Sokolov D.I., Mikhailova V.A., Selkov S.A. – manuscript editing; Oganyan K.A. – literature review, preparation of the manuscript for publication; Zazerskaya I.E. – the concept and design of the study, manuscript editing.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The study was supported by Almazov National Medical Research Centre of the Ministry of Health of Russia; State Assignment 123 021 000 051-2 “Development of risk assessment scale for abnormally adherent and invasive placenta in pregnant women with uterine scars”.
Ethical Approval: The protocol of the study was approved by the local Ethics Committee at Almazov National Medical Research Centre of the Ministry of Health of Russia (minutes of the meeting No. 11-22 of November 2022, protocol No. 2211-22)
Patient Consent for Publication: The patients have signed informed consent for participation in the study and publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Ponikarova N.Yu., Davydova A.A., Shelepova E.S., Osipova N.A., Sokolov D.I., Mikhailova V.A., Oganyan K.A., Selkov S.A., Zazerskaya I.E. Peripheral blood NK cells in patients with uterine scars and abnormally adherent and invasive placenta.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2025; (1): 66-72 (in Russian)
https://dx.doi.org/10.18565/aig.2024.303
Keywords
Currently, the pathogenetic mechanisms of abnormally adherent and invasive placenta (AAIP) remain not fully understood [1]. The main predictor of the development of AAIP is considered to be uterine scar after cesarean section in history. However, abnormal placental invasion occurs not in all cases. According to a number of authors, impaired immunological tolerance at the maternal-placental-fetal border underlies impaired angiogenesis and hypervascularization [2].
Natural killer cells (NK cells) are components of the innate immune system that provide protection against infections and tumor development, but have diverse functions during pregnancy [3].
Uterine natural killer cells (uNK cells) mediate the balance between activation and inhibition of extravillous trophoblast (EVT) invasion, regulate spiral artery remodeling through the synthesis of numerous chemokines, cytokines, as well as due to cytotoxic function [4]. Since uNK cells are involved in key phases of decidualization, implantation and placentation, the studies on peripheral and tissue-resident NK cells in pregnancy pathology have become widespread [5].
The conventional idea is that there is increased number of uNK cells in patients with recurrent miscarriage [6, 7]. The studies have shown that increased number of uNK cells in patients with recurrent miscarriage causes increased vascularization and impaired spiral artery remodeling, leading to preterm increase in placental blood flow and excessive oxidative stress and, as a result, to termination of pregnancy [8]. Other authors reported increased peripheral blood natural killer (pNK) cell activity in infertility or recurrent miscarriage [9]. At the same time, there is strong evidence in literature that NK cell deficiency or alterations in their function towards increase in the synthesis of tumor necrosis factor α (TNF)α and interferon γ (IFN)γ and decrease in secretion of vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) lead to impaired vascular remodeling in preeclampsia [10].
Given the available data on increased serum concentration of proangiogenic factors and decreased serum concentration of antiangiogenic factors in AAIP, which are synthesized by NK cells in the first trimester of pregnancy [8], it is interesting to study and evaluate the function of NK cells in abnormal placental invasion. Changes in the density of uNK cells in the uteroplacental complex in AAIP have been described in some studies [11–13].
The common origin of uNK and pNK cells by differentiation from hematopoietic cells in situ, as well as the similarity of their phenotypic characteristics [14] makes is possible to use pNK cells for investigation of interactions with chorionic cells. Сertainly, the differences between pNK and uNK cells with regard to membrane receptors CD16 and CD56 should also be taken into account.
The aim of the study was to evaluate cytotoxic activity of peripheral blood NK cells against trophoblast cells in pregnant women with AAIP.
Materials and methods
Controlled cross-sectional study and retrospective analysis was carried. The study included 18 pregnant women at 28–32 weeks of gestation. The main group included 6 patients with uterine scar after cesarean section and AAIP. The comparison group included 6 women with uterine scar and without AAIP. The control group included 6 pregnant women without uterine scar.
Inclusion criteria were: the age of patients at the time of enrollment in the study (24–25 years), spontaneous pregnancy, the chorion along the anterior wall of the uterus according to the first screening results, follow-up at the antenatal clinic starting from 11 weeks of pregnancy, voluntary informed consent signed by patients. Non-inclusion criteria were: the use of assisted reproductive technologies, recurrent miscarriage, isthmic-cervical insufficiency, placentomegaly, pre-existing or gestational diabetes mellitus, hypertensive diseases, preeclampsia, chronic heart failure, chronic kidney disease, antiphospholipid syndrome, exacerbation of chronic and acute inflammatory diseases.
Inclusion criteria in the main group were ultrasound and magnetic resonance imaging findings of placenta accreta according to current protocols for management of pregnant women with abnormal placentation [15, 16].
The study was approved by the Ethics Committee of Almazov National Medical Research Centre of the Ministry of Health of Russia.
Ultrasound examination was performed using ultrasound device Voluson E10, GE Healthcare. Magnetic resonance tomography was done in patients in the main group using magnetic induction of 1.5 T (Siemens, Germany) at 28–32 weeks of pregnancy in accordance with recommendations developed by the group of radiology researchers in perinatology and pediatrics at Almazov National Medical Research Centre of the Ministry of Health of Russia [17].
After anamnesis collection, general and obstetric examination, the women at 32–38 weeks of pregnancy were enrolled in the study at the Consultative and Diagnostic Department, the Perinatal Center of Almazov National Medical Research Centre of the Ministry of Health of Russia. Venous blood samples were collected from patients into vacuum tubes containing heparin in the morning on an empty stomach.
Сytotoxic activity of peripheral blood NK cells against trophoblast cells (JEG-3) was assessed using a method developed at the Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology (Head of the Department prof. S.A. Selkov). Trophoblast cells of the JEG-3 line (ATCC, Manssas, VA, USA), representing the main characteristics of first trimester trophoblast cells, were used as targets for NK cells. Cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Biolot, Russia) with addition of 10% inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/ml streptomycin, 2 mM glutamine, 1% essential amino acids and 1 mM sodium pyruvate (Sigma-Aldrich, США). NK cells were isolated from patients’ peripheral blood mononuclear cells using standard procedure for Ficoll/verografin density gradient centrifugation (Histopaque-1077, Sigma, USA).
Trophoblast cells were disintegrated, treated by carboxyfluorescein succinimidyl ester (CFSE) solution and incubated for 4 h in a humidified atmosphere at 37°C with 5% CO2 with addition of peripheral blood mononuclear cells in the effector to target cells ratio of 10:1. A portion of trophoblast cells were incubated in similar culture medium without addition of mononuclear cells to determine trophoblast cell death. After incubation, the cells were treated with a propidium iodide solution at a concentration of 2 μg/mL at 4°C for 10 minutes. Analysis of target cell death (PI positive staining) was performed using the FACSCanto ll flow cytometer system (Becton Dickinson, USA) [18].
Statistical analysis
IBM SPSS Statistics Version 26.0 was used for statistical analysis. The quantitative variables were tested for normality of distribution using the Shapiro–Wilk test. The quantitative variables with abnormal distribution are described as median (Me) and interquartile range (Q1–Q3). The Kruskal–Wallis test and Bonferroni correction was used to compare the groups.
Results
The groups of pregnant women were comparable in relation to age, gestational age and parity. There were differences between the groups in gravidity, number of intrauterine interventions and cesarean sections in history (Table). Pregnant women in the main group (with uterine scar and AAIP) had more pregnancies (p=0.002), uterine curettages due to abortion (p=0.023) and cesarean sections (p=0.02) in history compared with the women who had normal pregnancy. However, no differences were found between the women in the main and in the comparison groups. Threatened miscarriage in the first trimester occurred more often in the main group compared with the control and comparison groups (p=0.027).
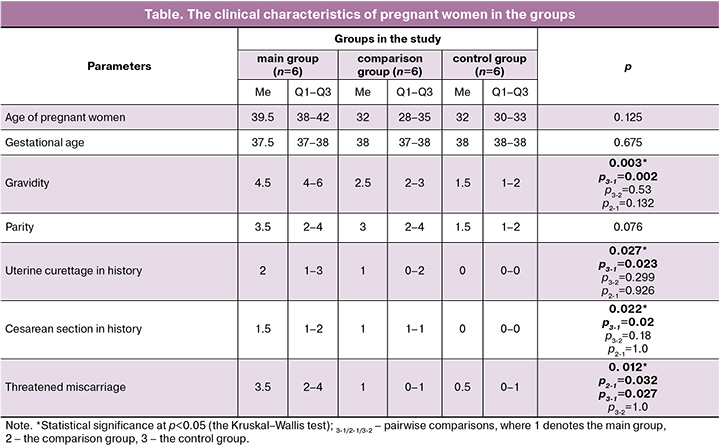
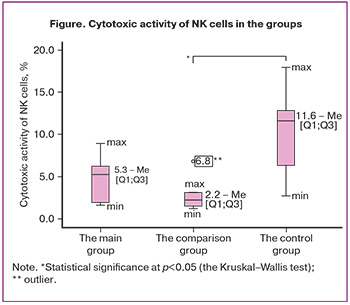
Comparison of cytotoxic activity of pNK cells in relation to trophoblast cells in the groups showed that cytotoxic activity of NK cells was higher in the group of healthy pregnant women with intact myometrium than in women with uterine scar (p=0.022). However, according to the results of ours study, there were no differences in cytotoxic activity compared with the main group (Figure).
Discussion
Currently, the issues of etiology and pathogenesis of AAIP remain controversial. Participation of uNK cells in the process of remodeling of spiral and radial arteries and trophoblast cell invasion [12, 19] suggests their dysfunction in AAIP.
According to literature data, two main NK cell subpopulations are distinguished in pregnancy: in the peripheral blood of pregnant women, 90% of NK cells (pNK) express CD56 (CD56dim) to a lesser degree and CD16 (CD16+bright) to a greater degree. CD 16+ has strong cytotoxic activity against tumor and virus infected cells. Uterine natural killer cells (or uNK cells) are characterized by predominant expression of CD56 (CD56bright), which are localized in the decidual tissue and endometrium and produce cytokines TNFα and IFNγ [20].
In early pregnancy, uNK cells represent the predominant lymphocytes – 70% of the total number of lymphocytes in the endometrium, and their number reduces gradually by the second trimester [21]. Recent studies have shown, that originating source of NK cells can be precursors of pNK cells, that makes it possible to use them for research on cell function in pregnancy pathology [22].
NK cells in the presence of trophoblast cells have the ability to alter expression of membrane receptors and, consequently, their functions [23]. For example, increased number of pNK cells with CD56dim phenotype, which have higher cytotoxic activity, and reduced number of CD56bright, as well as increased expression of activating receptors NKp46 and NKp30, perforin and granzymes by pNK cells is associated with recurrent miscarriage [24].
First of all, the functional characteristics of uNK cells depend on interactions with target cells through inhibitory or activating receptors [4]. Cytokines at the maternal-placental-fetal border may both enhance and reduce cytotoxic potency of NK-cells [25]. Non-classical major histocompatibility complex (MHC) proteins, such as human leukocyte antigens (HLA)-C, HLA-G and HLA-E provide trophoblast cells interaction with killer cell immunoglobulin-like receptors (KIRs), leukocyte immunoglobulin-like receptors (LILR), and natural killer group 2 receptor (NKG2D), and alter uNK cell function [26]. EVT cells express HLA-C and HLA-E, and interact with KIRs and NKG2 on uNK cells, blocking the cytotoxic response of NK cells to the invasive trophoblast [4]. On the other hand, EVT cells express HLA-C, interacting with uNK cell activation receptors KIR2DS1 or KIR2DS4С induce granulocyte-macrophage colony-stimulating factor (GM-CSF) secretion, which promote trophoblast cell invasion. [8].
There are some research papers in literature that reported quantitative assessment of the plasma NK cell counts in pregnant women with AAIP [12], but we found no studies describing the functional activity of pNK cells in placenta accreta. Some studies indicate weak expression of uNK cells in the decidual membrane in placental bed biopsy in women with AAIP compared with pregnant women without placentation abnormality [13, 27]. Other authors reported not only decreased expression of uNK cells in the placental bed in AAIP, but also direct positive correlation between risk factors for abnormal invasion and antiangiogenic factors compared with women in the control group [12].
Our study found that cytotoxic activity of pNK cells in relation to the number of trophoblast cells of the JEG-3 line was higher in women with normal pregnancy without uterine scar compared to women with cesarean section in history. However, no differences were found in comparison with the main group. Low cytotoxic activity of peripheral blood NK cells compared with intact myometrium of women with cesarean section in history is probably associated with specific natural killer cell-mediated cytotoxicity at the mother-placenta-fetus border in the area of the uterine scar without the signs of abnormal trophoblast invasion. Earlier studies by Skret-Magierlo J. et al. have shown that in patients with repeat cesarean section and normal placentation, expression of CD56+ cells in the scar tissue is higher than in the decidua in patients who underwent planned cesarean section for the first time, but the functional activity of the cells was not assessed in their study [28].
Interaction of HLA-G trophoblast cells with KIR2DL4 receptor triggers the synthesis of proangiogenic and proinflammatory factors. Via this interaction, production of NK cells is induced, they have low cytotoxicity but actively produce anti-inflammatory cytokines, angiogenic factors, such as VEGF and angiopoietins [29]. In addition, as a result of this interaction NK cells synthesize proteolytic factors, such as metalloproteinases, which stimulate trophoblast invasion and transformation of spiral arteries [30]. Thus, it can be assumed that cytotoxic activity of NK cells in patients with AAIP, which is comparable with cytotoxic activity of NK cells in the groups of pregnant women without placenta accreta, production of the proangiogenic factors and proteolytic enzymes is altered, that according to a number of authors pathogenetically determine placenta accreta [30, 31].
The results of our study were consistent with literature data indicating that the increase in frequency of pregnancies, curettage and cesarean section in history is associated with the risk of placenta accreta in subsequent pregnancies [32]. According to some authors, a decrease in the number of dNK cells is primarily due myometrial injury as a result of previous cesarean sections [33]. Other studies showed that with AAIP, perivascular infiltration of immune cells, including uNK cells, is a characteristic feature of not only remodeled vessels in the uteroplacental contact zone, but also vessels in the myometrium, which are not in contact with trophoblast, that indicates paracrine mechanisms of trophoblast invasion [11]. It is important to note that uNK cells contribute to initial structural changes in uteroplacental vessels starting before trophoblast invasion, that indicates NK cell dysfunction in the pathogenesis of AAIP [34].
Conclusion
This study analyzed for the first time cytotoxic activity of pNK cells in women with uterine scar and AAIP in comparison with pregnant women with uterine scar, but without placenta accreta and women with normal pregnancy without uterine scar. Low cytotoxic activity of NK cells in women with uterine scar without the signs of abnormal trophoblast invasion in comparison with normal pregnancy suggests that NK cell dysfunction plays and important role in the pathogenesis of placenta accreta.
Uterus damage as a result of previous cesarean section initiates morphological and functional changes in the uterine decidua. Relatively low cytotoxic activity of pNK cells in the group of women with uterine scar and without abnormal placental invasion is associated with the mechanism that prevents placenta accreta at the scar’s site. Thus, identification of women of risk group for developing AAIP with changes in the cytotoxic activity of NK cells is of interest to predict placenta accreta in early pregnancy.
References
- Kobayashi H., Matsubara S., Yoshimoto C., Shigetomi H., Imanaka S. Current understanding of the pathogenesis of placenta accreta spectrum disorder with focus on mitochondrial function. J. Obstet. Gynaecol. Res. 2024; 50(6): 929-40. https://dx.doi.org/10.1111/jog.15936.
- Xu X., Zhou Y., Wei H. Roles of HLA-G in the maternal-fetal immune microenvironment. Front. Immunol. 2020; 11: 592010. https://dx.doi.org/10.3389/fimmu.2020.592010.
- Kanter J.R., Mani S., Gordon S.M., Mainigi M. Uterine natural killer cell biology and role in early pregnancy establishment and outcomes. F. S. Rev. 2021; 2(4): 265-86. https://dx.doi.org/10.1016/j.xfnr.2021.06.002.
- Gaynor L.M., Colucci F. Uterine natural killer cells: functional distinctions and influence on pregnancy in humans and mice. Front. Immunol. 2017; 8: 467. https://dx.doi.org/10.3389/fimmu.2017.00467.
- El-Badawy O., Helmy A.S., Abbas A.M., Zahran A.M., Afifi N.A., Abdel-Rahim M.H. Concordance between peripheral and decidual NK cell subsets and killer immunoglobulin-like receptors in women with recurrent spontaneous miscarriages. J. Reprod. Immunol. 2020; 140: 103130. https://dx.doi.org/10.1016/j.jri.2020.103130.
- Chen X., Zhang T., Liu Y., Cheung W.C., Zhao Y., Wang C.C. et al. Uterine CD56+ cell density and euploid miscarriage in women with a history of recurrent miscarriage: A clinical descriptive study. Eur. J. Immunol 2021; 51(2): 487-9. https://dx.doi.org/10.1002/eji.202048868.
- Cooper S., Laird S.M., Mariee N., Li T.C., Metwally M. The effect of prednisolone on endometrial uterine NK cell concentrations and pregnancy outcome in women with reproductive failure. A retrospective cohort study. J. Reprod. Immunol. 2019; 131: 1-6. https://dx.doi.org/10.1016/j.jri.2018.10.001.
- Xie M., Li Y., Meng Y.Z., Xu P., Yang Y.G., Dong S. et al. Uterine natural killer cells: a rising star in human pregnancy regulation. Front. Immunol. 2022; 13: 918550. https://dx.doi.org/10.3389/fimmu.2022.918550.
- Dons'koi B.V., Osypchuk D.V., Chernyshov V.P., Khazhylenko K.G. Expression of natural cytotoxicity receptor NKp46 on peripheral blood natural killer cells in women with a history of recurrent implantation failures. J. Obstet. Gynaecol. Res. 2021; 47(3): 1009-15. https://dx.doi.org/10.1111/jog.14631.
- Deer E., Herrock O., Campbell N., Cornelius D., Fitzgerald S., Amaral L.M. et al. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 2023; 19(4): 257-70. https://dx.doi.org/10.1038/s41581-022-00670-0.
- Hecht J.L., Karumanchi S.A., Shainker S.A. Immune cell infiltrate at the utero-placental interface is altered in placenta accreta spectrum disorders. Arch. Gynecol. Obstet. 2020; 301(2): 499-507. https://dx.doi.org/10.1007/s00404-020-05453-1.
- El-Badawy O., Abbas A.M., Radwan E., Makboul R., Khamis A.A., Ali M. et al. Cross-talk between mucosal-associated invariant T, natural killer, and natural killer T cell populations is implicated in the pathogenesis of placenta accreta spectrum. Inflammation. 2023; 46(4): 1192-208. https://dx.doi.org/10.1007/s10753-023-01799-1.
- AbdelFattah S., Morsy M., Ahmed A.M., Abdelsalam H., Hosny G. Microcellular approach for the pathogenesis of placenta accreta spectrum inflammatory versus apoptotic pathways; a thorough look on Treg, dNK and VEGF. Pathol. Res. Pract. 2024; 254: 155153. https://dx.doi.org/10.1016/j.prp.2024.155153.
- Marron K., Harrity C. Endometrial lymphocyte concentrations in adverse reproductive outcome populations. J. Assist. Reprod. Genet. 2019; 36(5): 837-46. https://dx.doi.org/10.1007/s10815-019-01427-8.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Патологическое прикрепление плаценты (предлежание и врастание плаценты). 2023. [Ministry of Health of the Russian Federation. Clinical guidelines. Pathological attachment of the placenta (placenta previa and accreta). 2023. (in Russian)].
- ФГБУ «Северо-Западный федеральный медицинский исследовательский центр имени В.А. Алмазова» Министерства здравоохранения Российской Федерации. Клинические протоколы ведения пациентов по специальности «Акушерство и гинекология». Ч. I. 3‑е изд. СПб: Эко-Вектор; 2023. [V.A. Almazov North-Western Federal Medical Research Center of the Ministry of Health of the Russian Federation. Clinical protocols for patient management in the specialty "Obstetrics and Gynecology." Part I. 3rd ed. St. Petersburg: Eco-Vector; 2023. (in Russian)].
- Семенова Е.С., Мащенко И.А., Труфанов Г.Е., Фокин В.А., Ефимцев А.Ю., Лепёхина А.С., Горбунова Е.А., Сергиеня О.В., Шмедык Н.Ю., Тиллоев Т.А. Магнитно-резонансная томография при беременности: актуальные вопросы безопасности. REJR. 2020; 10(1): 216-30. [Semenova E.S., Mashchenko I.A., Trufanov G.E., Fokin V.A., Efimtsev A.Yu., Lepekhina A.S., Gorbunova E.A., Sergienya O.V., Shmedyk N.Yu., Tilloev T.A. Magnetic resonance imaging during pregnancy: current safety issues. REJR. 2020;10(1): 216-30. (in Russian)]. https://dx.doi.org/10.21569/2222-7415-2020-10-1-216-230.
- Загайнова В.А., Коган И.Ю., Беспалова О.Н., Сельков С.А., Соколов Д.И. Роль периферических и эндометриальных NK-клеток при повторных репродуктивных потерях. Акушерство и гинекология. 2021; 7: 19-27. [Zagainova V.A., Kogan I.Yu., Bespalova O.N., Selkov S.A., Sokolov D.I. The role of peripheral and endometrial natural killer cells in recurrent reproductive losses. Obstetrics and Gynecology. 2021; (7): 19-27 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.7.19-27.
- Bulmer J.N., Lash G.E. Uterine natural killer cells: Time for a re-appraisal? F1000Res. 2019; 8: F1000 Faculty Rev-999. https://dx.doi.org/10.12688/f1000research.19132.1.
- Vacca P., Chiossone L., Mingari M.C., Moretta L. Heterogeneity of NK cells and other innate lymphoid cells in human and murine decidua. Front. Immunol. 2019; 10: 170. https://dx.doi.org/10.3389/fimmu.2019.00170.
- Fu B., Wei H. Natural killer cells in reproduction: Before, during and after pregnancy. In: Mor G., ed. Reproductive Immunology: Basic Concepts. 2021: 55-72. https://dx.doi.org/10.1016/B978-0-12-818508-7.00009-9.
- Strunz B., Bister J., Jönsson H., Filipovic I., Crona-Guterstam Y., Kvedaraite E. et al. Continuous human uterine NK cell differentiation in response to endometrial regeneration and pregnancy. Sci. Immunol. 2021; 6(56): eabb7800. https://dx.doi.org/10.1126/sciimmunol.abb7800.
- Михайлова В.А., Давыдова А.А., Загайнова В.А., Сельков С.А., Соколов Д.И. NK-клетки в диагностике репродуктивных нарушений: долгий путь от теории к практике. Тезисы III Общероссийской научно-практической конференции для акушеров-гинекологов «Оттовские чтения». М.: Издательство журнала StatusPraesens; 2021: 18-9. [Mikhailova V.A., Davydova A.A., Zagainova V.A., Selkov S.A., Sokolov D.I. NK cells in the diagnosis of reproductive disorders: a long way from theory to practice. Theses of the III All-Russian Scientific and Practical Conference for Obstetricians and Gynecologists "Ott's Readings". Moscow: StatusPraesens; 2021: 18-9. (in Russian)].
- Toth B., Zhu L., Karakizlis H., Weimer R., Morath C., Opelz G. et al. NK cell subsets in idiopathic recurrent miscarriage and renal transplant patients. J. Reprod. Immunol. 2020; 138: 103098. https://dx.doi.org/10.1016/j.jri.2020.103098.
- Баженов Д.О., Михайлова В.А. Влияние клеток трофобласта на цитотоксический потенциал NK-клеток. Актуальные проблемы биомедицины - 2021: материалы XXVII Всероссийской конференции молодых ученых с международным участием, Санкт-Петербург, 25-26 марта 2021 года. СПб: Первый Санкт-Петербургский государственный медицинский университет им. академика И.П. Павлова; 2021: 144-5. [Bazhenov D.O., Mikhailova V.A. Effect of trophoblast cells on the cytotoxic potential of NK cells. Topical problems of biomedicine - 2021: materials of the XXVII All-Russian Conference of Young Scientists with International Participation, St. Petersburg, March 25-26, 2021. St. Petersburg: Academician I.P. Pavlov First St. Petersburg State Medical University; 2021: 144-5. (in Russian)].
- Li C., Houser B.L., Nicotra M.L., Strominger J.L. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc. Natl. Acad. Sci. U. S. A. 2009; 106(14): 5767-72. https://dx.doi.org/10.1073/pnas.0901173106.
- Laban M., Ibrahim E.A., Elsafty M.S., Hassanin A.S. Placenta accreta is associated with decreased decidual natural killer (dNK) cells population: a comparative pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014; 181: 284-8. https://dx.doi.org/10.1016/j.ejogrb.2014.08.015.
- Skret-Magierlo J., Wicherek L., Basta P., Galazka K., Sikora J., Wilk M. et al. RCAS1 decidual immunoreactivity during cesarean section in scar deciduosis: immune cell presence and activity. Gynecol. Obstet. Invest. 2008; 65(3): 187-94. https://dx.doi.org/10.1159/000111533.
- Smith A.L., Bole Aldo P., Racicot K.E. Placental regulation of immune functions. In: Mor G., ed. Reproductive Immunology: Basic Concepts. 2021: 335-48. https://dx.doi.org/10.1016/B978-0-12-818508-7.00004-X.
- Liu X., Wang Y., Wu Y., Zeng J., Yuan X., Tong C. et al. What we know about placenta accreta spectrum (PAS). Eur. J. Obstet. Gynecol. Reprod. Biol. 2021; 259: 81-9. https://dx.doi.org/10.1016/j.ejogrb.2021.02.001.
- Годзоева А.О., Зазерская И.Е., Васильева Е.Ю., Мащенко И.А., Яковлева Н.Ю., Ли О.А. Прогностическая значимость sFlt-1 и PlGF в диагностике глубокой инвазии плаценты. Журнал акушерства и женских болезней. 2022; 71(2): 39-48. [Godzoeva A.O., Zazerskaya I.E., Vasilyeva E.Y., Mashchenko I.A., Yakovleva N.Y., Li O.A. Prognostic value of sFlt-1 and PlGF in the diagnosis of abnormally deep placental invasion. Journal of obstetrics and women's diseases. 2022; 71(2): 39-48. (in Russian)]. https://dx.doi.org/10.17816/JOWD88697.
- Jauniaux E., Jurkovic D., Hussein A.M., Burton G.J. New insights into the etiopathology of placenta accreta spectrum. Am. J. Obstet. Gynecol. 2022; 227: 384-91. https://dx.doi.org/10.1016/j.ajog.2022.02.038.
- Zhou J., Chen H., Xu X., Liu Y., Chen S., Yang S. et al. Uterine damage induces placenta accreta and immune imbalance at the maternal-fetal interface in the mouse. Placenta. 2022; 119: 8-16. https://dx.doi.org/10.1016/j.placenta.2022.01.002.
- Harris L.K., Benagiano M., D'Elios M.M., Brosens I., Benagiano G. Placental bed research: II. Functional and immunological investigations of the placental bed. Am. J. Obstet. Gynecol. 2019; 221(5): 457-469. https://dx.doi.org/10.1016/j.ajog.2019.07.010.
Received 25.11.2024
Accepted 17.01.2025
About the Authors
Nataliya Yu. Ponikarova, PhD student, Department of Obstetrics and Gynecology, V.A. Almazov National Medical Research Center, Ministry of Health of Russia,197341, Russia, St. Petersburg, Akkuratova str., 2, +7(981)963-86-35, natalyponi@gmail.com, https://orcid.org/0000-0002-7230-3057
Alina A. Davydova, Junior Researcher at the Laboratory of Intercellular Interactions, Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute
for Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3, +7(812)328-98-50, alyadavydova@gmail.com,
https://orcid.org/0000-0001-5313-2910,
Ekatherina S. Shelepova, PhD, Teaching Assistant, Department of Obstetrics and Gynecology, V.A. Almazov National Medical Research Center, Ministry of Health of Russia, 197341, Russia, St. Petersburg, Akkuratova str., 2, shelepova_es@almazovcentre.ru, https://orcid.org/0000-0002-3233-8239
Nataliya A. Osipova, Dr. Med. Sci., Professor, Department of Obstetrics and Gynecology, V.A. Almazov National Medical Research Center, Ministry of Health of Russia, 197341, Russia, St. Petersburg, Akkuratova str., 2, osipova@almazovcentre.ru
Dmitry I. Sokolov, Dr. Bio. Sci., Professor, Head of the Laboratory of Intercellular Interactions, Department of Immunology and Intercellular Interactions,
D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3, +7(812)328-98-50,
falcojugger@yandex.ru, https://orcid.org/0000-0002-5749-2531
Valentina A. Mikhailova, Dr. Bio. Sci., Senior Researcher at the Laboratory of Intercellular Interactions, Department of Immunology and Intercellular Interactions,
D.O. Ott Research Institute for Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3, mva_spb@mail.ru,
https://orcid.org/0000-0003-1328-8157
Karina A. Oganyan, Resident Physician, Department of Obstetrics and Gynecology, V.A. Almazov National Medical Research Center, Ministry of Health of Russia,
197341, Russia, St. Petersburg, Akkuratova str., 2, oganyan_karina@bk.ru, https://orcid.org/0000-0002-2743-0882
Sergey A. Selkov, Merited Scolar of the Russian Federation, Dr. Med. Sci., Professor, Head of the Department of Immunology and Intercellular Interactions, D.O. Ott
Research Institute for Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3, +7(812)328-98-50, selkovsa@mail.ru,
https://orcid.org/0000-0003-1560-7529
Irina E. Zazerskaya, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, V.A. Almazov National Medical Research Center, Ministry of Health
of Russia, 197341, Russia, St. Petersburg, Akkuratova str., 2, zazera@mail.ru, https://orcid.org/0000-0003-4431-3917
Corresponding author: Nataliya Yu. Ponikarova, natalyponi@gmail.com



