The effectiveness of complex compression hemostasis in treating different grades of the placenta accreta spectrum disorders
Aim. To assess the effectiveness of complex compression hemostasis in treating different grades of the placenta accreta spectrum disorders.Zabelina T.M., Vasil'chenko O.N., Shmakov R.G., Pirogova M.M., Karimova G.N., Chuprynin V.D., Shchegolev A.I., Ezhova L.S., Gus A.I., Boikova Yu.V., Bychenko V.G., Uchevatkina P.V.
Materials and methods. A prospective analysis included 127 patients identified as having placenta accreta (n=62), placenta increta (n=58), and placenta percreta (n=7). All patients underwent a cesarean section and metroplasty with complex compression hemostasis.
Results. The blood loss volume was associated with the grades of the placenta accreta spectrum disorders making up 2943 (2039) ml, 1437 (694.5) ml and 1187 (632.6) ml, in patients with placenta percreta, placenta increta and placenta accreta, respectively.
Conclusion. Patients with different grades of the placenta accreta spectrum disorders had statistical differences in intraoperative blood loss volume and transfusion volume. Complex compression hemostasis was an effective hemostatic surgical technique for treating different grades of the placenta accreta spectrum disorders.
Keywords
It has been assumed that an increase in the rate of cesarean delivery is associated with a growing incidence of placenta accreta spectrum disorders [1]. The incidence of placenta accreta spectrum increased from 0.12% during the 1970s, to 0.31% during the 2000s [2]. Currently, abnormal placentation is the leading cause of severe bleeding, radical organ-removing surgery, maternal morbidity, and mortality. In 2016, according to the Russian Federal State Statistics Service, bleeding due to placental abruption and placenta previa were the fourth leading cause of maternal mortality [3].
Akker T.et al. analyzed the results of 128 studies, including 7,858 women who underwent an emergency peripartum hysterectomy. The most common indications were placental pathology (38%), uterine atony (27%), and uterine rupture (26%). The average blood loss was 3.7 l. Mortality was 5.2 per 100 hysterectomies [4]. Awan N. et al. (2011) analyzed all cases of emergency peripartum hysterectomy performed between the years 1999–2008 inclusive. The most common indications for emergency peripartum hysterectomy were placenta accreta spectrum disorders (54.8%), placenta previa (19.4%) [5].
To date, there are two main surgical approaches to delivery in women with placenta accreta spectrum. The first is an emergency or delayed hysterectomy (with embolization of uterine arteries and leaving the placenta in the uterus and / or using methotrexate); the second is organ-sparing surgery. The world’s leading clinics that accumulated vast institutional experience in managing patients with placenta accreta spectrum have been increasingly using organ-sparing [6–10].
In 2015–2017, in the V.I. Kulakov NMRC for OG&P, massive obstetric hemorrhage in patients placenta accreta spectrum, was managed by ligation of the internal iliac arteries or temporary occlusion of the common iliac artery. Since 2017, a new surgical method for managing of all placenta accreta spectrum disorders was developed, patented, and implemented. It was complex compression hemostasis, which is characterized by ease of implementation, effective reduction in blood loss, and low material costs [9, 11]. In this study, we evaluated the effectiveness of complex compression in patients with different grades of the placenta accreta spectrum.
The present study aimed to assess the effectiveness of complex compression hemostasis in treating different grades of the placenta accreta spectrum disorders.
Materials and methods
A prospective analysis was performed of the data of 127 pregnant women with placenta accreta spectrum, who were managed at the V.I. Kulakov NMRC for OG&P from January 2017 to October 2019. All patients were divided into three groups based on the grade of the placenta accreta spectrum. Patients in groups 1, 2, and 3 had placenta accreta (n=62), placenta increta (n=58), and placenta percreta (n=7). The grade of the placenta accreta spectrum was determined by the ultrasound examination and magnetic resonance imaging (MRI), followed by intraoperative visual and morphological verification of the diagnosis.
In all patients, surgery was performed under combined spinal-epidural anesthesia. During the operation, 4% of patients required conversion to general anesthesia owing to massive bleeding and hemodynamic instability. All patients underwent a cesarean section. The umbilical cord was ligated without traction, placed in the uterine cavity, and then the uterine incision was sutured.
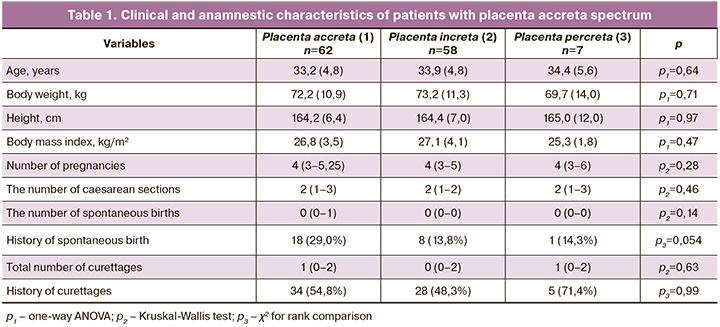
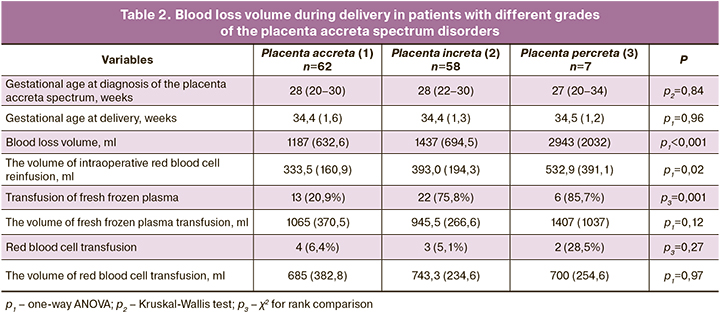
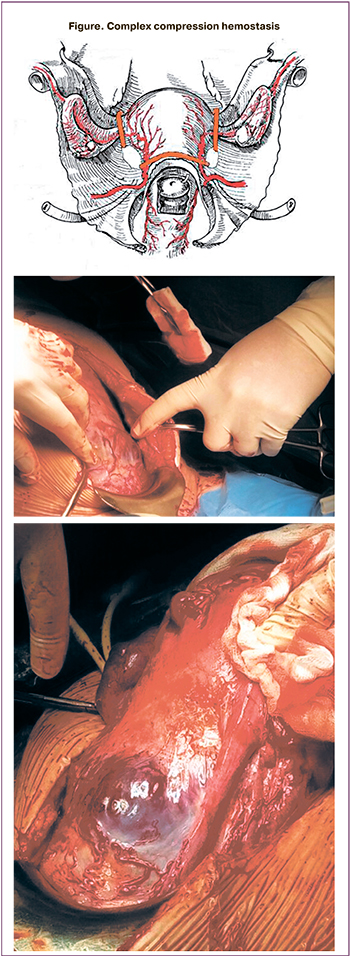 At the next stage, complex compression hemostasis was carried out. It included dissection of the peritoneum of the vesicoureteral fold, bringing down the bladder with its partial resection in some cases, targeted coagulation of blood vessels, the formation of artificial «windows» in broad ligaments of the uterus and the application of tourniquets through them bilaterally and on the cervical-interstitial region (Figure) [11]. Ovaries were displaced laterally of the tourniquets, and loops were created to place a tourniquet around the fallopian tubes, mesosalpinx, and the ovary ligaments. As a result, the tubal and communicative branches of the ovarian and uterine arteries were occluded. Next, a part of the anterior uterine wall with an abnormally adherent placenta was excised, followed by metroplasty using a controlled intrauterine balloon tamponade. This stage lasted an average of 24 minutes. Then the tourniquets were removed with the simultaneous injection of uterotonic agents [9].
At the next stage, complex compression hemostasis was carried out. It included dissection of the peritoneum of the vesicoureteral fold, bringing down the bladder with its partial resection in some cases, targeted coagulation of blood vessels, the formation of artificial «windows» in broad ligaments of the uterus and the application of tourniquets through them bilaterally and on the cervical-interstitial region (Figure) [11]. Ovaries were displaced laterally of the tourniquets, and loops were created to place a tourniquet around the fallopian tubes, mesosalpinx, and the ovary ligaments. As a result, the tubal and communicative branches of the ovarian and uterine arteries were occluded. Next, a part of the anterior uterine wall with an abnormally adherent placenta was excised, followed by metroplasty using a controlled intrauterine balloon tamponade. This stage lasted an average of 24 minutes. Then the tourniquets were removed with the simultaneous injection of uterotonic agents [9].
Statistical analysis
Statistical analysis and data visualization were carried out using GraphPad Prism (GraphPad Software, USA) statistical software. The normality of the distribution was tested by the generalized D’Agostino-Pearson test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (S.D.) and compared by one-way ANOVA with Tukey’s post-hoc multiple comparisons test when p < 0.05. Data with non-normal distribution were reported as the median (Me) and the quartiles Q1 and Q3 and compared using the Kruskal-Wallis test. Qualitative variables were summarized as counts and percentages and analyzed by a Chi-square test for rank comparison. Differences were considered statistically significant at p <0.05. Sensitivity and specificity were calculated using two-by-two contingency tables. The study was approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Results
The mean age of the patients was 33.8 (5.1), with no significant differences between the groups. The mean gestational age at delivery was 34.4 (1.6), 34.4 (1.3), and 34.5 (1.2) weeks in groups 1, 2, and 3, respectively, with no statistically significant differences between the groups. The number of previous pregnancies, cesarean deliveries, and uterine curettages also did not differ significantly. The diagnosis of placenta accreta spectrum disorders was established at the gestational age of 28 (20–30), 28 (22–30), and 27 (20–34) weeks in groups 1, 2, and 3, respectively. Therefore, pregnant women in the study groups did not differ statistically in the main anamnestic, anthropometric, and clinical data (Table 1).
Ultrasound examination and MRI were performed to determine the grade and the area of abnormal placental implantation. The sensitivity and specificity of MRI and ultrasound for preliminary diagnosis of placenta accreta spectrum in patients of the whole study cohort was 96.9% and 99.9%, 95.4% and 75.5%, respectively.
Blood loss volume of (Table 2) was statistically significantly higher in patients with placenta percreta [2943 (2032)] ml than that in patients with placenta increta [1437 (694.5)] ml (p <0.001) and placenta accreta [1187 (632.6)] ml (p <0.001). No statistically significant difference in blood loss volume was found between the groups of patients with placenta accreta and placenta increta (p = 0.19).
Intraoperative blood salvage and auto-transfusion of processed red cells were used during surgery in all patients, thus effectively minimizing or excluding the use of donor blood components. The volume of reinfusion was statistically significantly higher in patients with placenta percreta [532.9 (391.1)] ml, compared with patients from the placenta increta [393.0 (194.3)] ml (p = 0, 04) and placenta accreta [333.5 (160.9)] ml (p = 0.02). No statistically significant difference was found in reinfusion volumes between the groups of patients with placenta accreta and placenta increta (p = 0.22).
Thirteen (20.9%), 22 (75.8%), and 6 (85.7%) patients received transfusions of fresh frozen plasma in groups 1, 2, and 3, respectively. The higher was the grade of the placenta accreta spectrum, the more likely was the need for fresh frozen plasma transfusion (p = 0.001). Red blood cell transfusion was administered to 4 (6.4%), 3 (5.1%), and 2 (28.5%) patients in groups 1, 2, and 3, respectively.
Of the whole cohort, 13 (10.2%) patients underwent emergency delivery at a gestational age of 35.3 weeks. Indications in 10 and 3 of them were bleeding and the onset of labor, respectively. In 4 (6.5%) patients with placenta accreta, 2 (3.4%) with placenta increta, and in 2 (28.5%) with placenta percreta, the urinary bladder was resected, because it was impossible to bring it down due to a pronounced adhesive process or invasion of the placental villi into the posterior wall. These patients had no difference in gestational age at delivery of [34.6 (1.4) weeks].
Two patients with placenta accreta and two with placenta increta underwent an emergency postpartum hysterectomy due to uncontrollable postpartum hemorrhage. Delayed hysterectomy due to early postpartum hemorrhage [mean blood loss of 880 (506.1) ml] was performed in 2 patients with placenta increta. The absence of hysterectomies in patients with placenta percreta is probably due to a small number of cases. Another possible explanation for the absence of a relationship between hysterectomy and the grade of placenta accreta spectrum is the fact that it is not the grade, but the topography of abnormal placentation (cervix, lower urinary bladder, parametric fiber) that determines the possibility of preserving or removing the uterus.
The duration of surgery averaged 110 ± 32.2 minutes. Patients were discharged on the 7th (2) day.
Discussion
A general classification of placenta accreta spectrum includes placenta accreta in which the villi are attached directly to the surface of the myometrium without invading it; placenta increta, in which the villi penetrate deeply into the myometrium up to the uterine serosa; and placenta percreta, in which the invasive villous tissue penetrates through the uterine serosa and may reach the surrounding pelvic tissues, vessels, and organs. Placenta percreta is the rarest and most complicated form of placenta accreta spectrum [12]. The topography of invasion into the uterus and urinary bladder is an essential factor affecting pregnancy outcomes, intraoperative blood loss, and feasibility of organ-sparing operations. According to classification by Palacios J. M., the anterior placental invasion is divided into two sectors; the uterine body and upper posterior bladder wall are called S1 and the uterine sector adjacent to the lower posterior wall is called S2. A systematic review of 34 studies, exploring characteristics of near-miss cases of placenta accreta spectrum disorders showed that all near-miss cases showed S2 invasion. It must be emphasized that in all near-miss cases, massive bleeding occurred during pregnancy before delivery, and the placenta invaded the bladder posterior wall and parametrium. The authors note that high grade of placenta accreta spectrum is characterized not only by the presence of high and thick extrauterine anastomotic vessels but also, possibly, by new vessels developing due to the formation of a uterine hernia [6, 13].
Currently, some researchers are questioning the concept about the possibility of placenta to invade neighboring organs, in particular, in the urinary bladder due to the low malignant transformation potential of placental tissue [14, 15]. However, this assertion has not been supported by studies [16, 17]. In our study, in women with placenta percreta, during cystoscopy before surgery and according to MRI and ultrasound, no invasion of chorionic villi in the bladder mucosa was detected. This was confirmed by histological examination of the bladder wall after its resection.
In 2016, a meeting was held at Nottingham University (UK) to compare placental development and cancer growth. The human placenta was considered as the only «malignant» tissue that spreads, invading the myometrium, and develops without control of the maternal immune system, and trophoblast was defined as «physiological metastasis» [16]. Even though embryogenesis is a physiological and regulated process, and carcinogenesis is pathological and unregulated, they have many common features, including autonomous proliferative potential, low differentiation, relative resistance to apoptosis, active angiogenesis, invasive growth, and adhesive properties [17].
The main problem that occurs during delivery in women with placenta accreta spectrum disorders is the risk of massive blood loss and, therefore, the inability to perform organ-sparing surgery. Moreover, higher grades of abnormal placentation are associated with a higher risk of these problems. To date, it is not possible to determine the degree of placental invasion using only clinical and medical history data. In our study, there were no statistically significant differences between grades of placenta accreta spectrum in terms of primary anamnestic, anthropometric data, the number of previous deliveries, and manipulations on the uterus. It is important to emphasize that the history of cesarean deliveries is significantly associated with the incidence of placenta accreta spectrum disorders. However, in this study, no association was found between a history of cesarean delivery and the depth of invasion.
The results of the study once again confirmed the high diagnostic performance of ultrasound and MRI in detecting the grade of the placenta accreta spectrum, provided that an expert examiner performs the examinations.
The main methods of intraoperative hemostasis for the placenta accreta spectrum include complex compression hemostasis, ligation, and/or temporary occlusion of the iliac arteries or aorta [7, 8, 9, 10, 18]. According to some authors, balloon occlusion of the internal and common iliac arteries does not decrease blood loss volume, and only temporary balloon occlusion of the aorta results in a significant decrease in blood loss [19, 20]. It is worth noting that the use of these methods requires additional equipment and the presence of a vascular surgeon. Besides, there is a risk of developing arterial thrombosis [18, 21, 22].
The experiences gained in the V.I. Kulakov NMRC for OG&P also suggests that when using distal tourniquets, the mean blood loss was statistically lower by 713 ml, compared with temporary occlusion of the common iliac artery and by 1121 ml, compared with ligation of the internal iliac arteries [9].
Conclusion
The findings of this study showed that when using complex tourniquet hemostasis, patients with different grades of the placenta accreta spectrum disorders had statistical differences in intraoperative blood loss volume and transfusion volume. Particular attention should be paid to the topography of the placental invasion to choose an appropriate surgical technique and determine the possibility of organ-sparing surgery.
The results suggest that complex compression hemostasis is an effective hemostatic surgical technique for treating different grades of the placenta accreta spectrum disorders. The method is effective, feasible to perform in tertiary hospitals with sufficient experience in similar operations, and also may substantially reduce treatment costs compared with other existing methods.
References
- Creanga A.A., Bateman B.T., Butwick A.J., Raleigh L., Maeda A., Kuklina E. et al. Morbidity associated with cesarean delivery in the United States: Is placenta accreta an increasingly important contributor? Am. J. Obstet. Gynecol. 2015; 213(3): 384. e1-11. https://dx.doi.org/10.1016/j.ajog.2015.05.002.
- Marlando M., Sarno L., Napolitano R., Capone A., Tessitore G., Maruotti G.M. Martinelli Р. Placenta accreta: incidence and risk factors in an area with a particularly high rate of cesarean section. Acta Obstet. Gynecol. Scand. 2013; 92(4): 457-60. https://dx.doi.org/10.1111/aogs.12080.
- Здравоохранение в России 2017. Статистический сборник (Росстат). М.; 2017. 170 с. [Health care in Russia. Statistical digest. Federal State Statistics Service of the Russian Federation (Rosstat). Statistical Digest. Moscow, 2017: 172 p. (in Russian)].
- Akker T., Brobbel C., Dekkers O.M., Bloemenkamp K.W. Prevalence, indications, risk indicators, and outcomes of emergency peripartum hysterectomy worldwide: a systematic review and meta-analysis. Obstet. Gynecol. 2016; 128(6):1281-94. https://dx.doi.org/10.1097/AOG.0000000000001736.
- Awan N., Bennett M.J., Walters W.A. Emergency peripartum hysterectomy: a 10-year review at the Royal Hospital for Women, Sydney. Aust. N. Z. J. Obstet. Gynaecol. 2011; 51(3): 210-5. 10.1111/j.1479-828X.2010.01278.x.
- Palacios-Jaraquemada J.M., D’Antonio F., Buca D. Systematic review on near miss cases of placenta accreta spectrum disorders: correlation with invasion topography, prenatal imaging, and surgical outcome. J. Matern. Fetal Neonatal Med. 2019; Jan 30: 1-8. https://dx.doi.org/10.1080/14767058.2019.1570494.
- Matsubara S., Palacios J.M. Local uterine resection for placenta percreta: technical details are important. J. Matern. Fetal Neonatal Med. 2018; 31(17): 2338-9. https://dx.doi.org/10.1080/14767058.2017.1339228.
- Fitzpatric K.E., Sellers S., Spark P., Kurinczuk J.J., Brockchurst P., Knight M. The management and outcomes of placenta accreta, increta and percreta in the UK: a population based descriptive study. BJOG. 2014; 121(1): 62-70. https://dx.doi.org/10.1111/1471-0528.12405.
- Shmakov R.G., Vinitskiy A.A., Chuprinin V.D., Yarotskaya E.L., Sukhikh G.T. Alternative approaches to surgical hemostasis in patients with morbidity adherent placenta undergoing fertility-sparing surgery. J. Matern. Fetal Neonatal Med. 2019; 32 (12): 2042-8. https://dx.doi.org/10.1080/14767058.2018.1424821.
- Курцер М.А., Бреслав И.Ю., Латышкевич О.А., Григорьян А.М. Временная баллонная окклюзия общих подвздошных артреий у пациенток с рубцом на матке после кесарева сечнеия и placenta accrete, преимущества и возможные осложнения. Акушерство и гинекология. 2016; 12: 70-5. https://dx.doi.org/10.18565/aig.2016.12.70-5. [Kurtser M.A., Breslav I.Yu., Latyshkevich O.A., Grigor’yan A.M.; Temporary balloon occlusion of common iliac arthritis in patients with a post-cesarean uterine scar and placenta accrete, advantages and possible complications Obstetrics and gynecology. 2016; 70-5. (in Russian)].
- Шмаков Р.Г., Чупрынин В.Д., Виницкий А.А. Комплексный компрессионный гемостаз при выполнении органосохраняющего оперативного родоразрешения у пациенток с врастанием плаценты. Патент RU 2627633 C1. Заявлено 13.12.2016, опубликовано 09.08.2017. [Shmakov R.G., Chuprynin V.D., Vinitskii A.A. Comprehensive compression hemostasis during organ-preserving surgical delivery in patients with placental ingrowth. Patent RU 2627633 C1. December 13, 2016 (in Russian)].
- Милованов А.П., Буштарев А.В., Фокина Т.В. Особенности цитотрофобластической инвазии при полном предлежании и врастании плаценты. Архив патологии. 2017; 79(6): 30-5. [Milovanov A.P., Bushtarev A.V., Fokina T.V.; Features of cytotrophoblast invasion in complete placenta previa and increta. Pathology Archive. 2017; 79 (6): 30-5 (in Russian)].
- Calì G., D’Antonio F., Forlani F., Timor-Tritsch I.E., Palacios-Jaraquemada J.M. Ultrasound вetection of bladder-uterovaginal anastomoses in orbidly adherent. Placenta Fetal Diagn. Ther. 2017; 41(3): 239-40. https://dx.doi.org/10.1159/000445055.
- Burnirschke K., Burnirschke K., Burton G.J., Baergen R.N. Pathology of the human placenta. 6th ed. Springer; 2012. 941 p.
- Виницкий А.А., Шмаков Р.Г. Современные представления об этиопатогенезе врастания плаценты и перспективы его прогнозирования молекулярными методами диагностики. Акушерство и гинекология. 2017; 2: 5-10. https://dx.doi.org/10.18565/aig.2017.2.5-10. [Vinitskii A.A., Shmakov R.G. Modern ideas about the etiopathogenesis of placental growth and the prospects for its prediction by molecular diagnostics. Obstetrics and gynecology. 2017; 2: 5-11 (in Russian)].
- Kurlak L.O., Knoffer M., Mistry H.D. Common features between placental development and cancer growth. Placenta. 2017; 56: 2-4. https://dx.doi.org/10.1016/j.placenta.2017.04.012.
- Burton G.J., Jauniaux E., Murray A.J. Oxygen and placental development; parallels and differences with tumour biology. Placenta. 2017; 56: 14-8. https://dx.doi.org/10.1016/j.placenta.2017.01.130.
- Shahin Y., Pang C.L. Endovascular interventional modalities for haemorrhage control in abnormal placental implantation deliveries: a systematic review and meta-analysis. Eur. Radiol. 2018; 28(7): 2713-26. https://dx.doi.org/10.1007/s00330-017-5222-0.
- Feng S., Liao Z., Huang H. Effect of prophylactic placement of internal iliac artery balloon catheters on outcomes of women with placenta accreta: an impact study. Anaesthesia. 2017; 72(7): 853-8. https://dx.doi.org/10.1111/anae.13895.
- Chen M., Lv B., He G., Liu X. Internal iliac artery balloon occlusion during cesarean hysterectomy in women with placenta previa accreta. Int. J. Gynaecol. Obstet. 2019; 145(1): 110-5. https://dx.doi.org/10.1002/ijgo.12763.
- Bishop S., Butler K., Monaghan S., Chan K., Murphy G., Edozien L. Multiple complications following the use of prophylactic internal iliac artery balloon catheterisation in a patient with placenta percreta. Int. J. Obstet. Anesth. 2011; 20(1): 70-3. https://dx.doi.org/10.1016/j.ijoa.2010.09.012.
- Tokue H., Tokue A., Tsushima Y., Kameda T. Risk factors for massive bleeding based on angiographic findings in patients with placenta previa and accreta who underwent balloon occlusion of the internal iliac artery during cesarean section. Br. J. Radiol. 2019; 92(1102): 20190127. https://dx.doi.org/10.1259/bjr.20190127.
Received 06.02.2020
Accepted 25.03.2020
About the Authors
Tatyana M. Zabelina, Ph.D. Student at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel.: +7(977)957-17-29. E-mail: romashova-1993@bk.ru.4 Ac. Oparina str., Moscow, 117997, Russian Federation.
Oksana N. Vasil’chenko, Ph.D., Senior Researcher at the Department of Innovative Technologies, Institute of Obstetrics, V.I. Kulakov NMRC for OG&P
of Minzdrav of Russia. Tel.: +7(977)957-17-29. E-mail: o_vasilchenko@oparina4.ru. 4 Ac. Oparina str., Moscow, 117997, Russian Federation.
Roman G. Shmakov, Dr.Med.Sci., Professor of the RAS, Director of the Institute of Obstetrics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(977)957-17-29. E-mail: r_shmakov@oparina.ru. 4 Ac. Oparina str., Moscow, 117997, Russian Federation.
Maria M. Pirogova, Ph.D. Student at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel.: +7(977)957-17-29. E-mail: pirogovamaria@gmail.com.
4 Ac. Oparina str., Moscow, 117997, Russian Federation.
Galiya N. Karimova, Ph. D., Obstetrician-Gynecologist at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(977)957-17-29. E-mail: g_karimova@oparina.ru.
4 Ac. Oparina str., Moscow, 117997, Russian Federation.
Vladimir D. Chuprynin, Ph. D, Head of the Surgical Department, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(977)957-17-29. E-mail: v_chuprynin@oparina4.ru. 4 Ac. Oparina str., Moscow, 117997, Russian Federation.
Alexander I. Shchegolev, Dr.Med.Sci., Professor, Head of the Department of Anatomic Pathology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(977)957-17-29. E-mail: ashegolev@oparina4.ru. 4 Ac. Oparina str., Moscow, 117997, Russian Federation.
Larisa S. Ezhova, Senior Researcher at the Department of Anatomic Pathology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(977)957-17-29. E-mail: l_ezhova@oparina4.ru. 4 Ac. Oparina str., Moscow, 117997, Russian Federation.
Alexander I. Gus, Dr.Med.Sci., Professor, Head of the Department of Ultrasound and Functional Diagnostics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(977)957-17-29. E-mail: a_gus@oparina4.ru. 4 Ac. Oparina str., Moscow, 117997, Russian Federation.
Yulia V. Boikova, Ph.D., Physician at the Department of Ultrasound and Functional Diagnostics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(977)957-17-29. E-mail: j_boikova@oparina4.ru. 4 Ac. Oparina str., Moscow, 117997, Russian Federation.
Vladimir G. Bychenko, Ph. D., Head of the Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(977)957-17-29. E-mail: v_bychenko@oparina4.ru. 4 Ac. Oparina str., Moscow, 117997, Russian Federation.
Polina V. Uchvatkina, Physician at the Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(977)957-17-29. E-mail: p_uchevatkina@oparina4.ru. 4 Ac. Oparina str., Moscow, 117997, Russian Federation.
For reference: Zabelina T.M., Vasil’chenko O.N., Shmakov R.G., Pirogova M.M., Karimova G.N., Chuprynin V.D., Shchegolev A.I., Ezhova L.S., Gus A.I., Boikova Yu.V., Bychenko V.G., Uchevatkina P.V. The effectiveness of complex compression hemostasis
in treating different grades of the placenta accreta spectrum disorders.
Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2020; 6: 30-36 (in Russian):
https://dx.doi.org/10.18565/aig.2020.6.30-36



