Comparing the effectiveness of ultrasound and MRI in assessing cesarean uterine scar defects
Objective: To improve the effectiveness of uterine cesarean scar defect assessment using ultrasonography and magnetic resonance imaging (MRI). Materials and methods: This comparative study included 61 patients with uterine scar dehiscence after cesarean section (CS), who were interested in having another pregnancy. All patients underwent expert pelvic ultrasonography (eUS) and MRI at V.I. Kulakov NMRC for OG&P; 30 patients underwent echo-hysterosalpingography (eHSG). Each examination (eUS and MRI) was performed by two independent specialists. Scar parameters were measured using the standardized Delphi technique, irrespective of the examination method used. Measurements of uterine scar parameters obtained using eUS, MRI, and eHSG in the same patients were compared, and the consistency of measurements between specialists was assessed. Results: A comparative analysis showed a statistically significant difference in the minimum scar thickness (mST) between eUS [2.19(0.77) mm] and MRI [1.93 (0.7) mm] (p<0.05). A comparative analysis of MRI and eHSG data also revealed statistically significant differences in niche depth measurements of 3.29 (1.48) and 7.01 (2.97) mm, respectively (p<0.05); mST was significantly lower when measured by eHSG than MRI, 1.5 (0.4) mm and 1.81 (0.6) mm, respectively (p=0.03). There was no statistically significant difference between the eUS and MRI measurements taken by two independent specialists. However, according to the Bland–Altman analysis, MRI and eHSG showed less variability than eUS, indicating higher reproducibility. Conclusion: If a uterine scar is detected on ultrasound, MRI or eHSG is recommended to confirm the diagnosis and decide whether surgical treatment is necessary, as these methods are highly reproducible and reduce the risk of diagnostic errors. eHSG should be considered as the "gold standard" for diagnosing uterine scar defects after CS, due to its advantages of filling the uterine cavity with contrast and the possibility of detecting perforating scar defects. Authors' contributions: Martynov S.A., Adamyan L.V., Sukhareva T.A., Letunovskaya A.B., Kulabuhova E.A. – conception and design of the study; Letunovskaya A.B., Boykova Yu.V., Kulabuhova E.A., Uchevatkina P.V. – organisation and conduct of instrumental investigations; Sukhareva T.A., Martynov S.A. – data collection and analysis, statistical analysis; Sukhareva T.A., Martynov S.A. – manuscript drafting; Letunovskaya A.B., Boykova Yu.V., Kulabukhova E.A., Uchevatkina P.V., Adamyan L.V. – manuscript editing. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P. Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Sukhareva T.A., Martynov S.A., Adamyan L.V., Kulabukhova E.A., Uchevatkina P.V., Letunovskaya A.B., Boykova Yu.V. Comparing the effectiveness of ultrasound and MRI in assessing cesarean uterine scar defects. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (4): 78-86 (in Russian) https://dx.doi.org/10.18565/aig.2022.264Sukhareva T.A., Martynov S.A., Adamyan L.V., Kulabukhova E.A., Uchevatkina P.V., Letunovskaya A.B., Boykova Yu.V.
Keywords
Currently, cesarean section (CS) rates are increasing worldwide, reaching 40.5% in some countries [1, 2]. In Russia, this rate was 30.3% in 2020 [3].
Due to the increase in CS, there is an increased interest in both the assessment of scar status during pregnancy and delivery and the identification and study of uterine scar defects following CS outside of pregnancy.
A scar defect is defined as focal thinning of the myometrium at the site of the scar compared to the adjacent intact myometrium. This can occur with or without niche formation [4].
Uterine scar defects can cause menstrual dysfunction, chronic pelvic pain, secondary infertility, ectopic pregnancy in the scar, placenta accreta spectrum, placenta previa, and uterine rupture.
In this context, accurate assessment of uterine scars outside pregnancy is particularly important for timely surgical correction of severe defects.
The most accessible and common methods of investigation are ultrasound techniques, including 2D and 3D ultrasound (US) and echo-hysterosalpingography (eHSG) using contrast media [5–7].
Another informative and accessible diagnostic technique is magnetic resonance imaging (MRI), which allows fairly accurate assessment of all uterine scar parameters, including the anatomical characteristics of the scarred area. Contrast-enhanced MRI can also be used to assess perfusion in scar area [8–14].
There is no consensus among experts regarding the advantages and disadvantages of these methods, and there is no generally accepted measurement technique; therefore, there is no accepted 'gold standard' for diagnosing uterine scar defects.
The aim of our study was to improve the effectiveness of assessment of uterine scar after caesarean section by comparing different diagnostic methods.
Materials and methods
This comparative study was conducted at the V.I. Kulakov NMRC for OG&P. The study group included 61 patients with a uterine scar after CS in the lower uterine segment (> 1 year since the last CS), who were interested in having another pregnancy. The inclusion criteria were age from 18 to 45 years, pregnancy planning, suspicion of uterine scar thinning after CS in the lower uterine segment, and signed informed consent to participate in the study. Exclusion criteria were age < 18 and > 45 years, acute inflammatory diseases of the pelvic organs, severe comorbidities, and malignant neoplasms [15].
In the first stage, all the patients underwent expert ultrasound (eUS) of the pelvic organs. For further diagnostic search and verification of the diagnosis, all patients underwent pelvic MRI in the second stage, and 30 of these patients underwent eHSG in the third stage.
The eUS was performed in women during the proliferative phase on days 5-11 of the menstrual cycle using a Samsung WS80A Elite (Samsung Medison, South Korea) transvaginal 7.5 MHz transducer. For further comparative analysis, the studies were performed by two specialists at the Department of Ultrasound and Functional Diagnostics of the V.I. Kulakov NMRC for OG&P. The first investigator was a specialist with less than 15 years of experience, and the second investigator was a specialist with more than 25 years of experience. A standard description of the topography and anatomical features of the internal genitalia was performed [15]. Uterine scar parameters were measured according to the standardized methodology adopted in the Delphi study [16]. The measurements included maximum niche length and depth (in the sagittal plane), minimum scar thickness (mST) (in the sagittal plane, perpendicular to the serous membrane from the upper inner contour of the defect to the serous membrane; endometrium and serous membrane were not included in the measurement), maximum niche width (transverse plane), intact myometrium thickness (IMT), and thickness of the anterior uterine wall near the upper edge of the niche (sagittal plane).
MRI was performed on a GE Signa 1.5T HDX MRI scanner by General Electrics (USA) with 1.5T magnetic field strength, without contrast enhancement, with small and medium bladder filling, obtaining T1VI, T2VI, T2FS, FIESTA performed in Sag, Cor, and Ax planes. The topography and anatomical features of the internal genitalia were described, and uterine scar parameters were measured using the Delphi algorithm. The description of the images and the measurement of the uterine scar parameters were performed by two independent researchers with different levels of experience. The first researcher was a specialist with more than 25 years of experience, and the second researcher was a specialist with less than 10 years of experience. Until the measurements were completed, the results obtained by the first and second investigators were not known to one another.
In addition, 30 patients underwent eHSG to confirm diagnosis. eHSG was performed on days 7–11 of the menstrual cycle using a WS80A ultrasound scanner (Samsung Medison, South Korea) with a 7.5 MHz transvaginal transducer using a standard technique by an experienced specialist with over 15 years of experience specializing in eHSG (over 300 eHSG studies) [15]. The topography and anatomical features of the internal genitalia were described, and uterine scar parameters were measured using the Delphi algorithm [16].
Statistical analysis
Statistical analysis was performed using Microsoft Excel 2010 and STATISTICA 10. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Continuous variables showing normal distribution are presented as mean (M) and standard deviation (SD) and compared using Student’s t-test. The agreement between measurements obtained by two independent investigators (both ultrasound and MRI) was analyzed using Bland–Altman plotting [15]. The critical level of significance when testing the statistical hypotheses was set at p<0.05.
Results
The mean age of the patients was 35.4 (4.2) years, ranging from 25 to 44 years. Thirty-seven patients had a history of one CS, 19 had two CSs, and five had three CSs. If one patient had multiple scar defects after CS, the defect with the smallest mST was included in the study; if the mST values were equal, the defect with the largest niche parameter was included. At the time of the study, all women were more than one year post-CS. In 40/61 (65.6%) patients, the previous CS was performed as an emergency procedure, and in 21/61 (34.4%), it was an elective procedure.
The most common clinical manifestation of the uterine scar defect was trace post-menstrual vaginal spotting in 39 patients (from 2 to 20 days, averaging 7.6 days) and in the middle of the menstrual cycle in 2 patients (3.8 days each). The absence of pregnancy in a regular sexual life without contraception for more than 1 year was registered in 16 patients; algomenorrhea after CS developed in 9 patients (Table 1).
To compare the results of two diagnostic modalities (eUS and MRI), the mean values of uterine scar parameters obtained by two independent specialists using each method were calculated (Table 2) and the results were compared (Table 3).
According to the Kolmogorov–Smirnov and Shapiro–Wilk tests, mST values met the normality assumption, whereas niche parameters were not normally distributed. Comparative analysis of mST and niche parameters was performed using the Student's t-test.
Comparative analysis of eUS and MRI data showed statistically significant differences in mST measurements. Differences in niche width, length, and depth were not statistically significant (Table 3).
According to the eUS and MRI data, the uterine scar was identified in the lower third of the anterior uterine wall in three cases and in the projection of the internal cervical os in the remaining cases (Fig. 1, 2).
To assess the agreement between measurements of the same parameters obtained by two specialists with different levels of training in each examination method (eUS and MRI), a comparative analysis was performed for the independent variables, which showed no statistically significant differences. Therefore, Bland–Altman analysis was used. The mean of the differences between the two independent measures and the upper and lower bounds of the 95% confidence interval (CI) for the differences between the two measures for each method were calculated (Table 4). The closer the mean differences were to '0' and the narrower the 95% CI, the more homogeneous the measurements were.
As mST is fundamental in determining whether uterine scar surgery is required, Bland–Altman plots were generated from the data to show the agreement of uterine scar measurements (Fig. 3).
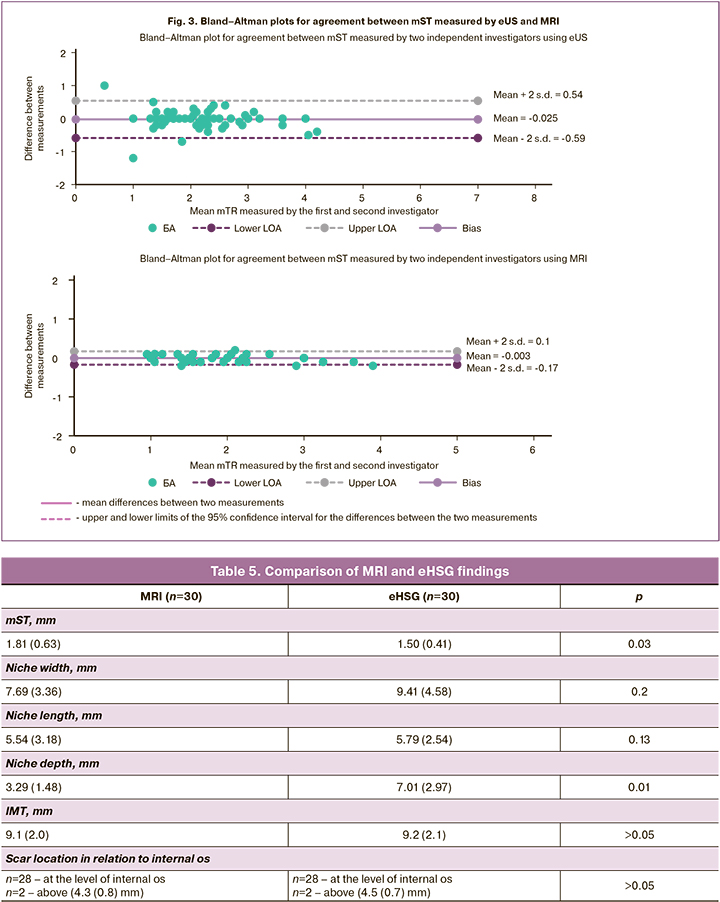
The analysis of the Bland–Altman plots showed that the mean difference between the first and second investigator values for both eUS and MRI was close to 0, indicating good agreement. Of particular note, however, was that the differences between the eUS results had a wider 95% CI than that of MRI, indicating a higher variability with eUS and correspondingly higher agreement and homogeneity with MRI.
In an earlier study [15] comparing eUS and eHSG findings by two specialists with different levels of training, we also found good agreement between the two methods. However, the differences between the results obtained with eHSG had a narrower 95% CI than those obtained with eUS, revealing a more pronounced agreement and homogeneity of results with eHSG.
Comparison of MRI and eHSG data showed differences in measurements of mST, width, length, and depth of the niche, with underestimation of mST and greater niche parameters in the eHSG data. Statistically significant differences were found in the mST (p=0.03) and niche depth (p=0.02) (Table 5).
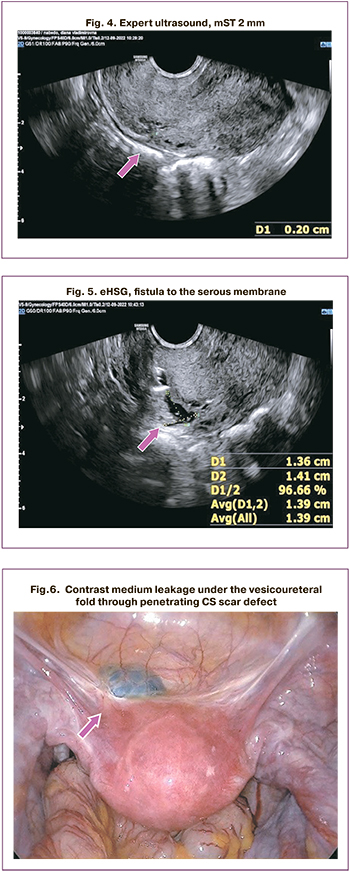
In one patient, eHSG revealed a fistula reaching the uterine serous membrane, with an mST of 2 mm, according to ultrasound and MRI (Fig. 4, 5). In another patient, a penetrating scar defect (leakage of contrast medium under the peritoneum of the vesicouterine fold) was detected; the mST was 3 mm according to ultrasound and 3.3 mm according to MRI. Additional findings in the eHSG were subsequently confirmed during surgery (Fig. 6).
Discussion
The importance of accurate assessment of cesarean uterine scars is unquestionable, as it influences the management of these patients, including surgical strategy.
The results of our previous study showed significant variability in uterine scar ultrasound parameters depending on the level of the medical institution where it was performed, as well as the advantages of eHSG owing to its high reproducibility. In this study, we studied the feasibility of MRI in diagnosing uterine scar defects compared to eUS and eHSG, and evaluated the agreement of MRI measurements.
According to our findings, MRI showed statistically significant differences in the measurement of some uterine scar parameters compared with ultrasound. The main parameter, mST, was found to be on average 0.26 mm smaller than that measured by ultrasound (p=0.023). There was also a greater niche width, as measured using MRI.
The difference in uterine scar measurements between ultrasound and MRI has also been confirmed in other studies [9, 10, 12]. Bolten K. et al. [9] in their study compared the effectiveness of MRI and transvaginal ultrasound in measuring the thickness of the anterior uterine wall in the post-CS scar area. According to the findings, mST was significantly smaller on MRI compared with ultrasound, both after planned CS (7.9 mm vs 12.0 mm, respectively) and after emergency CS (8.1 mm vs 9.5 mm, respectively).
Also, in a retrospective study by Yao M. et al. [10] including 282 patients, the authors observed that MRI showed greater niche length and width as well as a smaller mST compared to transvaginal ultrasound (2.91 mm and 2.98 mm, respectively, p=0.598).
In Tang X. et al. [12], a comparative evaluation of ultrasound and MRI showed that the mean defect length and depth were significantly greater with MRI than with ultrasound, but the authors found no significant difference in mST measurements.
In a study by Nozhniceva O.N. et al. [13], ultrasound had a sensitivity of 63% and specificity of 62%, whereas MRI had a sensitivity of 80% and specificity of 71%. The mST according to MRI was greater compared to ultrasound:3.44(1.64) mm and 3.3(1.54 mm), respectively, but the difference was statistically insignificant.
In a case-control study by Satpathy G. et al. [14], the authors also observed higher mST values on MRI than on ultrasound.
Thus, most authors confirm the lower mST values obtained with MRI than with ultrasound, which is consistent with our own findings. In our opinion, the resulting difference in measurements can be explained by the clearer tissue differentiation on MRI. MRI allows the identification of only the connective-tissue or connective-tissue-muscle component, without the basal endometrial layer and uterine serous membrane, which can be measured by ultrasound, to be included in the measurement of scar thickness.
The heterogeneity of the data obtained by different investigators [9, 10, 12–14] may be due to the lack of a uniform measurement technique. As our previous study has shown, performing ultrasonography on non-expert machines without using a standardized technique leads to significant variability in results [15]. It is the standardization of examinations that has been emphasized by Delphi group experts [16]. As MRI specialists, even those with considerable experience, often have little experience in post-cesarean uterine scar examination, particularly in multidisciplinary medical institutions, we believe it is important to introduce the Delphi technique not only into the practice of sonographers but also into the work of radiologists performing MRI.
To date, there have been no large comparative studies on the diagnosis of uterine scar defects using eHSG and MRI; therefore, we focused on the analysis of these two methods.
According to our findings, mST measured by eHSG was on average 0.31 mm smaller than measured by MRI, the difference was statistically significant (p=0.03), niche width and length were not significantly different and niche depth was significantly greater in eHSG versus MRI (7.01 mm and 3.29 mm, respectively, p=0.02).
The lower mST values on echo-HSG (compared to both MRI and ultrasound) may be related to tissue distension and niche expansion due to the injection of contrast into the uterine cavity. Intrauterine injection of contrast medium allows the detection of complex uterine scar defects in the form of branches and fistulas to the serous membrane.
We found greater agreement between MRI and eHSG measurements obtained by specialists with different levels of training than ultrasound. The high reproducibility of eHSG has been demonstrated in earlier studies [15, 17], but the reproducibility of MRI in assessing uterine scar defects has been evaluated by us for the first time. Even among clinicians with little experience, the findings are comparable to those of experienced specialists (if the Delphi algorithm is followed). MRI along with eHSG can be considered as a method for the definitive verification of uterine CS scars.
Conclusion
MRI and eHSG are highly effective methods for assessing CS uterine scar. Measurements of key parameters of uterine scars obtained by MRI and eHSG were statistically significantly different from those obtained by eUS. Statistically significant differences were found between MRI and eHSG in the measurement of mST and niche depth; both methods are highly reproducible to minimize ambiguous results.
Ultrasound should be considered as a 1st line research method, whereas eUS has the advantage of using the Delphi algorithm. If a uterine scar defect is detected by eUS, eHSG or MRI using the Delphi algorithm (2nd line modality) should be performed to decide whether surgical treatment is necessary, depending on the capabilities of the institution.
However, eHSG should be considered the "gold standard" for the diagnosis of uterine scar defects after CS because of its advantages in filling the uterine cavity with contrast and its ability to detect perforating scar defects. Due to the statistically significant differences in the assessment of the main parameters of the uterine scar obtained with eUS, MRI, and eHSG, this fact should be considered when deciding whether to refer patients for surgical treatment.
References
- Robson S.J., de Costa C.M. Thirty years of the World Health Organization's target caesarean section rate: time to move on. Med. J. Aust. 2017; 206(4): 181-5. https://dx.doi.org/10.5694/mja16.00832.
- Betrán A.P., Ye J., Moller A.B., Zhang J., Gülmezoglu A.M., Torloni M.R. The increasing trend in caesarean section rates: global, regional and national estimates: 1990-2014. PLoS One. 2016; 11(2): e0148343.https://dx.doi.org/10.1371/journal.pone.0148343.
- Федеральная служба государственной статистики. Здравоохранение в России: статистический сборник. 2021. [Federal State Statistics Service. Health care in Russia: statistical collection. 2021. (in Russian)].
- Мартынов С.А., Адамян Л.В. Рубец на матке после кесарева сечения: терминологические аспекты. Гинекология. 2020; 22(5): 70-5. [Martynov S.A., Adamyan L.V. Cesarean scar defect: terminological aspects. Gynecology. 2020; 22(5): 70-5. (in Russian)].
- Ludwin A., Martins W.P., Ludwin I. Evaluation of uterine niche by three-dimensional sonohysterography and volumetric quantification: techniques and scoring classification system. Ultrasound Obstet. Gynecol. 2019; 53(1): 139-43. https://dx.doi.org/10.1002/uog.19181.
- Rasheedy R., Sammour H., Elkholy A., Fadel E. Agreement between transvaginal ultrasound and saline contrast sonohysterography in evaluation of cesarean scar defect. J. Gynecol. Obstet. Hum. Reprod. 2019; 48(10): 827-31.https://dx.doi.org/10.1016/j.jogoh.2019.05.013.
- di Pasquo E., Kiener A.J.O., DallAsta A., Commare A., Angeli L., Frusca T., Ghi T. Evaluation of the uterine scar stiffness in women with previous Cesarean section by ultrasound elastography: A cohort study. Clin. Imaging. 2020; 64: 53-6. https://dx.doi.org/10.1016/j.clinimag.2020.03.006.
- Wong W., Fung W.T. Magnetic resonance imaging in the evaluation of cesarean scar defect. Gynecol. Minim. Invasive Ther. 2008; 7(3): 104-7.https://dx.doi.org/10.4103/GMIT.GMIT_23_18.
- Bolten K., Fischer T., Bender Y.Y., Diederichs G., Thomas A. Pilot study of MRI/ultrasound fusion imaging in postpartum assessment of Cesarean section scar. Ultrasound Obstet. Gynecol. 2017; 50(4): 520-6. https://dx.doi.org/10.1002/uog.17349.
- Yao M., Wang W., Zhou J., Sun M., Zhu J., Chen P., Wang X. Cesarean section scar diverticulum evaluation by saline contrast-enhanced magnetic resonance imaging: the relationship between variable parameters and longer menstrual bleeding. J. Obstet. Gynaecol. Res. 2017; 43(4): 696-704.https://dx.doi.org/10.1111/jog.13255.
- Макиян З.Н., Быченко В.Г., Павлович С.В., Адамян Л.В. Метод функциональной магнитно-резонансной томографии для определения перфузионного кровотока в области рубца после кесарева сечения. Патент 2187888 Российская Федерация. МПК A61B5/55 A61K49/06. № RU2727313C1. Заявлено 11.02.20; опубл. 21.07.20. [Makiyan Z.N., Bychenko V.G., Pavlovich S.V., Adamyan L.V. Method of functional magnetic resonance imaging to determine perfusion blood flow in the scar area after cesarean section. Patent 2187888 Russian Federation. MPK A61B5/55 A61K49/06.No. RU2727313C1. Application 11.02.20; Publ. 21.07.20. (in Russian)].
- Tаng X., Wang J., Du Y., Xie M., Zhang H., Xu H., Hua K. Caesarean scar defect: risk factors and comparison of evaluation efficacy between transvaginal sonography and magnetic resonance imaging. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 242: 1-6. https://dx.doi.org/10.1016/j.ejogrb.2019.09.001.
- Ножницева О.Н., Семенов И.А., Беженарь В.Ф. Рубец на матке после операции кесарева сечения и оптимальный алгоритм диагностики его состояния. Лучевая диагностика и терапия. 2019; 2: 85-90. [Nozhnitseva O.N., Semenov I.A., Bezhenar V.F. The scar on the uterus after cesarean section and the optimal algorithm for diagnostics. Diagnostic Radiology and Radiotherapy. 2019; (2): 85-90. (in Russian)]. http://dx.doi.org/10.22328/2079-5343-2019-10-2-85-90.
- Satpathy G., Kumar I., Matah M., Verma A. Comparative accuracy of magnetic resonance morphometry and sonography in assessment of post-cesarean uterine scar. Indian J. Radiol. Imaging. 2018; 28(2): 169-74. https://dx.doi.org/10.4103/ijri.IJRI_325_17.
- Сидорова Т.А., Мартынов С.А., Адамян Л.В., Летуновская А.Б., Бойкова Ю.В. Сравнение эффективности ультразвуковых методов диагностики в оценке дефектов рубца на матке после операции кесарева сечения. Акушерство и гинекология. 2022; 4: 132-40. [Sidorova T.A., Martynov S.A., Adamyan L.V., Letunovskaya A.B., Boykova Yu.V. Comparison of the effectiveness of ultrasound diagnosis in assessment of uterine scar defets after cesarean section. Obstetrics and Gynecology. 2022; (4): 132-40. (in Russian)].https://dx.doi.org/10.18565/aig.2022.4.132-140.
- Jordans I.P.M., de Leeuw R.A., Amso N.N., de Leeuw R.A., Stegwee S.I., Barri-Soldevila P.N. et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet. Gynecol. 2019; 53(1): 107-15. https://dx.doi.org/10.1002/uog.19049.
- Baranov A., Gunnarsson G., Salvesen K.Å., Isberg P.E., Vikhareva O. Assessment of Cesarean hysterotomy scar in non-pregnant women: reliability of transvaginal sonography with and without contrast enhancement. Ultrasound Obstet. Gynecol. 2016; 47(4): 499-505. https://dx.doi.org/10.1002/uog.14833.
Received 07.11.2022
Accepted 04.04.2023
About the Authors
Tatyana A. Sukhareva, PhD Student at the Gynecological Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-77-83,t_sidorova@oparina4.ru, https://orcid.org/0000-0002-5508-3611, 117997, Russia, Moscow, Ac. Oparina str., 4.
Sergey A. Martynov, Dr. Med. Sci., Leading Researcher at Gynecological Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
+7(495)438-77-83, s_martynov@oparina4.ru, https://orcid.org/0000-0002-6795-1033, 117997, Russia, Moscow, Ac. Oparina str., 4.
Elena A. Kulabukhova, PhD, Radiologist at the Department of Radiation Diagnostics, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
+7(495)438-76-47, e_kulabukhova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Polina V. Uchevatkina, Radiologist at the Department of Radiation Diagnostics, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-76-47, p_uchevatkina@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Anna B. Letunovskaya, PhD, Obstetrician-Gynecologist, Obstetric Diagnostic Sonographer, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, annletynovskaya@yandex.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Yulia V. Boykova, PhD, Obstetrician-Gynecologist, Obstetric Diagnostic Sonographer, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
j_boikova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Leyla V. Adamyan, Dr. Med. Sci., Professor, Academician of RAS, Deputy Director for Science, Head of Gynecological Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-77-83, l_adamyan@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Corresponding author: Tatiana A. Sukhareva, t_sidorova@oparina4.ru



