Delivery of patients with a uterine scar who have undergone cell technology in a previous caesarean section
Objective: To determine the likelihood of successful spontaneous delivery in women with myometrial scars who had undergone cell technology, that is, mesenchymal stromal cell derived exosomes (MSCE), during a previous caesarean section.Pekarev O.G., Baranov I.I., Pekareva E.O., Silachev D.N., Pozdnyakov I.M.
Materials and methods: Group 1 (study group) included 60 pregnant and parturient women who underwent cell technologies. Group 2 (control group) consisted of 100 pregnant and parturient women without exosomal support. In addition, an intra-natal assessment of the scar condition was performed in 71 parturient women, including 19 and 22 women from groups 1 and 2, respectively. In addition, the condition of the lower uterine segment was evaluated by ultrasound in 30 women without a post-caesarean uterine scar in the control group. A suprapubic arrangement of a RAB6-D volumetric convex transducer with a frequency of 2–8 MHz and an intracavitary IC5-9-D transducer with a frequency of 4–9 MHz from the GE Voluson E8 device (USA) was used. The primary endpoints were the course of pregnancy and childbirth in patients in the study and control groups. Secondary endpoints included the results of repeated deliveries of patients with uterine scars with and without the use of cell technologies, i.e., exosomal support.
Results: No infectious or inflammatory complications were observed in postpartum women who underwent cell technologies, while 6/100 (6 %) patients in group 2 had symptoms of metroendometritis, which required hospitalization and inpatient treatment. Two patients in group 2 (2%) developed lochiometra, as confirmed by ultrasound and office hysteroscopy. The rate of successful vaginal deliveries was 63.6% (14/22) in the group with prior exosomal support, compared to 20.7% (6/29) in the control group.
Conclusion: This study demonstrated the feasibility of using cell technologies, specifically exosomes derived from mesenchymal stromal cells of placental origin, to improve the repair of postoperative myometrial scars and increase the likelihood of safe spontaneous delivery in women with a history of abdominal delivery. This finding suggests the potential of reducing the cesarean section rate in patients with a post-cesarean uterine scar.
Authors' contributions: Pekarev O.G. – study design, drafting of the manuscript; Baranov I.I., Pozdnyakov I.M. – editing of the manuscript; Pekareva E.O. – data collection and analysis, statistical analysis; Silachev D.N. – study design, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Novosibirsk City Clinical Perinatal Center.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Pekarev O.G., Baranov I.I., Pekareva E.O., Silachev D.N.,
Pozdnyakov I.M. Delivery of patients with a uterine scar
who have undergone cell technology in a previous caesarean section.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (9): 91-97 (in Russian)
https://dx.doi.org/10.18565/aig.2023.186
Keywords
Modern obstetric science and practice are currently witnessing a pandemic of increased cesarean delivery rates driven by various subjective and objective factors. This increase in rates is evident in nearly double the number of cesarean sections, increasing from 250.7 thousand in 2005 to 390.4 thousand in 2022. Unfortunately, this surge in operative interventions has led to a subsequent increase in infectious and inflammatory complications as well as the occurrence of niches, isthmoceles, uterine ruptures, and more severe complications of placenta previa and placenta accreta. In the present context, a previous myometrial scar remains a significant indication for subsequent abdominal deliveries. However, there is growing interest among obstetricians regarding the possibility of achieving spontaneous vaginal birth in women who have previously undergone uterine surgery. As a result, efforts are underway to explore methods that can enhance myometrial regeneration after a previous cesarean section. Cell technologies have been investigated for over 15 years, with a focus on optimizing reparative processes, although some challenges still need to be overcome for their wider application. Among various pathogenetic mechanisms, the attention is now on the role of exosomes in intercell communication at both the protein and genetic levels, which have shown great promise in enhancing tissue regenerative abilities as modulators.
A series of previous clinical studies conducted at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology, Ministry of Health of the Russian Federation (directed by Academician of the Russian Academy of Sciences G.T. Sukhikh), and at the Novosibirsk City Clinical Perinatal Center (led by Professor I.M. Pozdnyakov), have clearly demonstrated the beneficial impact of cell technologies, specifically the administration of mesenchymal stromal cell exosomes (MSCE), on the postpartum period. These studies aimed to reduce the need for abdominal delivery by increasing the likelihood of achieving spontaneous vaginal birth in women with uterine scars following cesarean sections. The basis for this statement lies in experimental studies using a rat model of cesarean section, which convincingly showed that exosomes can persist in the myometrium for at least eight days.
The objective of this study was to determine the probability of successful spontaneous delivery in women with myometrial scars who had previously received cell technology, specifically mesenchymal stromal cell-derived exosomes (MSCE), during their previous cesarean section.
Materials and methods
The study included 160 patients who were divided into 2 groups depending on the management of the postoperative period.
Group 1 (study group) included 60 pregnant and parturient women who underwent a cesarean section. After suturing the uterus with a single-row continuous vicryl suture, 500 μl of MSCE was injected into the incision area, obtained in the laboratory of cell technologies of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology [15–20, 22]. Permission to use cell technologies was obtained based on the decision of the Research Ethics Committee of Novosibirsk City Clinical Perinatal Center.
Group 2 (control group) consisted of 100 pregnant and parturient women who underwent traditional suturing of the lower segment of the uterus with a single-row continuous Vicryl suture during cesarean section.
In addition, an intrapartum assessment of the scar condition was performed in 71 patients, including 19 from the study group, 22 from the control group, and the condition of the lower segment in 30 parturient women (control group) without a scar on the uterus after cesarean section who underwent ultrasound examination [23]. We used the suprapubic location of a volumetric convex sensor RAB6-D with a frequency of 2–8 MHz and an intracavitary sensor IC5-9-D with a frequency of 4–9 MHz from a GE Voluson E8 device (USA).
The primary end points were the outcomes of pregnancy and childbirth in the study and control groups. The secondary endpoints included the outcomes of repeated deliveries of patients with uterine scars with and without the use of cell technologies, that is, exosomal support.
All patients provided informed consent for the administration of exosomes.
Obtaining a culture of MSCs
The material was obtained from healthy women 25–30 years old who underwent cesarean section at Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
Umbilical cord samples obtained after birth were washed several times in phosphate-buffered saline (PBS; Paneco, Moscow, Russia). After removing the blood vessels, the umbilical cord tissue was crushed into 1–2 mm3 fragments. Cells were grown in a complete nutrient medium (CPM) consisting of DMEM/F12 culture medium (BIOLOT, Moscow, Russia) (1:1), 7% fetal bovine serum (HyClone, Cytiva, Austria) with penicillin (100 U/ml), streptomycin (100 μg/ml) (Gibco, New York, USA), and 2 mM L-glutamine (Paneko, Moscow, Russia) and incubated in a humidified atmosphere with 5% CO2 at 37°C. The nutrient medium was replaced every 3–4 days. The same medium was used to obtain the EMWs produced by MSCs. To do this, it was pre-centrifuged (108,000×g for 1.5 hours at 4°C) to purify the EMV present in fetal bovine serum. The centrifuged medium was added to MSCs at the 3rd passage, which reached 80–90% confluence and was collected after 24 h. Cell growth and morphology were monitored daily using an inverted light microscope. Upon reaching 80% confluence, the cells were trypsinized, centrifuged (1600×g for 3 min), resuspended in PPS, and transferred to new culture flasks. The MSCs used in this study were positive for mesenchymal stem cell markers (CD73 – 98%; CD90 – 100%, CD105 –100%) and contained a small admixture of hematopoietic cells (approximately 4.6% CD14, CD20, CD45, and CD34).
Statistical analysis
Statistical analysis was performed using Microsoft Excel spreadsheets and the GraphPad Prism 6 software package (GraphPad Software, USA). Quantitative variables showing a normal distribution are expressed as mean (M) and standard deviation (SD). When analyzing the parametric data, the mean value and standard deviation were calculated. Differences were considered statistically significant at p<0.05.
Results
The clinical and demographic characteristic analysis of pregnant and parturient women did not reveal significant differences in the comparison groups either in age, or in the frequency of extragenital and genital pathology, or in reproductive history [16].
In the group of patients who underwent cell technology, a previously planned caesarean section was performed in 26/60 (43.3%) pregnant women. In the structure of planned cesarean sections, patients with complete (mixed) breech presentation were predominated, 21/26 (80.8%). In addition, the first planned cesarean section in patients with exosome support was performed in 5/26 (19.2%) pregnant women with marginal placenta previa who had a history of two medical abortions [16]. In all pregnant and parturient women, abdominal delivery was performed using epidural anesthesia under conditions of intraoperative antibacterial prophylaxis, according to clinical recommendations [24]. Among the patients in the control group who did not receive MSCE intraoperatively, there were no significant differences in either the duration of the operation or the total blood loss. Additionally, the study groups did not differ in terms of the duration of the first stage of labor. At the same time, there was a significant difference in the duration of postoperative hospital stay in postoperative women who underwent cell technologies, which was significantly less than that in women without previous use of exosomes (p=0.03). Additionally, patients without exosome support showed a more significant expansion of the uterine cavity (p=0.006). A similar result was observed during the analysis of the blood leukocyte count (p=0.03). At the same time, in patients in the study group, despite a significantly longer duration of ruptured membranes, there were no postpartum infectious or inflammatory complications, while in postpartum women without the administration of exosomes, complications during the postpartum period were recorded in 8/100 (8%) [16].
Since 2019, 22/60 (36.7%) women who received MSCE during their first caesarean section and 29/100 (29%) patients from the control group achieved the desired pregnancies in the natural cycle, which proceeded in both groups without complications. Almost every second, 12/22 (54.5%) patients with a uterine scar from the study group were admitted for childbirth with spontaneous development of labor. In the control group, regular labor was observed in 17/29 (58.6%) pregnant women. The remaining 22/51 (43.1%) pregnant women were hospitalized to prepare for childbirth in a planned manner at 40 weeks of pregnancy and delivered at the Novosibirsk City Clinical Perinatal Center. Their age and gestational ages were comparable and did not differ significantly (Table 1).
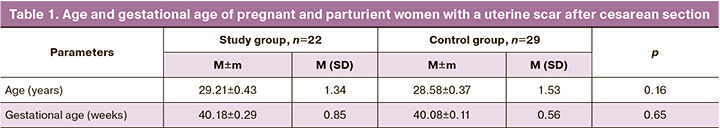
The only indication for pre-induction of labor was the tendency for post-term pregnancy. This condition occurred in 11/22 (50%) pregnant women in the study group and 12/29 (41.4%) patients in the control group. These 23/51 (45.1%) patients with a uterine scar after cesarean section underwent pre-induction using a balloon to accelerate cervical ripening, which does not contradict the Clinical recommendations of the Ministry of Health of the Russian Federation “Singleton birth, delivery by cesarean section” [24].
In the first period, patients after abdominal delivery, for whom spontaneous delivery was programmed in the labor management plan to assess the condition and usefulness of the uterine scar, as well as 30 parturient women with intact myometrium, underwent dynamic ultrasound during both the latent and active phases of labor (Table 2). Analysis of ultrasound parameters showed no significant differences in the thickness of the lower segment between patients who used cell technologies and parturient women with an intact uterus throughout almost the entire active phase of labor (4 and 8 cm – 6.85±0.13 mm and 3.13±0.13 mm, respectively). At the same time, the thickness of the myometrium in the area of the lower segment in women with exosome support in the latent phase (2 cm) and in the middle of the active phase (6 cm) was significantly lower compared to patients without a uterine scar (7.66±0.16 mm and 3.93±0.18 mm, respectively), but significantly higher than in patients without exosome support (p<0.05). At the same time, in patients who did not undergo cell technologies during the first cesarean section, the myometrium remained significantly thinner throughout the entire first stage of labor, both compared to patients in the study group and women with an intact uterus.
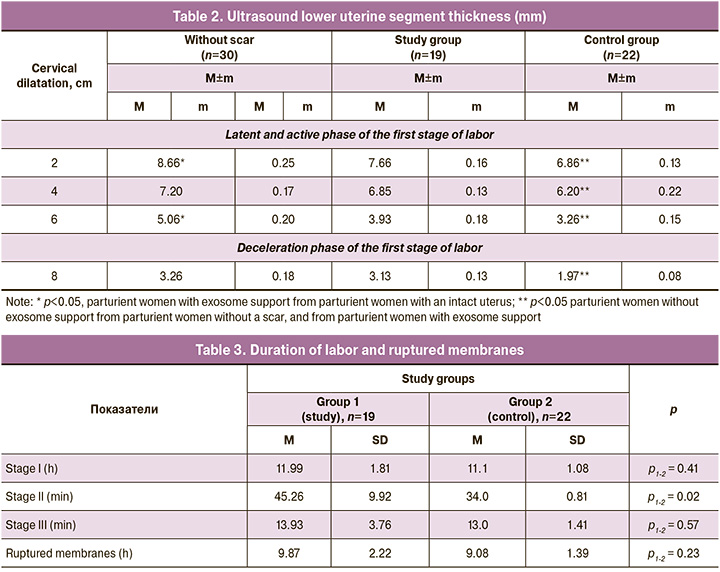
The duration of the first and third stages of labor, as well as the duration of ruptured membranes, both in the group with exosome support and in the control group, were almost the same (Table 3) and corresponded to the normative values specified in the Clinical recommendations of the Russian Academy of Medical Sciences “Singleton birth, spontaneous delivery in occipital presentation (normal birth)” approved in 2021 by the Russian Ministry of Health [24].
Thus, the duration of the first period was in the time range from 11.08±0.32 in group 1 to 11.99±0.41 hours in the control group (p1-2=0.41). It is noteworthy that in 2/19 (10.5%) patients, that is, every tenth patient with a uterine scar after cesarean section who ultimately gave birth spontaneously, the first stage of labor was complicated by primary/secondary weakness of labor, which was the basis for the use of oxytocin under continuous cardiac monitoring.
According to the RSAG Clinical Guidelines (2021), the use of uterotonic drugs, with the exception of prostaglandin E1, is not contraindicated for parturient women with uterine scars after cesarean section. All parturient women with a uterine scar after a cesarean section in the first stage of labor were administered epidural analgesia to relieve the pain of contractions, including 1/19 (5.2%) parturient women to correct uterine incoordination, which also does not contradict the Clinical Guidelines (2021).
As for the duration of the third stage of labor, in groups 1 and 2 it ranged from 13.93±0.97 to 14.71±0.92 minutes (p1-2=0.57). At the same time, in 2/19 (10.5%) patients in the study group and 3/22 (13.6%) in the control group, the third stage of labor was complicated by partial/complete abnormally invasive placenta, which required manual separation and removal of the placenta. The rest of the postpartum women, according to the Clinical Guidelines of the ROAG “Postoperative scar on the uterus, requiring the provision of medical care to the mother during pregnancy, childbirth and the postpartum period,” approved by the Ministry of Health of the Russian Federation in 2021, did not require routine manual inspection of the walls of the uterine cavity. These patients underwent direct ultrasound examination of the uterus in the delivery room. The integrity of the organs was confirmed in all cases.
At the same time, women in the study group had a significantly longer second stage of labor, 45.26±2.56 minutes, compared to the control group, p1-2=0.02). This can be explained by the accumulation of experience and an increase in obstetric patience, despite the fact that 1/19 (5.3%) parturient women in the study group and 1/22 (4.5%) patients in the control group required fetal vacuum extraction because of weakness in pushing when the head was localized on the pelvic floor.
Conclusion
At the time of article submission, 51 pregnant women with uterine scar gave birth. In 14/22 (63.6%) patients in whom cell technologies were used in a previous abdominal delivery, namely, MSCE of placental origin, the delivery was successfully completed through the vaginal canal. The obtained prospective data on the delivery of patients with a uterine scar after cesarean section demonstrated the high effectiveness of the intraoperative use of cell technologies, specifically MSCE, compared with the traditional management of the postpartum period. This is indicated by the absence of infectious and inflammatory complications and a significant increase in scar thickness on intrapartum ultrasound. Patients with exosome support were three times more likely to give birth spontaneously than those in the control group (63.6% and 20.7%, respectively).
References
- Майбородин И.В., Якимова Н.В., Матвеева В.А., Пекарев О.Г., Майбородина Е.И., Пекарева Е.О. Ангиогенез в рубце матки крыс после введения аутологичных мезенхимальных стволовых клеток костномозгового происхождения. Бюллетень экспериментальной биологии и медицины. 2010; 150(12): 705-11. [Mayborodin I.V., Yakimova N.V., Matveeva V.A., Pekarev OG, Mayborodina E.I., Pekareva E.O. Angiogenesis in the uterine rumen of rats after administration of autologous mesenchymal stem cells of bone marrow origin. Bulletin of Experimental Biology and Medicine. 2010; 150(12): 705-11. (in Russian)].
- Майбородин И.В., Якимова Н.В., Матвеева В.А., Пекарев О.Г., Майбородина Е.И., Пекарева Е.О., Ткачук О.К. Морфологический анализ результатов введения аутологичных стволовых стромальных клеток костномозгового происхождения в рубец матки крыс. Морфология. 2010; 138(6): 47-55. [Mayborodin I.V., Yakimova N.V., Matveeva V.A., Pekarev O.G., Maiborodina E.I., Pekareva E.O., Tkachuk O.K. Morphological analysis of the results of the introduction of autologous stromal stem cells of bone marrow origin into the rat uterine scar. Morphology. 2010; 138(6): 47-55. (in Russian)].
- Майбородин И.В., Оноприенко Н.В., Частикин Г.А. Морфологические изменения тканей матки крыс и возможность самопроизвольных родов в результате введения мультипотентных мезенхимных стромальных клеток на фоне гидрометры. Бюллетень экспериментальной биологии и медицины. 2015; 159(4): 511-6. [Mayborodin I.V., Onoprienko N.V., Chastikin G.A. Morphological changes in the tissues of the uterus of rats and the possibility of spontaneous delivery as a result of the introduction of multipotent mesenchymal stromal cells against the background of a hydrometer. Bulletin of Experimental Biology and Medicine. 2015; 159(4): 511-6. (in Russian)].
- Rodrigues M., Yates C.C., Nuschke A., Griffith L., Wells A. The matrikine tenascin-C protects multipotential stromal cells/mesenchymal stem cells from death cytokines such as FasL. Tissue Eng. Part A. 2013; 19(17-18): 1972-83. https://dx.doi.org/10.1089/ten.TEA.2012.0568.
- Майбородин И.В., Матвеева В.А., Маслов Р.В., Оноприенко Н.В., Кузнецова И.В., Частикин Г.А., Аникеев А.А. Некоторые реакции регионарных лимфатических узлов крыс после имплантации в дефект костной ткани мультипотентных стромальных клеток, адсорбированных на полигидроксиалканоате. Морфология. 2016; 149(2): 21-6. [Mayborodin I.V., Matveeva V.A., Maslov R.V., Onoprienko N.V., Kuznetsova I.V., Chastikin G.A., Anikeev A.A. Some reactions of rat regional lymph nodes after implantation of multipotent stromal cells adsorbed on a polyhydroxyalkanoate into a bone defect. Morphology. 2016; 149(2): 21-6. (in Russian)].
- Майбородин И.В., Морозов В.В., Аникеев А.А., Фигуренко Н.Ф., Маслов Р.В., Частикин Г.А., Матвеева В.А., Майбородина В.И. Макрофагальный ответ у крыс на введение мультипотентных мезенхимальных стромальных клеток в регион хирургической травмы. Новости хирургии. 2017; 25(3): 233-41. [Mayborodin I.V., Morozov V.V., Anikeev A.A., Figurenko N.F., Maslov R.V., Chastikin G.A., Matveeva V.A., Mayborodina V.I. Macrophage response in rats to the introduction of multipotent mesenchymal stromal cells into the region of surgical injury. Surgery News. 2017; 25(3): 233-41. (in Russian)].
- Yates C.C., Nuschke A., Rodrigues M., Whaley D., Dechant J.J., Taylor D.P., Wells A. Improved transplanted stem cell survival in a polymer gel supplemented with Tenascin C accelerates healing and reduces scarring of murine skin wounds. Cell Transplant. 2017; 26(1): 103-13.https://dx.doi.org/10.3727/096368916X692249.
- Takeda Y.S., Xu Q. Neuronal differentiation of human mesenchymal stem cells using exosomes derived from differentiating neuronal cells. PLoS One. 2015;10(8): e0135111. https://dx.doi.org/10.1371journal.pone.0135111.
- Furuta T., Miyaki S., Ishitobi H., Ogura T., Kato Y., Kamei N. et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med. 2016; 5(12): 1620-30. https://dx.doi.org/10.5966/sctm.2015-0285.
- Narayanan R., Huang C.C., Ravindran S. Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int. 2016; 2016: 3808674. https://dx.doi.org/10.1155/2016/3808674.
- Van der Pol E., Böing A. N., Harrison P., Sturk A., Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012; 64(3): 676-705. https://dx.doi.org/10.1124/pr.112.005983.
- Akers J.C., Gonda D., Kim R., Carter B.S., Chen C.C. Biogenesis of extra-cellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J.f Neurooncol. 2013; 113(1): 1-11. https://dx.doi.org/10.1007/s11060-013-1084-8.
- Février B., Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004; 16(4): 415-21.https://dx.doi.org/10.1016/j.ceb.2004.06.003.
- Пекарева Е.О., Баранов И.И., Пекарев О.Г. Применение клеточных технологий в экспериментальной и клинической акушерской практике (обзор литературы). Акушерство и гинекология: новости, мнения, обучение. 2022; 10(4): 31-7. [Pekareva E.O., Baranov I.I., Pekarev O.G. Use of cell technologies in experimental and clinical obstetric practice (literature review). Obstetrics and Gynecology: News, Opinions, Training. 2022; 10(4): 31-7.(in Russian)]. https://dx.doi.org/10.33029/2303-9698-2022-10-4-31-37.
- Pekarev O.G., Pekareva E.O., Mayborodin I.V., Silachev D.N., Baranov I.I., Pozdnyakov I.M. et al. The potential of extracellular microvesicles of mesenchymal stromal cells in obstetrics. J. Matern. Fetal Neonatal Med. 2022; 35(25): 7523-5. https://dx.doi.org/10.1080/14767058.2021.1951213.
- Сухих Г.Т., Пекарева Е.О., Пекарев О.Г., Силачев Д.Н., Майбородин И.В., Баранов И.И., Поздняков И.М., Бушуева Н.С. Возможности родоразрешения пациенток, которым в ходе предшествующего кесарева сечения вводились экстрацеллюлярные микровезикулы мезенхимальных стромальных клеток. Акушерство и гинекология. 2022; 4: 103-14. [Sukhikh G.T., Pekareva E.O., Pekarev O.G., Silachev D.N., Maiborodin I.V., Baranov I.I., Pozdnyakov I.M., Bushueva N.S. Feasibility of delivery in patients receiving mesenchymal stromal cell-derived extracellular microvesicles during the previous caesarean section. Obstetrics and Gynecology. 2022; (4): 103-14.(in Russian)]. https://dx.doi.org/10.18565/aig.2022.4.103-114.
- Сухих Г.Т., Пекарев О.Г., Майбородин И.В., Силачев Д.Н., Шевцова Ю.А., Горюнов К.В., Оноприенко Н.В., Майбородина В.И., Галенок Р.В., Новиков А.М., Пекарева Е.О. К вопросу о сохранности экстрацеллюлярных микровезикул мезенхимных стромальных клеток после абдоминального родоразрешения в эксперименте. Клеточные технологии в биологии и медицине. 2020; 1: 3-11. [Sukhikh G.T., Pekarev O.G., Mayborodin I.V., Silachev D.N., Shevtsova Yu.A., Goryunov K.V., Onoprienko N.V., Mayborodina V.I., Galenok R.V., Novikov A.M., Pekareva E.O. On the question of the preservation of extracellular microvesicles of mesenchymal stromal cells after abdominal delivery in the experiment. Cell Technologies in Biology and Medicine. 2020; (1): 3-11. (in Russian)].
- Sukhikh G.T., Pekarev О.G., Maiborodin I.V., Silachev D.N., Shevtsova Y.А., Gоrуunоv K.V., Onoprienko N.V., Maiborodina V.I., Galenok R.V., Novikov A.M., Pekareva Е.О. Preservation of mesenchymal stem cell-derived extracellular vesicles after abdominal delivery in the experiment. Bull. Exp. Biol. Med. 2020; 169(1): 122-9. https://dx.doi.org/10.1007/s10517-020-04838-1.
- Сухих Г.Т., Пекарев О.Г., Пекарева Е.О., Майбородин И.В., Силачев Д.Н., Баранов И.И., Поздняков И.М., Бушуева Н.С., Новиков А.М. Первые результаты клинического применения экстрацеллюлярных микровезикул мезенхимальных стромальных клеток после абдоминального родоразрешения. Акушерство и гинекология. 2021; 1: 52-60. [Sukhikh G.T., Pekarev O.G., Pekareva E.O., Maiborodin I.V., Silachev D.N., Baranov I.I., Pozdnyakov I.M., Bushueva N.S., Novikov A.V. Initial results of clinical application of mesenchymal stromal stem cell-derived extracellular microvesicles after abdominal delivery. Obstetrics and Gynecology. 2021; (1): 52-60.(in Russian)]. https://dx.doi.org/10.18565/aig.2021.1.52-60.
- Pekarev O.G., Pekareva E.O., Mayborodin I.V., Silachev D.N., Baranov I.I., Pozdnyakov I.M., Bushueva N.S., Novikov A.M., Sukhikh G.T. The potential of extracellular microvesicles of mesenchymal stromal cells in obstetrics. J. Matern. Fetal. Neonatal. Med. 2022; 35(25): 7523-5. https://dx.doi.org/10.1080/14767058.2021.1951213.
- Пекарева Е.О. Предварительные итоги экспериментального и клинического применения экстрацеллюлярных микровезикул мезенхимальных стромальных клеток после кесарева сечения. Акушерство и гинекология: новости, мнения, обучение. 2021; 9(4): 36-43. [Pekareva E.O. The results of experimental and clinical application of extracellular microvesicles of mesenchymal stromal cells after caesarean section. Obstetrics and Gynecology: News, Opinions, Training. 2021; 9(4): 36-43. (in Russian)].https://dx.doi.org/10.33029/2303-9698-2021-9-4-36-43.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации «Роды одноплодные, родоразрешение путем кесарева сечения». М.; 2021. 98с. [Russian Society of Obstetricians-Gynecologists, Association of Anesthesiologists-Resuscitators, Association of Obstetric Anesthesiologists-Resuscitators. Clinical guidelines "Childbirth is singular, delivery by caesarean section". Moscow; 2021. 98p. (in Russian)].
- Silachev D.N., Goryunov K.V., Shpilyuk M.A., Beznoschenko O.S., Morozova N.Y., Kraevaya E.E. et al. Effect of MSCs and MSC-derived extracellular vesicles on human blood coagulation. Cells. 2019; 8(3): 258. https://dx.doi.org/10.3390/cells8030258.
- Министерство здравоохранения Российской Федерации «Роды одноплодные, самопроизвольное родоразрешение в затылочном предлежании (нормальные роды)». М.; 2021. 66с. [Ministry of Health of the Russian Federation "Single births, spontaneous delivery in the occipital region (normal childbirth)". Moscow; 2021. 66p. (in Russian)].
Received 09.08.2023
Accepted 01.09.2023
About the Authors
Oleg G. Pekarev, Dr. Med. Sci., Professor, Deputy Director of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, o_pekarev@oparina4.ru, https://orcid.org/0000-0001-7122-6830, 117997, Russia, Moscow,Аc. Oparin str., 4.
Igor I. Baranov, Dr. Med. Sci., Professor, Head of the Department of Scientific and Educational Programs, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, i_baranov@oparina4.ru, https://orcid.org/0000-0002-9813-2823,
117997, Russia, Moscow, Аc. Oparin str., 4.
Evgenia O. Pekareva, Dr. Med. Sci., obstetrician-gynecologist, Novosibirsk City Clinical Perinatal Center, https://orchid.org/0000-0003-0581-9755, 630090, Russia,
Novosibirsk, A. Lezhen str., 32.
Denis N. Silachev, Dr. Bio. Sci., Head of the Cell Technologies Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, https://orcid.org/0000-0003-0581-9755, 117997, Russia, Moscow, Аc. Oparin str., 4.
Ivan M. Pozdnyakov, Dr. Med. Sci., Professor, Chief Physician, Novosibirsk City Clinical Perinatal Center, https://orcid.org/0000-0003-0600-3053, 630090, Russia,
Novosibirsk, A. Lezhen str., 32.



