Feasibility of delivery in patients receiving mesenchymal stromal cell-derived extracellular microvesicles during the previous caesarean section
Objective: To investigate the postoperative course of patients receiving exosome – extracellular microvesicles (EMV) of mesenchymal stromal cells (MSC) during a previous cesarean section and the results of subsequent delivery.Sukhikh G.T., Pekareva E.O., Pekarev O.G., Silachev D.N., Maiborodin I.V., Baranov I.I., Pozdnyakov I.M., Bushueva N.S.
Materials and methods: The study group included 60 women who received 500 μl of MSC EMV. The control group consisted of 100 patients without exosomal support. At the stage of delivery, patients were further divided into subgroup a (spontaneous delivery) and b (repeat cesarean section).
Results: The study group had no septic complications, while in the control group 6% and 2% of the patients developed postpartum endometritis and lochimetra, respectively. In the study group, 5/8 (62.5%) patients had spontaneous labor, while in the control group 16/20 (80%) women underwent a repeat cesarean section. Nineteen biopsy specimens from the dissected scars were submitted for pathomorphological examination.
Conclusion: Intraoperative injection of EMV was highly effective in complete scar formation compared to the control group. In 5/8 (62.5%) patients with exosomal support resulted in spontaneous delivery; in the control group, this figure was 4/20 (20%). The study showed the feasibility of using MSC EMV to reduce abdominal delivery rate in patients with post-cesarean uterine scar.
Keywords
In the 21st century, there has been a long-awaited paradigm shift in terms of repeat cesarean abdominal delivery in patients with a uterine scar. An almost twofold increased cesarean section rate from 250.7 thousand in 2005 to 422.5 thousand in 2020 was associated with an increased frequency of septic complications and uterine ruptures. The myometrial scar ranks first among the indications for a repeat cesarean section. In this context, new methods to improve myometrium repair after surgery so that 2 to 3 years after cesarean birth, a woman could give birth naturally. Cellular technologies have been successfully used in clinical medicine for 15 years to increase tissue regeneration [1–3]. However, there are certain difficulties with their wide implementation [4–7]. Among the variety of unique mechanisms of action of stem cells, their ability to interact and exchange both protein and genetic material through the secretion of microvesicles deserves the closest attention. These intercellular communicators are promising in terms of improving tissue repair, since they can participate in the transmission of intercellular information and work as a modulator [8–13].
Based on the above, we believe that the use of extracellular microvesicles (EMVs) can improve the postoperative course and increase the likelihood of further spontaneous delivery in patients with a uterine scar from the cesarean section. This optimism is based on experimental studies in which a Caesarean section model was created in inbred Wag laboratory rats. Already 4 years ago, the experimental studies on the cesarean birth model using an inbred line of Wag rats showed that both in intact animals and after a cesarean section, exosomes remain in the myometrium for at least eight days [14–18].
The present study aimed to investigate the postoperative course of patients receiving exosome – extracellular microvesicles (EMV) of placenta-derived mesenchymal stromal cells (MSC) during a previous cesarean section and the results of subsequent delivery.
Materials and methods
The study included 160 patients divided into two groups categorized by type of postoperative care. Group 1 (study group) consisted of 60 pregnant and parturient women who received 500 μl EMV MSC in the incision area during abdominal delivery and continuous closure of the low transverse uterine incision with vicryl suture. EMV MSCs were obtained in the Cell Technologies Laboratory of the V.I. Kulakov NMRC for OG&P [14, 15, 19]. Patients in the control group (n=100) underwent traditional continuous single-layer closure of the uterine incision with vicryl suture.
Depending on the delivery management strategy, pregnant women in the study and control groups were further divided into subgroups: a – vaginal delivery and b – repeat cesarean section. All patients signed an informed consent for exosome injection.
MSC culture
Human umbilical cord samples were obtained from healthy women aged 25 to 30 years who gave birth by cesarean section to healthy full-term babies at the V.I. Kulakov NMRC for OG&P. Umbilical cords obtained after delivery were washed several times with PBS (PanEco) (PanEco, Moscow, Russia). After removal of blood vessels, umbilical cord tissue was minced to 1–2 mm3 fragments. The cells were grown in complete culture medium consisting of DMEM/F-12 (1:1) media (PanEco) and supplemented with 7% fetal calf serum (Biosera), penicillin (100 U/ml), streptomycin (100 pg/ml) (Gibco), and 2 mM L-glutamine (PanEco) in a humid atmosphere with 5% C02 at 37°C. The medium was replaced every 3–4 days. The same medium was used to obtain MSC-derived EMV. For this purpose, it was pre-centrifuged (108000×g for 1.5 h at 4°C) to purify fetal bovine serum from EMVs. Centrifuged medium was added to MSC at the third passageway, reaching a confluence of 80–90% and was collected after 24 h. Cell growth and morphology were monitored daily under an inverted light microscope. After attaining 80% confluence, the cells were trypsinized, centrifuged (1600g, 3 min), resuspended in complete culture medium, and transferred to new culture vials. The MSCs used were positive for MSC markers (CD73 – 98%, CD90 – 100%, CD105 – 100%) and contained a small admixture of hematopoietic cells (about 4.6% CD14, CD20, CD45, and CD34).
Isolation of EMVs from conditioned medium
EMVs were isolated from conditioned medium by differential centrifugation [19]. The medium collected 24 h after addition was subjected to successive centrifugations to remove dead cells and debris (400×g for 10 min and 10000×g at 4°C for 30 min). The resulting supernatant was used for EMV isolation by ultracentrifugation at 108000×g for 1.5 h at 4°C on an Avanti JXN-30 high-speed centrifuge (Beckman Coulter Inc., USA), followed by washing the EMV precipitate in PBS and re-centrifugation under similar conditions. The resultant EMV precipitate was resuspended in 500 μl of PBS. Vesicle samples were stored at -80°C.
Quantitative and qualitative analysis of EMV
The EMVs were characterized by nanoparticle tracking analysis (NTA) in liquid samples. For this purpose, an NTA LM10 system equipped with a blue laser (405 nm, 60 MW, NanoSight technology, London, UK) and a CMOS camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan) was used. Each EMV sample was diluted 10,000 times with PBS to achieve the optimum linear measurement range of the instrument (12–22 particles/frame). Imaging was performed in 12 repetitions of 1 minute, each at 30 frames per second. The NTA 2.3 software was used for the analysis of concentration and size distribution with the following video processing settings: calibration, 166 nm/pixel; blur, automatic; detection threshold, 8; minimum track length, automatic; minimum expected particle size, 30 nm; temperature, 24.7°C; viscosity, 0.90 cp. Calibration (size and fluorescence) was performed using the manufacturer's standards (Malvern Panalytical, UK). NTA showed that an EMV population consisting mainly of exosomes (40–150 nm) with an admixture of microvesicles with an average nanoparticle size of 124.2 ± 6.67 nm and a concentration of 4.1×1010 particles/ml was obtained. At the same time, the highest proportion of microparticles (more than 54%) in the EMV population was distributed in the range of 60–160 nm.
Morphological analysis
In 19/28 (67.8%) patients, biopsy specimens of excised scars and sections of the lower uterine segment were submitted for morphological analysis. All patients signed an informed consent for biopsy. The material was fixed in 4% paraformaldehyde solution in phosphate buffer (pH 7.4) for at least 24 hours, dehydrated in a series of increasing concentrations of ethanol, cleared in xylene, and encased in paraffin. Sections 5 µm thick were stained with hematoxylin and eosin, Van Gieson and Romanowsky [20] and studied with a Triton light microscope (Seti, Belgium). Morphometric study of the structure of different sections of the uterine scar in the patients was performed in accordance with the recommendations on theoretical substantiation and specific examples of the application of these methods [21].
Statistical analysis
Statistical analysis was performed using Microsoft Excel spreadsheets and the GraphPad Prism 6 software (GraphPad Software, USA). Quantitative variables showing a normal distribution were expressed as means (M) and standard deviation (SD). Differences were considered significant at p<0.05.
Results
The first intraoperative administration of placenta-derived EMV in world obstetric practice was performed on November 14, 2019. The administration of EMV MSC was approved by the Research Ethics Committee of the Novosibirsk City Clinical Perinatal Center and patient K., 24 years old (delivery notes No. 5894), provided informed consent. The 60 patients included in the study who received intraoperative exosomal support wished to give birth repeatedly and spontaneously in 2–3 years.
The ages (in years) of pregnant and puerperal women are presented in Table 1. As can be seen in the presented table, the patients were young [27.25 (4.13) years in the main group and 26.28 (3.64) years in the control group], and the age did not differ significantly between the groups (p=0.12).
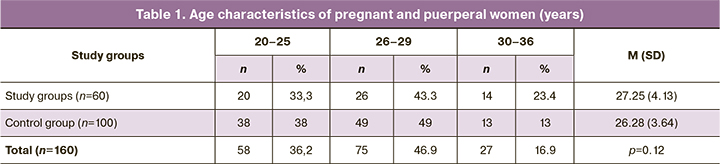
The clinical and demographic characteristics of the study participants are presented in Table 2.
Regarding somatic pathology, there were no significantly significant differences between pregnant and puerperal women in the comparison groups. Almost every second woman had chronic tonsillitis (51 to 56.6%). More than 40% had been diagnosed with chronic kidney inflammation, 19% had bronchitis, and one in six (16%) was obese. Nearly half of the pregnant women (45 to 48.3%) who underwent Cesarean section were not working and were engaged in household chores.
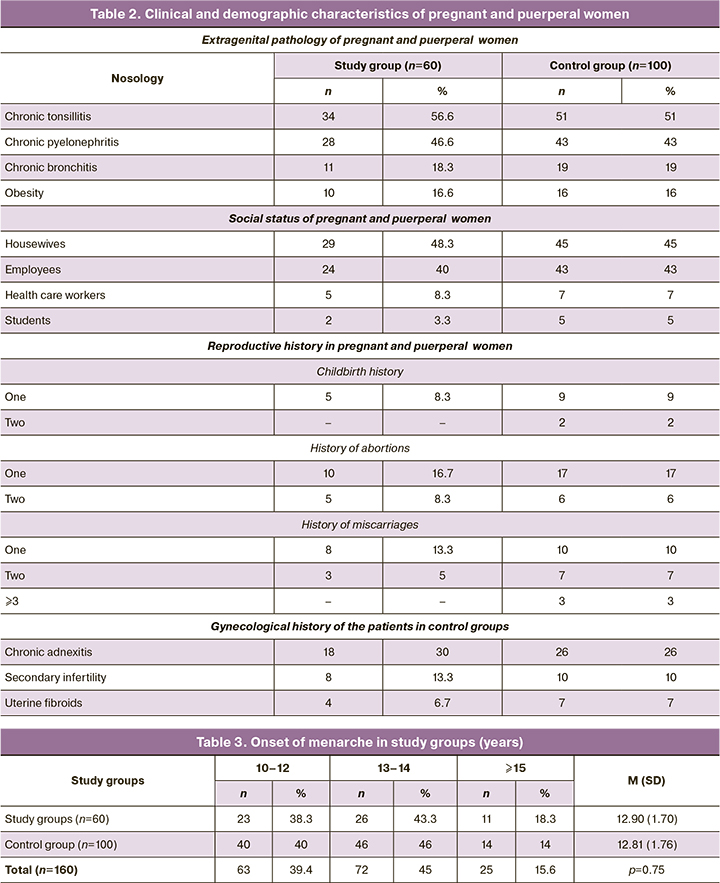
It should be noted that 15/60 (25%) patients in the exosomal-supported group and 23/100 (23%) in the control group had a history of voluntary induced termination of pregnancy. This could be the cause of spontaneous miscarriage in 11/60 (18.3%) and 17/100 (17%) women in the study and control groups, respectively.
Baseline clinical evaluation and history collection revealed a high incidence of previous gynecological infections and inflammatory diseases, which could have been a cause of infertility. Almost one in eight pregnant women in the study group complained of this condition, 8/60 (13.3%), and one in ten, 10/100 (10%), in the control group.
The age of onset of menarche was practically statistically average for women in our country and was 12.9 (1.7) years in the exosomal support group and 12.81 (1.76) years in the control group (Table 3), not significantly different from each other (p=0.75).
Our analysis of the clinical data of pregnant and puerperal women did not reveal any significant differences between the study groups. In the study group, 26/60 (43.3%) pregnant women underwent the first prior planned abdominal delivery (Table 4).
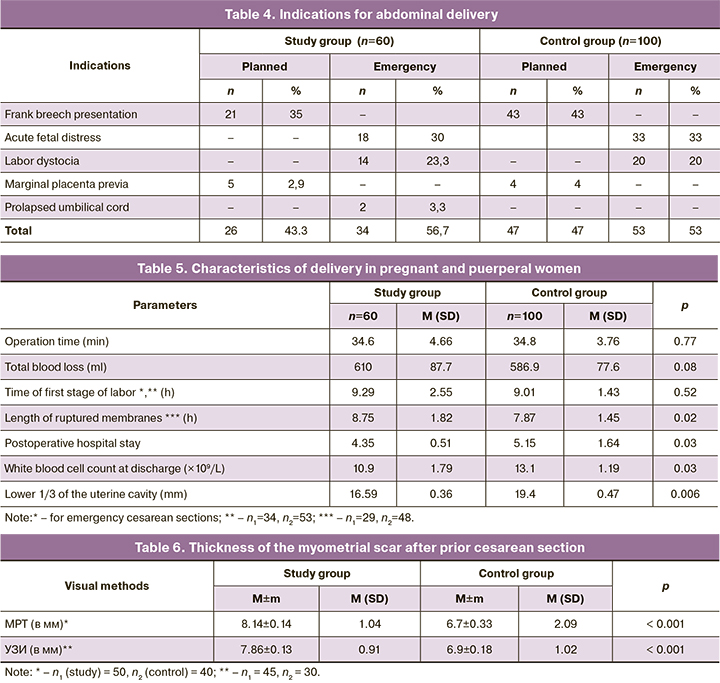
Among patients undergoing elective cesarean sections, 21/26 (80.8%) had frank breech presentation. Moreover, 5/26 (19.2%) patients with exosomal support who had a history of two induced abortions had their first cesarean section due to partial placenta previa (Table 4). In the study group, 34/60 (56.7%) women had an emergency delivery and received 500 μL of placenta-derived EMV intraoperatively during the preceding cesarean section. Almost one in three women in the study group, 18/60 (30%), was operated on for acute fetal distress, one in four, 14/60 (23.3%), for labor dystocia that could not be medically corrected, and 2/60 (3.3%) women were operated on for umbilical cord prolapse. The pattern of indications for the first abdominal delivery in the control group patients did not differ significantly.
All women underwent cesarean section under epidural anesthesia with single-dose antibiotic prophylaxis. There were no significant differences between the group in operating time [(34.8 (3.76) vs. 34.6 (4.66) min, p=0.77)], intraoperative blood loss [(586.9 (77.6) vs. 610.0 (87.7) ml, p=0.08)], or duration of the first period of labor [(9.01 (1.43) vs. 9.29 (2.55) h, p=0.52)] (Table 5).
There were significant differences between the study and control groups in the duration of ruptured membranes [(8.75 (1.82) h in the exosomal support group vs 7.87 (1.45) h in the control group, p=0.02)]. Significant differences were also recorded in the duration of the postoperative hospital stay, which was significantly shorter in patients with exosomal support than in the control group [(4.35 (0.51) vs. 5.15 (1.64), p=0.03)]. In our opinion, this could be due to delayed uterine involution in the control group patients. The enlargement of the uterine cavity was significantly greater in women without exosomal support compared with those in the control group [(19.4 (0.47) vs 16.59 (0.36) mm, respectively, p=0.006)]. Similar dynamics were seen in the leukocyte count at discharge, which was 10.9 (1.79)×109/l in the group with intraoperative injection of exosomes, and their number was significantly lower than that in the control group [13.1 (1.19)×109/l, p=0.03)]. Patients in the study group, despite a significantly longer ruptured membranes, had no postpartum infectious and inflammatory complications after delivery, while 8/100 (8%) of women giving birth without exosome administration had a complicated postpartum course, including endometritis diagnosed in 6/100 (6%) cases and lochimetra in 2/100 (2%) cases.
As women in the control groups were planning repeat pregnancies, magnetic resonance imaging (MRI) was performed 6 months after a previous cesarean section in 50 patients with prior exosomal support and in 40 patients with traditional postpartum management. Patients who received placenta – derived EMV during the first cesarean section had no signs of uterine scar dehiscence and niche. The thickness of myometrium in the scar area was 8.14 (1.04) mm, whereas in the control group it was 6.7 (2.09) mm (p<0.001). At the same time, 6/40 (15%) women in the control group who had postpartum metroendometritis had less than 2 mm thinning of the postoperative scar with the formation of a 4–6 mm niche of (Table 6).
To objectify the condition of the postoperative uterine scar, 45 patients in the study group and 30 women in the control group underwent ultrasound examination and office hysteroscopy 6–12 months after the previous cesarean section. Ultrasound findings showed that even at the stage of preconception care, an absolutely homogeneous myometrium without dense inclusions and that did not differ in appearance from other uterine walls was detected in 39/45 (86.7%), that is, in the overwhelming majority of patients in the study group (Table 6). The myometrial thickness in the scar area in this cohort of women was 7.86 (0.91) mm, which was significantly higher than in the control group [6.9 (1.02) mm, p<0.001)]. In the control group, 7/30 (23.3%) had large masses in combination with echo-negative surrounding tissue, larger echo-negative inclusions in the scar area and surrounding tissue, and between the uterus and bladder with local thinning of the scar.
Hysteroscopy in the study group revealed internal endometriosis in 4/45 (8.9%) women. There were no other signs of intrauterine pathology: the uterine scar area was similar in structure to other areas, and there were few connective-tissue fibers with a weak vascular network in these areas. In the control group, 8/30 (26.6%) women had ultrasound signs of incompleteness: straightening of the scar, formation of angles and asymmetry, presence of depressions and bulges, indentation along part or all scars, and change of color above the scar, up to white color.
Since 2019, 8 women who received placenta-derived EMV during their first cesarean section and 20 patients in the control group have conceived in the natural cycle, which proceeded without complications in both study groups. Every second [4/8 (50%)] patient with a uterine scar in the study group entered labor with spontaneous development of labor activity. In the control group, regular labor was observed in 12/20 (60%) pregnant women. The remaining 12/28 (42.8%) pregnant women from the control groups were hospitalized for planned labor preparation at 40 weeks of gestation and delivered at Novosibirsk City Clinical Perinatal Center. Their age characteristics and gestational age were comparable and did not differ significantly (Table 7).
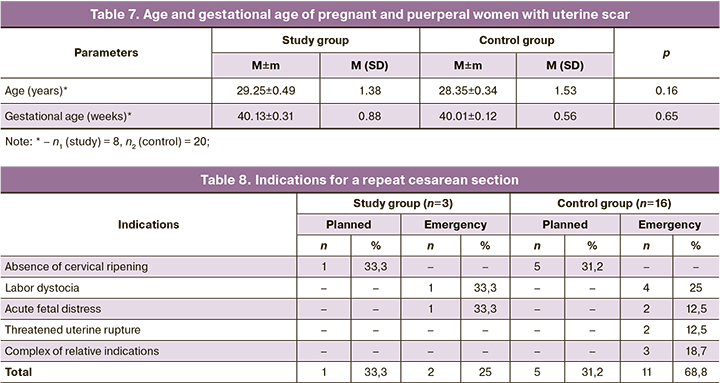
The only indication for induction of labor was a tendency toward prolonged gestationб which occurred in 4/8 (50%) pregnant women in the study group and 8/20 (40%) patients in the control group. These 12/28 (42.8%) patients with a uterine scar after cesarean section underwent balloon induction for labor to accelerate cervical ripening, which does not contradict the Clinical Recommendations of Ministry of Health of Russia "Singleton Pregnancy Labor, Delivery by Cesarean Section" [22].
Thus, at the time of the manuscript drafting, 28 pregnant women with a uterine scar delivered their babies. In 5/8 (62.5%) patients who had received placenta-derived EMV during a previous abdominal delivery, pregnancy ended successfully with vaginal delivery of healthy babies. At the same time, 16/20 (80%) pregnant and puerperal women in the control group underwent a second cesarean section. The indications for repeat abdominal delivery are presented in Table 8.
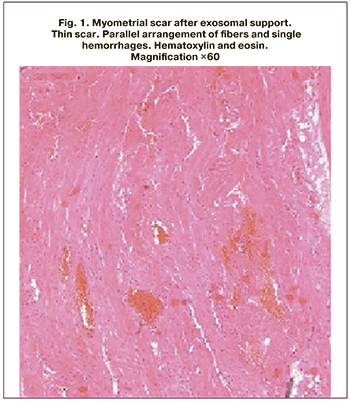
Since 19/28 (67.8%) patients could not avoid a second abdominal delivery, biopsy samples of excised scars and sections of the lower uterine segment of all of these women were submitted to pathomorphological examination. The tissues of the lower uterine segment, fragments of which were obtained during scar excision after exosomal support, were characterized by blood vessel congestion. Small hemorrhages were present in some sections of the myometrium, varying in time of occurrence. The vessels in the myometrium were slightly dilated; lymphatic vessels were dilated in some areas. The connective tissue content was not expressed; small interlayers of this tissue were present mainly along the course of the vessels. The number of tissue leukocytes in the myometrium was assessed as moderate. Leukocytic infiltrates with a predominance of neutrophils and lymphocytes were present. The numerical density of tissue leukocytes in the cervix did not differ significantly between women with and without exosomal support. The fragments from the study group were characterized by a parallel arrangement of fibers and single hemorrhages, dilated full blood vessels, and single lymphocytes in the myometrial scar tissue (Fig. 1, 3, 5). While the control group patients showed chaotic arrangement of fibers and extensive hemorrhages in the scar tissue, with many vessels with sclerotic walls and increased numerical density of all leukocytes with large number of neutrophils and erythrocytes in the myometrial scar tissue (Fig. 2, 4, 6).
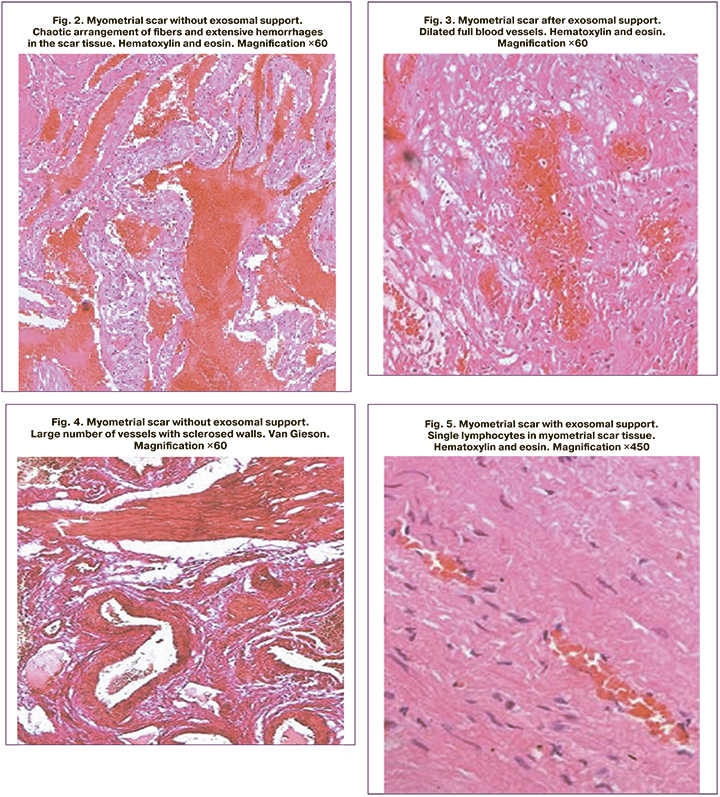
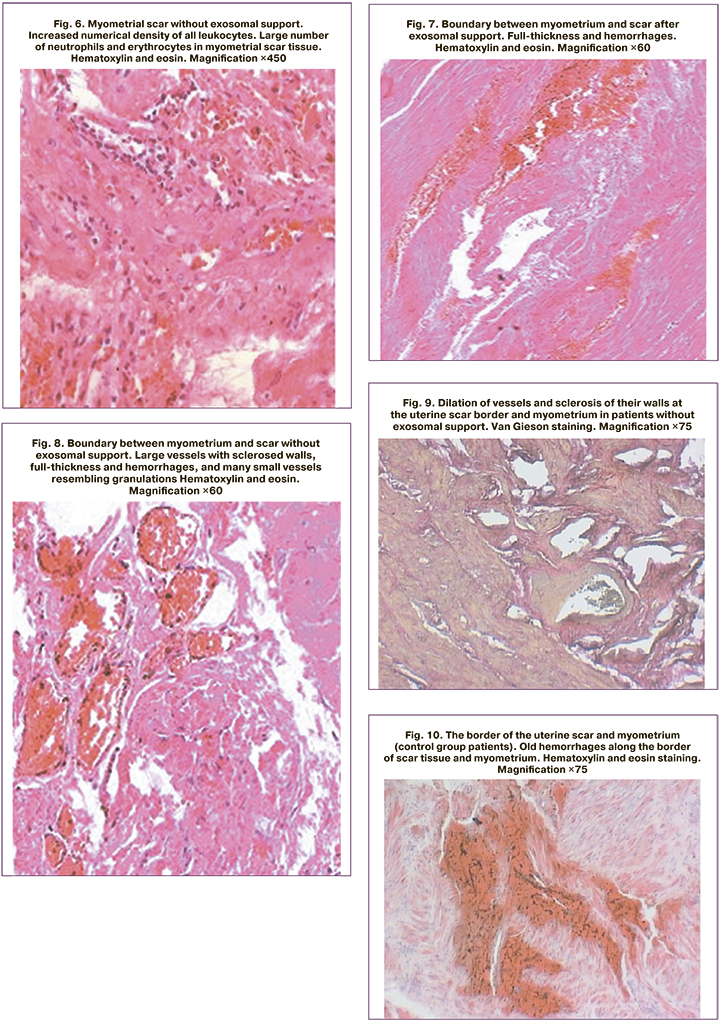
The border between myometrium and scar in the patients of the study group was characterized by full-thickness and single hemorrhages (Fig. 7). Unlike the study group, the border between the uterine scar and the myometrium in the control group patients clearly showed sclerosis of the vascular wall and a sharp dilation of the vessels themselves, mainly in the scar area (Fig. 8). Both in the uterine scar and in the myometrium of the control group, there was vasodilation and sclerosis of their walls at the border of the uterine scar, varying in magnitude and timing of hemorrhage. Furthermore, there were numerous ruptures of the vessels themselves, old hemorrhages along the border of the scar tissue and myometrium, mainly in the scar zone and along the border of different tissues (Fig. 9, 10). At the same time, the histological fragments were represented by a scar consisting of well-formed coarse fibrous connective tissue. Besides hemorrhage, various sized cysts lined with endothelium and filled with transparent contents were found on the uterine scar border. It was on the border of the scar with myometrium that tissue strength was strongly reduced and there was dehiscence of the border with myometrium rather than of the scar itself. Due to the presence of hemorrhages and cysts on the border of the uterine scar and myometrium, the durability of these uterine regions was significantly reduced even after a single delivery in the control group patients.
Conclusion
The study findings indicate a higher efficacy of intraoperative EMV administration compared to traditional postpartum management. The efficacy was confirmed by the absence of septic complications, a significant increase in scar thickness according to MRI and ultrasound, as well as the histological pattern of dissected fragments of the lower uterine segment at the border of the scar and myometrium. Patients with exosomal support were three times more likely to deliver spontaneously compared to the control group (62.5% and 20%, respectively).
References
- Майбородин И.В., Якимова Н.В., Матвеева В.А., Пекарев О.Г., Майбородина Е.И., Пекарева Е.О. Ангиогенез в рубце матки крыс после введения аутологичных мезенхимальных стволовых клеток костномозгового происхождения. Бюллетень экспериментальной биологии и медицины. 2010; 150(12): 705-11. [Mayborodin I.V., Yakimova N.V., Matveeva V.A., Pekarev OG, Mayborodina E.I., Pekareva E.O. Angiogenesis in the uterine rumen of rats after administration of autologous mesenchymal stem cells of bone marrow origin. Bulletin of Experimental Biology and Medicine. 2010; 150(12): 705-11. (in Russian)].
- Майбородин И.В., Якимова Н.В., Матвеева В.А., Пекарев О.Г., Майбородина Е.И., Пекарева Е.О., Ткачук О.К. Морфологический анализ результатов введения аутологичных стволовых стромальных клеток костномозгового происхождения в рубец матки крыс. Морфология. 2010; 138(6): 47-55. [Mayborodin I.V., Yakimova N.V., Matveeva V.A., Pekarev O.G., Maiborodina E.I., Pekareva E.O., Tkachuk O.K. Morphological analysis of the results of the introduction of autologous stromal stem cells of bone marrow origin into the rat uterine scar. Morphology. 2010; 138(6): 47-55 (in Russian)].
- Майбородин И.В., Оноприенко Н.В., Частикин Г.А. Морфологические изменения тканей матки крыс и возможность самопроизвольных родов в результате введения мультипотентных мезенхимных стромальных клеток на фоне гидрометры. Бюллетень экспериментальной биологии и медицины. 2015; 159(4): 511-6. [Mayborodin I.V., Onoprienko N.V., Chastikin G.A. Morphological changes in the tissues of the uterus of rats and the possibility of spontaneous delivery as a result of the introduction of multipotent mesenchymal stromal cells against the background of a hydrometer. Bulletin of Experimental Biology and Medicine. 2015; 159(4): 511-6. (in Russian)].
- Rodrigues M., Yates C.C., Nuschke A., Griffith L., Wells A. The matrikine tenascin-C protects multipotential stromal cells/mesenchymal stem cells from death cytokines such as FasL. Tissue Eng. Part A. 2013; 19(17-18): 1972-83. https://dx.doi.org/10.1089/ten.TEA.2012.0568.
- Майбородин И.В., Матвеева В.А., Маслов Р.В., Оноприенко Н.В., Кузнецова И.В., Частикин Г.А., Аникеев А.А. Некоторые реакции регионарных лимфатических узлов крыс после имплантации в дефект костной ткани мультипотентных стромальных клеток, адсорбированных на полигидроксиалканоате. Морфология. 2016; 149(2): 21-6. [Mayborodin I.V., Matveeva V.A., Maslov R.V., Onoprienko N.V., Kuznetsova I.V., Chastikin G.A., Anikeev A.A. Some reactions of rat regional lymph nodes after implantation of multipotent stromal cells adsorbed on a polyhydroxy alkanoate into a bone defect. Morphology. 2016; 149(2): 21-6. (in Russian)].
- Майбородин И.В., Морозов В.В., Аникеев А.А., Фигуренко Н.Ф., Маслов Р.В., Частикин Г.А., Матвеева В.А., Майбородина В.И. Макрофагальный ответ у крыс на введение мультипотентных мезенхимальных стромальных клеток в регион хирургической травмы. Новости хирургии. 2017; 25(3): 233-41. [Mayborodin I.V., Morozov V.V., Anikeev A.A., Figurenko N.F., Maslov R.V., Chastikin G.A., Matveeva V.A., Mayborodina V.I. Macrophage response in rats to the introduction of multipotent mesenchymal stromal cells into the region of surgical injury. Surgery news. 2017; 25(3): 233-41. (in Russian)].
- Yates C.C., Nuschke A., Rodrigues M., Whaley D., Dechant J.J., Taylor D.P., Wells A. Improved transplanted stem cell survival in a polymer gel supplemented with Tenascin C accelerates healing and reduces scarring of murine skin wounds. Cell Transplant. 2017; 26(1): 103-13. https://dx.doi.org/10.3727/096368916X692249.
- Takeda Y.S., Xu Q. Neuronal differentiation of human mesenchymal stem cells using exosomes derived from differentiating neuronal cells. PLoS One. 2015; 10(8): e0135111. https://dx.doi.org/10.1371journal.pone.0135111.
- Furuta T., Miyaki S., Ishitobi H., Ogura T., Kato Y., Kamei N. et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med. 2016; 5(12): 1620-30. https://dx.doi.org/10.5966/sctm.2015-0285.
- Narayanan R., Huang C.C., Ravindran S. Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int. 2016; 2016: 3808674.
- Van der Pol E., Böing A. N., Harrison P., Sturk A., Nieuwland R. Classifica-tion, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012; 64(3): 676-705. https://dx.doi.org/10.1124/pr.112.005983.
- Akers J.C., Gonda D., Kim R., Carter B.S., Chen C.C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013; 113(1): 1-11. https://dx.doi.org/10.1007/s11060-013-1084-8.
- Février B., Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004; 16(4): 415-21. https://dx.doi.org/10.1016/j.ceb.2004.06.003.
- Сухих Г.Т., Пекарев О.Г., Майбородин И.В., Силачев Д.Н., Шевцова Ю.А., Горюнов К.В., Оноприенко Н.В., Майбородина В.И., Галенок Р.В., Новиков А.М., Пекарева Е.О. К вопросу о сохранности экстрацеллюлярных микровезикул мезенхимных стромальных клеток после абдоминального родоразрешения в эксперименте. Клеточные технологии в биологии и медицине. 2020; 1: 3-11. [Sukhikh G.T., Pekarev O.G., Mayborodin I.V., Silachev D.N., Shevtsova Yu.A., Goryunov K.V., Onoprienko N.V., Mayborodina V.I., Galenok R.V., Novikov A.M., Pekareva E.O. On the question of the preservation of extracellular microvesicles of mesenchymal stromal cells after abdominal delivery in the experiment. Cell technologies in biology and medicine. 2020; 1: 3-11. (in Russian)].
- Sukhikh G.T., Pekarev О.G., Maiborodin I.V., Silachev D.N., Shevtsova Y.А., Gоrуunоv K.V., Onoprienko N.V., Maiborodina V.I., Galenok R.V., Novikov A.M., Pekareva Е.О. Preservation of mesenchymal stem cell-derived extracellular vesicles after abdominal delivery in the experiment. Bull. Exp. Biol. Med. 2020; 169(1): 122-9. https://dx.doi.org/10.1007/s10517-020-04838-1.
- Сухих Г.Т., Пекарев О.Г., Пекарева Е.О., Майбородин И.В., Силачев Д.Н., Баранов И.И., Поздняков И.М., Бушуева Н.С., Новиков А.М. Первые результаты клинического применения экстрацеллюлярных микровезикул мезенхимальных стромальных клеток после абдоминального родоразрешения. Акушерство и гинекология. 2021; 1: 52-60. [Sukhikh G.T., Pekarev O.G., Pekareva E.O., Maiborodin I.V., Silachev D.N., Baranov I.I., Pozdnyakov I.M., Bushueva N.S., Novikov A.V. Initial results of clinical application of mesenchymal stromal stem cell-derived extracellular microvesicles after abdominal delivery. Obstetrics and Gynecology. 2021; 1: 52-60. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.1.52-60.
- Pekarev O.G., Pekareva E.O., Mayborodin I.V., Silachev D.N., Baranov I.I., Pozdnyakov I.M., Bushueva N.S., Novikov A.M., Sukhikh G.T. The potential of extra-cellular microvesicles of mesenchymal stromal cells in obstetrics. J. Matern. Fetal Neonatal Med. 2021 Aug 4: 1-3. https://dx.doi.org/10.1080/14767058.2021.1951213.
- Пекарева Е.О. Предварительные итоги экспериментального и клиниче-ского применения экстрацеллюлярных микровезикул мезенхимальных стромальных клеток после кесарева сечения. Акушерство и гинекология: новости, мнения, обучение. 2021; 9(4): 36-43. [Pekareva E.O. The results of experimental and clinical application of extracellular microvesicles of mesenchymal stromal cells after caesarian section. Akuserstvo i ginekologiya: novosti, mneniya, obuchenie/Obstetrics and Gynecology: News, Opinions, Training. 2021; 9(4): 36-43. (in Russian)]. https://dx.doi.org/10.33029/2303-9698-2021-9-4-36-43.
- Silachev D.N., Goryunov K.V., Shpilyuk M.A., Beznoschenko O.S., Morozova N.Y., Kraevaya E.E., Popkov V.A., Pevzner I.B., Zorova L.D., Evtushenko E.A., Starodubtseva N.L., Kononikhin A.S., Bugrova A.E., Evtushenko E.G., Plotnikov E.Y., Zorov D.B., Sukhikh G.T. Effect of MSCs and MSC-derived extracellular vesicles on human blood coagulation. Cells. 2019; 8(3): 258. https://dx.doi.org/10.3390/cells8030258.
- Саркисов Д.С., Перов Ю.Л. Микроскопическая техника. Руководство для врачей и лаборантов. М.: Медицина; 1996. 544 с. [Sarkisov D.S., Perov Yu.L. Microscopic Technique: Manual for Physicians and Laboratory Technicians. Moscow: Medicine, 1996. 544 p. (in Russian)].
- Горчаков В.Н. Морфологические методы исследования сосудистого русла. Новосибирск: СО РАМН; 1997. 440 с. [Gorchakov V.N. Morphological methods of vascular bed study. Novosibirsk: SB RAMS; 1997. 440 p. (in Russian)].
- Клинические рекомендации «Роды одноплодные, родоразрешение путем кесарева сечения». Утверждены Министерством здравоохранения Российской Федерации 4 июня 2021 г. 98 с. [Clinical Recommendations "Single birth, delivery by cesarean section". Approved by the Ministry of Health of the Russian Federation on June 4, 2021. 98 p. (in Russian)].
Received 24.02.2022
Accepted 26.03.2022
About the Authors
Gennady T. Sukhikh, Dr. Med. Sci., Professor, Academician of the RAS, Director of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, g_sukhikh@oparina4.ru, https://orcid.org/0000-0002-7712-1260, 4, Oparina str., Moscow, 117997, Russia.Evgenia O. Pekareva, Ph.D., Obstetrician-Gynecologist, Novosibirsk City Clinical Perinatal Center, pekareva_e@mail.ru, https://orcid.org/0000-0002-6335-2121,
32, Lezhena str., Novosibirsk, 630090, Russia.
Oleg G. Pekarev, Dr. Med. Sci., Professor, Deputy Director of the Institute of Obstetrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
o_pekarev@oparina4.ru, https://orcid.org/0000-0001-7122-6830. 4, Oparina str., Moscow, 117997, Russia.
Denis N. Silachev, Ph.D. (Bio), Head of the Cell Technologies Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, silachevdn@genebee.msu.ru,
https://orcid.org/0000-0003-0581-9755. 4, Oparina str., Moscow, 117997, Russia.
Igor V. Maiborodin, Dr. Med. Sci., Professor, Chief Researcher of the Stem Cell Laboratory, Institute of Chemical Biology and Fundamental Medicine, SB of the RAS,
imai@mail.ru, https://orcid.org/0000-0002-8182-5084, 8, Ac. Lavrentyeva str., Novosibirsk, 630090, Russia.
Igor I. Baranov, Dr. Med. Sci., Professor, Head of the Department of Scientific and Educational Programs, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, i_baranov@oparina4.ru, https://orcid.org/0000-0002-9813-2823, 4, Oparina str., Moscow, 117997, Russia.
Ivan M. Pozdnyakov, Dr. Med. Sci., Professor, Chief Physician, Novosibirsk City Clinical Perinatal Center, https://orcid.org/0000-0003-0600-3053,
32, Lezhena str., Novosibirsk, 630090, Russia.
Natalya S. Bushueva, M.D., Head of the Maternity Department, Novosibirsk City Clinical Perinatal Center, https://orcid.org/0000-0002-1036-6925,
32, Lezhena str., Novosibirsk, 630090, Russia.
Authors' contributions: Sukhikh G.T. – conception and design of the study, manuscript editing; Pekareva E.O. – material collection and processing, statistical analysis; Pekarev O.G. – design of the study, manuscript drafting; Silachev D.N. – design of the study, manuscript editing; Mayborodin I.V. – conducting pathomorphological study, manuscript drafting; Baranov I.I., Pozdnyakov I.M. – manuscript editing; Bushueva N.S. – material collection and processing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Novosibirsk City Clinical Perinatal Center.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Sukhikh G.T., Pekareva E.O., Pekarev O.G.,
Silachev D.N., Maiborodin I.V., Baranov I.I., Pozdnyakov I.M., Bushueva N.S. Feasibility of delivery in patients receiving mesenchymal stromal cell-derived
extracellular microvesicles during the previous caesarean section.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 4: 103-114 (in Russian)
https://dx.doi.org/10.18565/aig.2022.4.103-114



