Risk factors for cesarean uterine scar defect
Aim. To identify risk factors for developing cesarean uterine scar defect using sonographic and the scar's morphological characteristics. Materials and methods. This retrospective study of 164 women with a cesarean uterine scar included 98 (59.7%) women with normal uterine scar thickness (group I) and 66 (40.3%) with thinned uterine scars (group II). We used the data of hospital medical records, antenatal care cards, and the morphology reports of uterine scars after repeat cesarean section. Results. The groups had significant differences in uterine position, active phase of labor, suture technique, infectious complications before or after surgery, intraoperative blood loss, and operating time (all P<0.001). There were also significant differences in uterine scar morphological characteristics (P<0.001). Conclusion. Longer operating time, infectious complications, and uterine retroflection are risk factors for the development of a dehiscent scar. In contrast, intraoperative blood loss < 400 ml and double-row uterine closure technique are factors that reduce the risk of a scar defect.Malysheva A.A, Matukhin V.I., Rukhlyada N.N., Taits A.N., Novitskaya N.Yu.
Keywords
Recently, there has been a tendency towards an increase in the number of cesarean deliveries. According to the World Health Organization (WHO) estimates, cesarean section rates should be between 10–15%, which is associated with a decrease in maternal, neonatal, and infant mortality. An increase in cesarean delivery rates above this level is not associated with a reduction in mortality. However, an increase in cesarean delivery rates of up to 50% can lead to higher complication rates [1]. In most regions of the Russian Federation, abdominal delivery rates have been increasing annually. From 2006 to 2016, these figures have in Russia have increased by 52.2% (from 18.4% to 28%). However, increasing the cesarean section rate does not always reduce perinatal and infant mortality [2].
High cesarean section rates are associated with the risk of short- and long-term complications of subsequent pregnancies, such as uterine rupture and placental abnormalities. In this regard, there has been growing interest in the consequences of a uterine scar after cesarean section [3, 4].
Despite all the advantages of the operation, it results in damage to the myometrium, which inevitably leads to uterine scar formation. Due to regenerative mechanisms, scar tissue replaces the normal myometrium [5]. The scar's connective tissue cannot completely replace uterine smooth muscle tissue in terms of its functional and structural properties, which can lead to complications such as uterine rupture, abnormal placentation, and embryonic implantation in a uterine scar during the next pregnancy [6].
Uterine scar dehiscence after cesarean section also referred to as “niche,” “isthmocele,” “diverticulum,” “uterine cesarean scar defect,” is a defect in the anterior wall of the uterine isthmus at the site of the previous cesarean incision [7]. An increase in operative delivery rate is associated with a higher likelihood of post-cesarean uterine scar dehiscence. Still, currently, there is no clear understanding of the underlying mechanisms of this condition [8]. Also, no significant risk factors have been identified that influence the formation of uterine cesarean scar defect [9].
According to Xiaoyan Tang et al. (2019), the prevalence of thinned uterine cesarean scar ranged from 24% to 70% when assessed by transvaginal sonography and was even higher when combined with sonohysterographic evaluation (56–84%) [10, 11]. Patients with thinned uterine scars are usually asymptomatic, and the most common complaints associated with uterine scar defect are prolonged menstrual bleeding, chronic pelvic pain, dysmenorrhea, and secondary infertility [3, 12, 13].
At present, it is generally believed that cesarean scar defect is the result of incomplete healing of the isthmic myometrium after cesarean section [4]. Although not all women with a history of cesarean section develop cesarean scar defects, it is necessary to determine the risk factors that may influence its development [14, 15].
There is still no consensus about a standardized approach to detecting and measuring the dehiscent post-cesarean uterine scar. There are no reliable morphological characteristics of a thinned uterine scar after cesarean section [6, 8].
The present study aimed to identify risk factors for developing cesarean uterine scar defect using sonographic and the scar's morphological characteristics.
Materials and methods
The study of 164 women with the post-cesarean uterine scar was conducted at the perinatal center of the Saint Petersburg State Pediatric Medical University and the Saint Petersburg I.I. Dzhanelidze Research Institute of Emergency Medicine from January to December 2019. Of them, 98 (59.7%) women had normal uterine scar thickness (group I), and 66 (40.3%) had thinned uterine scars (group II). We used the data of hospital medical records, antenatal care cards, and the morphology reports of uterine scars after repeat cesarean section. The parameters of the scar thickness and signs of scar dehiscence were assessed by ultrasound examination (US) in the third trimester of a pregnancy before the cesarean section. A scar with a residual myometrial thickness of less than 3 mm with a “niche” was considered dehiscent. A surgical specimen of the uterine scar for histological examination was obtained during a repeat cesarean section.
The study inclusion criteria were a history of cesarean section, diagnosis of uterine scar dehiscence after cesarean section confirmed by abdominal ultrasonography, no operations on the uterus, except for the previous cesarean section. Women with a history of a cesarean section who had normal myometrial thickness in the uterine scar area, confirmed by ultrasound, were included in the control group. Detailed obstetric characteristics were collected from each patient's medical records. The characteristics of cesarean uterine scar before the index pregnancy were also evaluated in the presence of ultrasound and magnetic resonance imaging (MRI) findings.
Ultrasound and MRI measurements of a thinned uterine scar included uterine flexion, length (the transverse distance of the thinned scar at its base), width (length of the thinned scar at its base along the cervicoisthmic canal), depth (vertical distance between the base and apex of the thinned scar) and residual myometrial thickness of the thinned scar in accordance with Naji et al. [16]. The volume of a “niche” was defined as the product of length, width, and depth [10, 16].
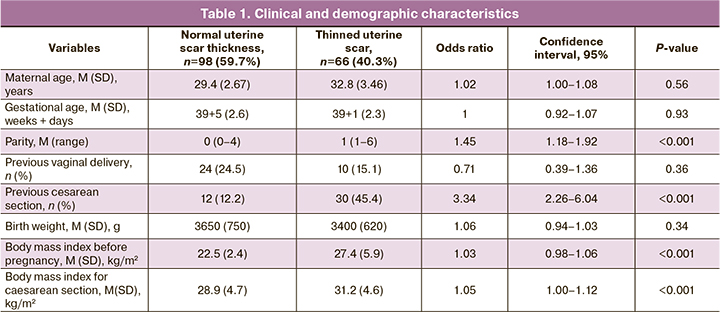
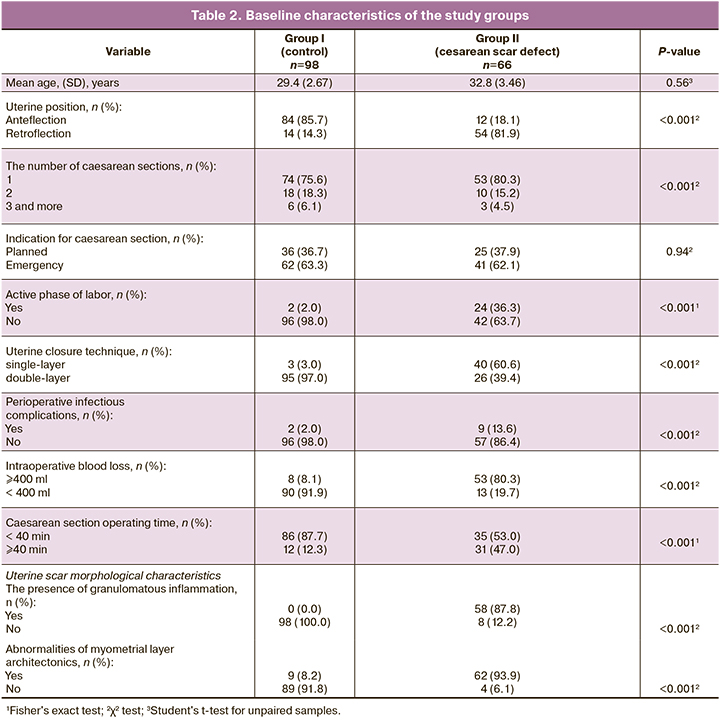
The presence of infectious complications after previous cesarean sections were assessed using medical records by an increase in body temperature of more than 38°C every 4 hours before and after a cesarean section, leukocyte count more than 15x109/l, neutrophils 90% or more, C-reactive protein more than 8 mg/l. All data obtained from previous cesarean section analysis are presented in tables 1 and 2.
Statistical analysis
Statistical analysis was performed using Microsoft Excel 2010 and STASTICA 10. The student's t-test was used to compare continuous variables of two unpaired samples with normal distribution, or Mann–Whitney U-test when they were not normally distributed. Levene's test was used to evaluate the homogeneity of group variances with P>0.05, showing that the compared groups' variances were equal. All qualitative variables were compared using the χ2 test or Fisher's exact test. P<0.001 were considered statistically significant. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD).
Results
The previous cesarean section's data were evaluated and compared with the morphological characteristics of the cesarean uterine scar. The groups with and without cesarean scar dehiscence had significant differences in uterine position, the active phase of labor, suture technique, infectious complications before or after surgery, intraoperative blood loss, and operating time, and the number of cesarean sections (all P<0.001). There were no significant differences between the two groups in age and surgery indications (P>0.05). Overall, 51 women (31.0%) underwent an elective cesarean section, and 113 women (69.0%) had emergency abdominal delivery. There were also significant differences in uterine scar morphological characteristics (P<0.001).
Factors preventing the development of a thinned cesarean uterine scar included intraoperative blood loss <400 ml, operating time <40 minutes, the absence of postoperative infectious complications, and double-row uterine closure technique. Risk factors for the development of a dehiscent scar included blood loss >400 ml, operating time >40 minutes, infectious complications, and a single-row uterine closure technique. It should be noted that among group 2 patients an increase in operating time > 40 minutes (n=31) was associated with technical difficulties during the intervention. Of these, in 14 (45.1%) cases there was uterine hypotension with massive bleeding (> 1000 ml) with the need for blood transfusion in 8 (57%) cases. In 8 cases (25.8%), there was abnormal placental implantation, also accompanied by blood loss (> 1000 ml) with the need for blood transfusion in all these cases. Many women with cesarean scar dehiscence were in the active phase of labor (36.3%), compared with the control group (2.0%).
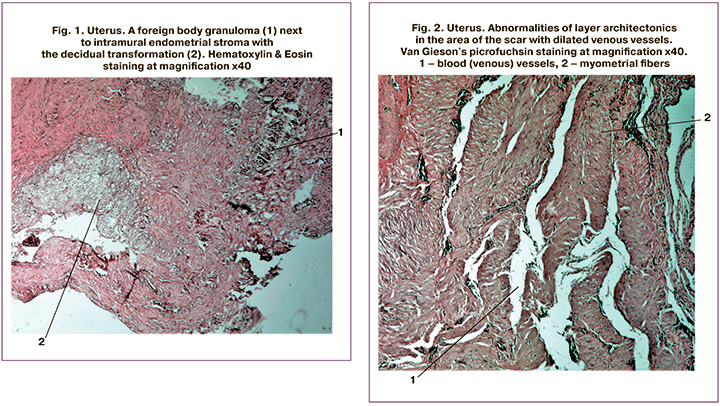
According to reports of morphological examination of the, in 58/66 (87.8%) of thinned scar tissue had granulomatous inflammation with foreign inclusions (Fig. 1), most likely the remnants of suture material. Conversely, none of the specimens of scars with normal thickness had traces of granulomatous inflammation. In 62/ 66 (94%) specimens of thinned scars, there were abnormalities of myometrial layer architectonics (Fig. 2), while among normal scar specimens, these abnormalities were observed only in 9/98 (8.2%) cases.
Discussion
To date, the exact pathophysiology underlying the development of cesarean scar dehiscence is unknown [17]. The present study demonstrated that longer operating time, postpartum infectious complications, and uterine retroflection are risk factors for developing a dehiscent scar. In contrast, intraoperative blood loss < 400 ml and double-row uterine closure technique are factors that reduce the risk of a scar defect. However, this hypothesis needs to be confirmed by further research. It is assumed that a high degree of mechanical stress in the lower uterine segment in a retroflexed position can reduce blood perfusion and oxygenation, which may have a negative effect on healing after cesarean section [4, 9]. Moreover, the active phase of labor contributes to additional overstretching and tension of the lower uterine segment, which leads to a decrease in oxygenation of this area and creates an additional risk for the development of a dehiscent scar, which is consistent with the data of Osser et al. [5].
Single-layer and double-layer uterine closure technique and the type of suture material can affect cesarean scar dehiscence [14]. Vikhareva O.O. et al. (2010) found that cesarean scar dehiscence was more common in women with single-layer (90.9%) than with double-layer uterine closure (9.1%) but the difference was not statistically significant [18].
Further research is needed to confirm this hypothesis. Our study showed that cesarean scar dehiscence is more common after cesarean section with longer operating time and in the presence of fever or infectious complications, both before and after cesarean section.
Histological examination of excised cesarean scar showed morphological differences between normal and thinned scar. In most cases (90%), morphometry of thinned scars showed abnormalities of myometrial layer architectonics, manifested mainly in increased angiogenesis (Fig. 2).
More severe tissue ischemia and slowly absorbable sutures are more likely to lead to the formation of a dehiscent scar [13, 17, 19]. A dehiscent scar's occurrence depends on many factors, including cervical dilatation and, possibly, uterine muscle contractile force, which leads to thinning at the uterine incision site [4, 5, 19]. In 93.9% of dehiscent scar specimens, there was granulomatous inflammation with a foreign body (Fig. 1), most likely a remnant of suture material. Also, the endometrial stroma in the thickness of the myometrium was found in 87.8% of cases. According to our study, the materials for suturing the uterus and the uterine closure technique influence a normal uterine scar formation.
Among suture material properties, the critical factor is its reactivity in relation to tissues and biodegradation rate [14, 20]. Until 2005, the transverse incision of the lower segment of the uterus was traditionally sutured in two layers [2, 3, 10]. Double-layer uterine closure improves hemostasis and uterine wound healing and, possibly, reduces the risk of uterine rupture during subsequent pregnancy [17]. Single-layer uterine closure may be associated with reduced operating time, less tissue destruction, and less foreign material in the wound [1, 10].
Until about the 80s of the XX century, the most commonly used uterine closure technique was double-layer suturing [21]. However, over the past 20 years, a continuous suture, which is not inferior to individual suture effectiveness, has been used. Currently, the more widely used uterine closure technique during cesarean section is single-layer suturing [22]. The advantage of this technique is considered a smaller area of tissue hypoxia, which disrupts the function of myometrial cells, which may contribute to impaired regeneration [18].
When studying the histological specimens of uterine scars, it was shown that the single-layer technique was associated with a lower incidence of postoperative morbidity [15]. These scars were better supplied with blood due to multiple formed vessels. However, according to C. Durnwald (2003), single-row uterine closure was associated with a higher risk of the "niche" of the scar formation [22, 23].
According to research evidence, double-layer uterine closure, compared with a single-row, leads to increased myometrial thickness at the cesarean scar site [13, 22, 23]. This may reduce the development of a "niche," which is the risk of myometrial rupture at the cesarean scar site [3, 19].
Conclusion
Our study's findings suggest that longer operating time, an operation in the active phase of labor, infectious complications, and uterine retroflection are risk factors for the development of a dehiscent scar. In contrast, intraoperative blood loss < 400 ml and double-row uterine closure technique are factors that reduce the risk of a scar defect. It can be assumed that a planned cesarean section reduces the risk of uterine scar dehiscence by preventing an active phase of labor, but this factor requires further study.
In summary, these results demonstrate that the optimal uterine closure technique in terms of preventing "niches" and associated symptoms has not been established and requires additional research. The optimal technique of uterine closure and materials for suturing the uterus could be determined only after the influence of the individual stages of the cesarean section are reliably known (i.e., single or double layer suturing, peritonization, the use of long-term or rapidly absorbable suture material, a relatively high uterine incision in the case of an active phase of labor.
References
- Алиева Э.Н., Кулбаева С.Н. Кесарево сечение – резервы снижения частоты. Вестник Казахского национального медицинского университета. 2015; 4: 5-6. [Alieva E.N., Kulbaeva S.N. Caesarean section – the reserves of frequency reduction. Bulletin of the Kazakh National Medical University. 2015; 4: 5-6. (in Russian)].
- Гурьев Д.Л., Охапкин М.Б., Гурьева М.С., Кабанов И.В., Гурьева Д.Д., Асадова С.А. Снижение частоты кесарева сечения и перинатальных потерь в стационаре уровня 3А с использованием классификации Робсона. Доктор.Ру. 2019; 4: 8-13. [Gur’ev D.L., Ohapkin M.B., Gur’eva M.S., Kabanov I.V., Gur’eva D.D., Asadova S.A. Reduction of Caesarean section and perinatal loss rates in a level 3A hospital using the Robson classification. Doctor.Ru. 2019; 4: 8-13. (in Russian)]. https://dx.doi.org/10.31550/1727-2378-2019-159-4-8-13.
- Gubbini G., Casadio P., Marra E. Resectoscopic correction of the “isthmocele” in women with postmenstrual abnormal uterine bleeding and secondary infertility. J. Minim. Invasive Gynecol. 2008; 15(2): 172-5. https://dx.doi.org/10.1016/j.jmig.2007.10.004.
- Osser O.V., Jokubkiene L., Valentin L. High prevalence of defects in Cesarean section scars at transvaginal ultrasound examination. Ultrasound Obstet. Gynecol. 2009; 34(1): 90-7. https://dx.doi.org/10.1002/uog.6395.
- Luo L., Niu G., Wang Q., Xie H.Z., Yao S.Z. Vaginal repair of cesarean section scar diverticula. J. Minim. Invasive Gynecol. 2012; 19(4): 454-8. https://dx.doi.org/10.1016/j.jmig.2012.03.012.
- Van der Voet L.F., Bij de Vaate A.M., Veersema S., Brölmann H.A.M., Huirne J.A. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014; 121(2): 236-44. https://dx.doi.org/10.1111/1471-0528.12542.
- Abalos E., Oyarzun E., Addo V., Sharma J.B., Matthews J., Oyieke J. et al. CORONIS Collaborative Group. CORONIS – International study of caesarean section surgical techniques: the follow-up study. BMC Pregnancy Childbirth. 2013; 13: 215. https://dx.doi.org/10.1186/1471-2393-13-215.
- Wang C.J., Huang H.J., Chao A., Lin Y.P., Pan Y.J., Horng S.G. Challenges in the transvaginal management of abnormal uterine bleeding secondary to cesarean section scar defect. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011; 154(2): 218-22. https://dx.doi.org/10.1016/j.ejogrb.2010.10.016.
- Parra-Herran C., Djordjevic B. Histopathology of placenta creta: chorionic villi intrusion into myometrial vascular spaces and extravillous trophoblast proliferation are frequent and specific findings with implications for diagnosis and pathogenesis. Int. J. Gynecol. Pathol. 2016; 35(6): 497-508. https://dx.doi.org/10.1097/pgp.0000000000000250.
- Tang X., Wang J., Du Y., Xie M., Zhang H., Xu H. et al. Caesarean scar defect: risk factors and comparison of evaluation efficacy between transvaginal sonography and magnetic resonance imaging. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019: 242: 1-6. https://dx.doi.org/10.1016/j.ejogrb.2019.09.001.
- Park I.Y., Kim M.R., Lee H.N., Gen Y., Kim M.J. Risk factors for Korean women to develop an isthmocele after a cesarean section. BMC Pregnancy Childbirth. 2018; 18(1): 162. https://dx.doi.org/10.1186/s12884-018-1821-2.
- Nezhat C., Grace L., Soliemannjad R., Razavi G.M., Nezhat A. Cesarean scar defect: what is it and how should it be treated? OBG Management. 2016; 28(4): 32, 34, 36, 38-39, 53.
- Dodd J.M., Anderson E.R., Gates S., Grivell R.M. Surgical techniques for uterine incision and uterine closure at the time of caesarean section. Cochrane Database Syst. Rev. 2014; (7): CD004732. https://dx.doi.org/10.1002/14651858.CD004732.pub3.
- Ананьев В.А. Результаты кесарева сечения при наложении однорядного и двухрядного шва на матку. Акушерство и гинекология. 2000; 4: 26-9. [Anan’ev V.A. Results of cesarean section with single-row and double-row suture on the uterus. Obstetrics and Gynecology. 2000; 4: 26-9.(in Russian)].
- Гребенкин Б.Е., Заплатина В.С., Беда Ю.В. Кесарево сечение в современных условиях. Практическая медицина. 2009; 2: 72-6. [Grebenkin B.E., Zaplatina V.S., Beda Yu.V. Cesarean section in modern conditions. Practical Medicine. 2009; 2: 72-6. (in Russian)].
- Naji O., Abdallah Y., Bij de Vaate A.J., Smith A., Pexsters A., Stalder C. et al. Standardized approach for imaging and measuring cesarean section scars using ultrasonography. Ultrasound Obstet. Gynecol. 2012; 39(3): 252-9. https://dx.doi.org/10.1002/uog.10077.
- Ofili-Yebovi D., Ben-Nagi J., Sawyer E., Yazbek J., Lee C., Gonzalez J. et al. Deficient lower-segment Cesarean section scars: prevalence and risk factors. Ultrasound Obstet. Gynecol. 2008; 31(1): 72-7. https://dx.doi.org/10.1002/uog.5200.
- Vikhareva Osser O., Valentin L. Risk factors for incomplete healing of the uterine incision after caesarean section. BJOG. 2010; 117(9): 1119-26.https://dx.doi.org/10.1111/j.1471-0528.2010.02631.x.
- Stegwee S.I., Jordans I.P.M., van der Voet L.F., Bongers M.Y., de Groot C.J.M., Lambalk C.B. et al. Single- versus double-layer closure of the caesarean (uterine) scar in the prevention of gynaecological symptoms in relation to niche development - the 2 Close study: a multicentre randomised controlled trial. BMC Pregnancy Childbirth. 2019; 19(1): 85. https://dx.doi.org/10.1186/s12884-019-2221-y.
- Vervoort A.J.M.W., Uittenbogaard L.B., Hehenkamp W.J.K., Brölmann H.A., Mol B.W., Huirne J.A. Why do niches develop in Caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum. Reprod. 2015; 30(12): 2695-702. https://dx.doi.org/10.1093/humrep/dev240.
- Стрижаков А.Н., Тимохина Т.Ф., Баев О.Р., Рыбин М.В., Христофорова А.В. Модификация кесарева сечения. Акушерство и гинекология. 1997; 5: 33-7. [Strizhakov A.N., Timokhina T.F., Baev O.R. et al. Modification of the cesarean section. Obstetrics and Gynecology. 1997; 5: 33-8.(in Russian)].
- Малышева А.А., Матухин В.И., Резник В.А., Рухляда Н.Н., Тайц А.Н. Опыт оперативной коррекции несостоятельности рубца на матке после операции кесарева сечения на этапе предгравидарной подготовки. Проблемы репродукции. 2018; 24(6): 46-50. [Malysheva A.A., Matukhin V.I.,Reznik V.A. et al. Experience of operative correction of uterine scar failure after cesarean section at the stage of pre-gravidar preparation. Problems of reproduction. 2018; 24(6): 46-50. (in Russian)]. https://dx.doi.org/10.17116/repro20182406146.
- Durnwald C., Mercer B. Uterine rupture, perioperative and perinatal morbidity after single-layer and double-layer closure at cesarean delivery. Am J Obstet Gynecol. 2003; 189(4): 925-9. https://doi.org/10.1067/s0002-9378(03)01056-1.
Received 19.06.2020
Accepted 24.11.2020
About the Authors
Anna A. Malysheva, Gynecologist at the Department of Obstetrics and Gynecology, St. Petersburg State Pediatric Medical University, Ministry of Health of Russia,St. Petersburg, Russia. E-mail: annataits1@rambler.ru. 194100, Russia, Saint Petersburg, Litovskaya str., 2.
Valeriy I. Matukhin, Teaching Assistant at the Department of Obstetrics and Gynecology, St. Petersburg State Pediatric Medical University, Ministry of Health of Russia,
Saint Petersburg, Russia. E-mail: val-matukhin@mail.ru. ORCID: 0000-0002-8906-8356. 194100, Russia, Saint Petersburg, Litovskaya str., 2.
Natalia Yu. Novitskaya, Pathologist at the I.I. Dzhanelidze Research Institute of Emergency Medicine, Saint Petersburg, Russia. E-mail: natnovicf@gmail.com.
192242, Russia, Saint Petersburg, Budapeshtskaya str., 3А.
Nikolai N. Rukhliada, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, St. Petersburg State Pediatric Medical University, Ministry of Health
of Russia, Saint Petersburg, Russia. E-mail: nickolasr@ mail.ru. 194100, Russia, Saint Petersburg, Litovskaya str., 2.
Anna N. Taits, Ph.D., Associate Professor at the Department of Obstetrics and Gynecology, St. Petersburg State Pediatric Medical University, Ministry of Health of Russia,
Saint Petersburg, Russia. E-mail: annataits1@ rambler.ru. 194100, Russia, Saint Petersburg, Litovskaya str., 2.
For citation: Malysheva A.A., Matukhin V.I., Rukhlyada N.N., Taits A.N., Novitskaya N.Yu. Risk factors for cesarean uterine scar defect.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 2: 77-83 (in Russian)
https://dx.doi.org/10.18565/aig.2021.2.77-83



