Histological determinants of trial of labor after cesarean delivery
Objective: To identify histological determinants (uterine scar specifics) of trial of labor after cesarean section (CS). Materials and methods: This prospective study was conducted in 2013–2019 in Moscow maternity hospitals No. 68 and No. 29. The study included 272 women who wanted to have vaginal delivery but underwent CS. Of them, 182 underwent prelabor CS (vaginal delivery was either not considered or not attempted), and 90 had intrapartum CS (vaginal delivery was attempted but converted to CS). Results: Scar histology was correlated with a combination of highly diverse clinical, anamnestic, and maternal ultrasound factors. They included number of years lived, age of menarche, pre-pregnancy body mass index (BMI), number of phenotypic manifestations of undifferentiated connective tissue dysplasia, ordinal number of deliveries, interval between previous CS and present pregnancy, estimated fetal weight, amniotic fluid index, minimal scar thickness, right uterine artery resistance index before labor (Wilks' Lambda=0.006, p<0.001). In the attempted trial of labor, the predominance of fibrous tissue was associated with a lower age of menarche, higher prepregnancy BMI, and fifth-minute Apgar score. Conclusion: Myometrial reparation after CS characterizes the body as a whole. The histology of the uterine scar after CS combines prepregnancy, gestational, and intrapartum factors beyond those that were surgically determined. Myometrium histology is not an argument for post factum justifying or challenging attempted trial of labor: the prevalence of muscle tissue, muscle tissue with foci of fibrosis, or fibrous tissue is comparable.Vuchenovich Yu.D., Novikova V.A., Radzinsky V.E., Vasilchenko M.I., Trykina N.V., Yarotskaya I.A.
Keywords
Vaginal birth after a previous cesarean section carries a risk of labor complications. This risk is associated with incomplete healing of the lower uterine myometrium and further weakening of the uterine scar [1, 2]. Myometrial restitution is possible, which justifies subsequent vaginal birth. Attempted vaginal birth, referred to worldwide by the acronym TOLAC (Trial of Labor After Cesarean), requires a deliberate decision. The noninvasive diagnosis of scar integrity (ultrasound, MRI, etc.) is informative, but is based on subjective evaluation [3] and is incomparable with the accuracy of histological examination [4]. Prediction of uterine scar integrity is determined by multidirectional factors, including CS technique, suture material, uterine closure technique, and characteristics of the postoperative period, especially postpartum endometritis as a result of generalized infection [5, 6]. Today, the genetic pathways of myometrial reparation after CS, dependence on neoangiogenesis of the wound area, immunohistochemical quality of the scar-modified myometrium, and other factors [2, 7] have been identified. The scar after CS, like the whole myometrium, contains type VI collagen, elastin, Smooth muscle myosin heavy chain (SMMHC) Alpha Smooth Muscle Actin (ASMA), endothelial cell marker CD31 (differentiation cluster 31 or platelet-endothelial cell adhesion molecule 1 (PECAM1) [7]. However, type VI collagen, elastin, endothelial cell marker, CD31, aSMA, and SMMhc are active components of both ruptured and unruptured uterine scars. The mechanism of incomplete myometrial healing remains unclear [4]. The integrity of the uterine scar was studied in women undergoing prelabor, early labor, and late labor cesarean delivery [8]. Experimental therapeutic strategies are being developed to improve myometrial regeneration, scar collagen degradation, and de novo vessel formation based, for example, on transplantation of human amniotic epithelial cells [9], and local injection of multipotent mesenchymal stromal cells (MMSC) with the transfected gene of green fluorescent protein (GFP) [10], etc. However, most state-of-the-art studies will not replace the analysis of clinical and anamnestic data, the unshakable basis for predicting a healthy uterine scar after CS [1, 2, 11, 12]. Today, trial of labor after cesarean is undertaken almost blindly, because the absence of a myometrial defect on ultrasound or magnetic resonance imaging does not guarantee uterine scar integrity. The preoperative histological diagnosis of the myometrium is used in research centers but not routinely.
This study aimed to identify histological determinants (uterine scar specifics) of a trial of labor after cesarean section.
Materials and methods
This prospective multicenter cohort study was conducted between January 2013 and July 2019 in the maternity hospitals No. 68 and No. 29 in Moscow. The study included 272 women who wanted to have vaginal delivery but underwent CS. Of them, 182 underwent prelabor CS (vaginal delivery was either not considered or not attempted), and 90 had intrapartum CS (vaginal delivery was attempted but converted to CS).
Inclusion criteria were a history of CS, transverse uterine incision. 1 uterine scar, regardless of the number of CS, full-term pregnancy, singleton pregnancy, placenta location outside the uterine scar, patient's desire to have natural childbirth. Exclusion criteria were more than one uterine scar after CS, uterine scar of other origin, absolute contraindications to vaginal birth, placenta previa or abnormal invasion, and multiple pregnancies.
Vaginal delivery was attempted according to the available clinical protocols [11, 12] exclusively after the woman's informed consent and ex consilio decision. The delivery was performed in a full operating room setting, continuously monitoring the functional status of the fetus and the contractile activity. Fetal size, amniotic fluid index, condition, and thickness of the lower uterine segment in the scar area were evaluated by ultrasound. The uterine artery resistance index (D, right; S, left) was determined by Doppler ultrasound.
Myometrial fragments were obtained from 117 women during scar dissection during repeated CS, fixed in 10% neutral formalin solution, and embedded in paraffin. Histological examination was performed on serial deparaffinized stained sections. We used high-tech medical equipment using a Leica DM1000 microscope with a Leica EC3 camera, at magnifications of 4×/0.10 and 10×/0.25. We used stains: 1) hematoxylin-eosin as a tissue review stain, resulting in blue or purple nuclei staining and pink or orange staining of cytoplasm; 2) Masson staining to detect fibrosis, resulting in: nuclei stained black, protoplasm stained pink, fibrin stained red, collagen and reticular fiber stained blue.
Statistical analysis
Statistical analysis was performed using Statistica v12.0 and Microsoft Excel 2013. Quantitative variables were expressed as means (M), standard deviation (SD), median (Me) with interquartile range (Q1; Q3), and minimum (Min) and maximum (Max). The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test; the distribution was considered normal at p>0.05. Continuous variables showing normal distribution and unequal variance were compared using Student’s t-test; otherwise, Mann–Whitney U-test was used.
To compare two paired samples, the nonparametric one-sample Wilcoxon criterion was used. Categorical variables were compared between groups by chi-square test (χ2), with n<10 by χ2 with Yates correction. Quantitative assessment of the association between a risk factor and an outcome was determined by odds ratio (OR ) with 95% confidence interval (CI). A stepwise logistic regression model (logit model) was used to binary classify the outcome based on the independent variables. Regression coefficients (β-coefficient) for each independent variable with p values, Wald test, and odds ratio (OR) and 95% CI for OR were determined to identify statistically significant predictors. By constructing a univariate logistic curve, a threshold value of the probability of the outcome (y) was established, one of which was assigned the response variable 0, the other 1. The value of y=0.5 was taken as the threshold value for accepting the hypothesis of high probability of the outcome. A stepwise discriminant analysis was used to discriminate a non-binary outcome for a set of independent variables. Highly informative discriminants of the outcome were selected, Wilks' Lambda score was calculated (approaching zero value increases the significance of the studied factor to discriminate groups), F-criterion reflecting the significance of excluding the studied feature from the analysis, p-level – its level of statistical significance; parameters of the discriminant function equation (k – discriminant function coefficient, constant) to calculate classification weights (G) by the formula:
G = constant + k1×Y1 + k2×Y2 + ...,
where k – regression coefficient, Y – values of initial variables.
Substitution of the values and calculated coefficients into the system of equations allows for obtaining the final value of G for the categorical factor. The highest of all G values indicates to which group the outcome belongs. The demonstration of differences between groupings was based on canonical roots, the canonical discriminant functions. The significance of discrimination was confirmed by the Mahalanobis distance square and its statistical significance (F-criterion, p). Discrimination accuracy (percent of correct answers in the training sample) of at least 80% was considered informative.
Results and discussion
The proportion of women with a history of one CS was 61.77%; almost 1/3 (27.94%) had two CS and 1/10 (10.29%) had more than two (Fig. 1).
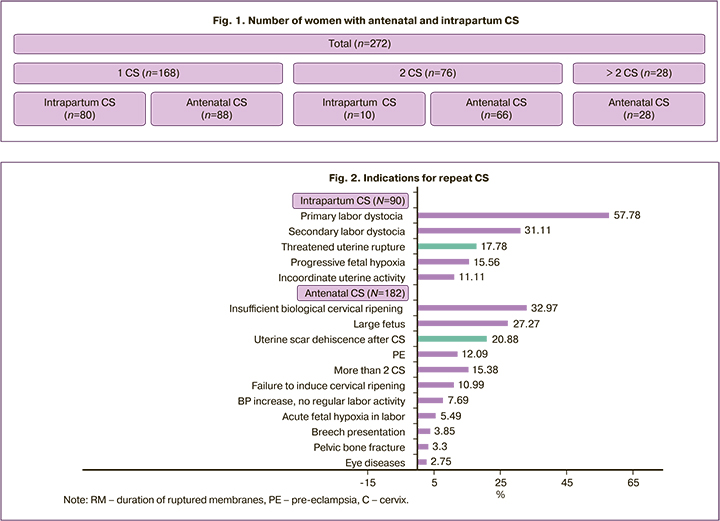
A history of two CS versus one CS significantly increased the chance of antenatal CS (66 of 76 and 88 of 168, respectively, OR =6.0; 95% CI 2.89-12.46). The indications for antenatal and intrapartum CS (individual or combination) differed markedly (Figure 2).
Note: RM – duration of ruptured membranes, PE – pre-eclampsia, С – cervix.
Most of indications for antenatal CS was related to the uterine scar (e.g., combination with insufficient biological ripening of the cervix, failure to induce cervical ripening, etc.), but not directly to scar dehiscence. Ultrasonic criteria of uterine scar dehiscence (presence of niches, changes in the thickness and structure of the scar tissue, presence of connective tissue) were an obstacle to a trial of labor in 20.88%, and threatened uterine rupture interrupted it in 16 (17.78%) women. Threatened uterine rupture was diagnosed in 16 women out of 90, but was not confirmed in almost 1/2 (n=6). In 74 women, there were no signs of threatened uterine rupture, but in 22 of them they were masked by progressive fetal hypoxia or labor anomalies, which required abdominal delivery and revealed myometrial inconsistency. The association of uterine scar dehiscence after CS with the clinical presentation of threatened uterine rupture was high but not absolute (OR=3.94 (95% CI 1.28–12.17)).
A similar trend was observed in intrapartum fetal distress (progressive hypoxia), which was observed in 8 of 32 women with a myometrial defect and was absent in 24. In intact myometrium (n=58), fetal distress was seen in 6 and absent in 52. The association of scar dehiscence with functional signs of intrapartum fetal hypoxia was significant but not absolute (OR=3.27 (95% CI 1.02–10.53)).
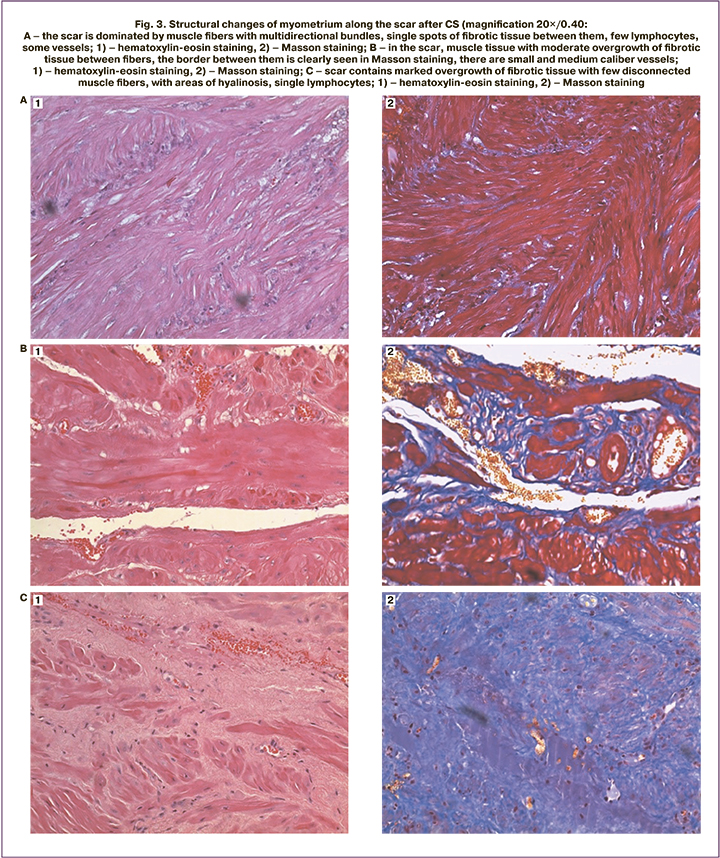
We distinguished three variants of the histological characteristics of the myometrium in the scar area after CS [3] (Fig. 3): predominance of muscle tissue (A); muscle tissue containing dense fibrotic foci (B); predominance of fibrotic tissue (C).
All three histologic variants occurred in women with different numbers of previous CSs, antenatal CS, and intrapartum CS during trial of labor after CS (Figure 4).
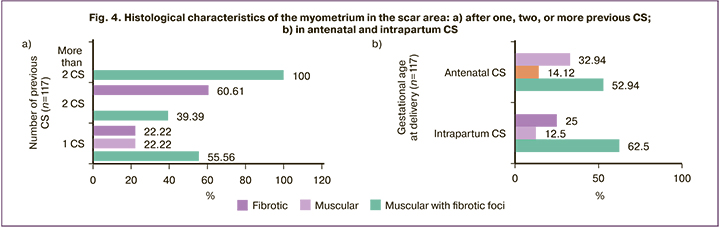
The predominance of muscle tissue appeared to be an exceptional marker of a history of single CS (Fig. 4a). The predominance of muscle tissue with fibrotic foci did not significantly distinguish one CS from two CS (40 of 72 and 13 of 33, respectively, χ2=2.36; p=0.13). The predominance of fibrotic tissue was significantly more frequently diagnosed in patients with two CSs than one CS (20 of 33 and 16 of 72, respectively, χ2=14.80, p<0.001; OR=5.39 (95% CI 2.21–13.14). Surprisingly, women with a history of more than two CS had predominant muscle tissue with fibrotic foci, which was significantly more common compared to one CS (χ2=8.62, p=0.003) and two CSs (χ2=13.09, p=0.0003).
Importantly, the prevalence of muscle (χ2=0.01, p=0.94), muscle with fibrotic foci (χ2=0.86, p=0.35), or fibrotic tissue (χ2=0.37, p=0.55) was comparable in not attempted (antenatal CS) and attempted (intrapartum CS) vaginal births after CS (Fig. 4).
The search for clinical and anamnestic determinants of scar histology came up with a total lack of information about the surgical features, inflammatory, and postoperative complications of the previous CS. Therefore, the key attention was paid to the correlation of the histological picture with two recognized risk factors of uterine scar failure - its ultrasound thickness (minimum and maximum) and the time interval from the previous CS to the present pregnancy. The expectations were partially fulfilled. Histology of the uterine scar is associated with its ultrasound thickness, both minimum (F=6.73, p=0.002) and maximum (F=8.11, p=0.0007) and both (Wilks lambda=0.76, F=5.01, p=0.0009). However, the ultrasound thickness of the scar was not informative to differentiate the predominance of muscle tissue from muscle tissue with fibrotic foci, as it was comparable [minimum 2.16 (0.42) mm and 2.17 (0.63) mm, p=0.93; maximum 2.19 (0.43) mm and 2.34 (0.66) mm, p=0.33]. The difference determined the predominance of fibrotic tissue with the lowest ultrasound thickness [minimum 1.68 (0.43) mm and maximum 1.8 (0.35) mm], significantly less compared to the predominance of muscle (minimum p=0.0001) and maximum p=0.0002) or muscle tissue with fibrotic foci (minimum p<0.001 and maximum p<0.001).
In our study, the time interval was not a universal criterion for the differentiation of histological patterns. It was comparable for the predominant muscle tissue and muscle tissue with fibrotic foci [6.63 (4.53) years and 5.32 (3.48) years, p=0.21], the predominant muscle tissue and fibrotic tissue [6, 63 (4.53) years and 6.61 (4.60) years, p=0.99], the predominant muscle with fibrotic foci and fibrotic tissue [5.32 (3.48) years and 6.61 (4.60) years, p=0.12].
In not attempted vaginal births (antenatal CS) and attempted (intrapartum CS), this parameter differed, and the latter were at least 2 years. In antenatal CS, the longest time interval characterized the predominance of muscle tissue [7.83 (4.65) years], being significantly superior only to muscle tissue with fibrotic foci [5.09 (3.87), p=0.04]. In contrast, in intrapartum CS, the predominance of muscle tissue was characterized by the shortest time interval [3.00 (0.0) years], significantly shorter compared with both muscle tissues with fibrotic foci [5.80 (2.46) years, p=0.04] and fibrotic tissue [8.50 (2.45) years, p=0.001]. In contrast to antenatal, the longest time interval of intrapartum CS corresponded to the predominance of fibrotic tissue, significantly exceeding that of muscular tissue with foci of fibrosis (p=0.01).
Logistic regression identified additional pre-gestational, gestational, and postpartum criteria for differentiation (independent variables) of one of the outcomes - predominance of muscle or fibrous tissue in the scar after CS (Table 1).
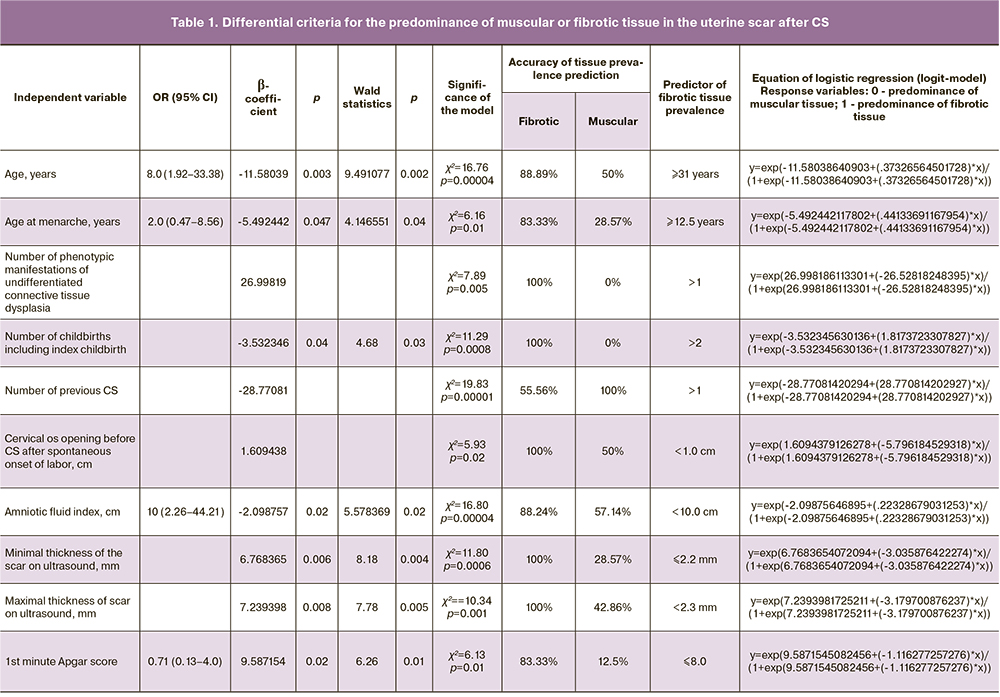
We note the correlation of the prevalence of fibrous tissue not only with maternal pre-gestational clinical and anamnestic characteristics, immaturity of the birth canal, tendency to oligohydramnios, but also with the trend of a lower Apgar score at the first minute.
The established correlation of scar histology not with one, but with a combined set of extremely diverse factors (clinical and anamnestic, maternal ultrasound) seems important. A stepwise general discriminant analysis selected 10 highly informative criteria from all available, the aggregate of which differentiates (discriminates) women with a history of CS by histological scar characteristics (Wilks Lambda =0.006, F (20.60)=35.96, p<0.001). The discriminant analysis also presented the mean predictor values for each type of tissue, calculated the parameters of the discriminant function equation to predict (calculating classification weights) uterine scar histology after CS, and evaluated its prognostic significance (Table 2).
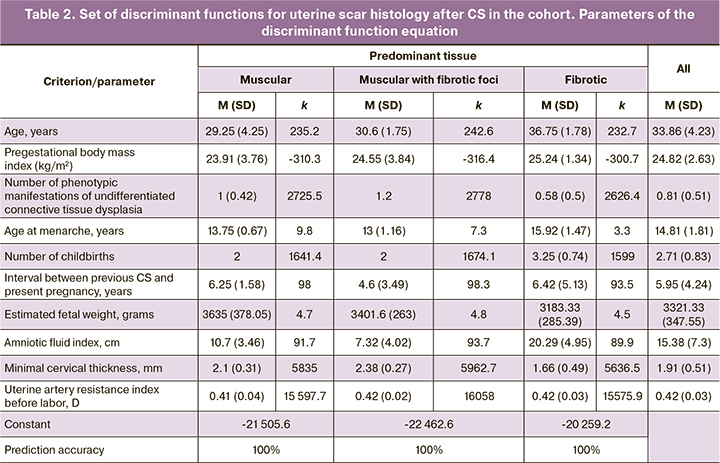
The accuracy of the distribution of women in the entire cohort with three variants of histology of the uterine scar according to the set of 10 common predictors was absolute (100%). The graphic visualization of these differences is the canonical root plot shown in Figure 5a.
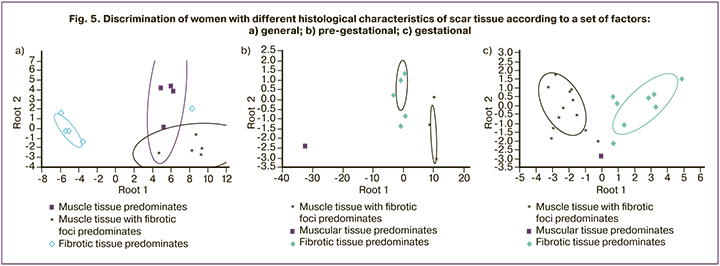
The classification of women according to the histological variant of the uterine scar after CS is evident: insignificant dispersion of points relative to the centroid within each grouping and sufficient distance between the centroids of different groups. The best discrimination was noted for fibrotic tissue, compared with both muscular tissue with fibrotic foci (Mahalanobis distance square=176.30, F=95.73, p<0.001) and muscular tissue alone (Mahalanobis distance square=125.86, F=58.089, p<0.001); less pronounced, but statistically significant, for muscular and muscular tissue with fibrotic foci (Mahalanobis distance square=33.72, F=11.53, p<0.001).
For the pre-gestational factors (Fig. 5b) maximum discrimination corresponded to muscle tissue vs. muscle tissue with fibrotic foci (Mahalanobis distance square=1830.88, F=414.26, p<0.001); slightly inferior for muscle versus fibrotic tissue (Mahalanobis distance square=1051.10, F=276.05, p<0.001); was smallest, but significant, for fibrotic and muscle with fibrotic foci (Mahalanobis distance square=117.05, F=276.05, p<0.001).
For gestational factors (Fig. 5c), the highest discrimination referred to muscle with fibrotic foci versus fibrotic tissue (Mahalanobis distance square=25.39, F=55.11, p<0.001); then to muscle versus fibrotic tissue (Mahalanobis distance square=15.61, F=3.82, p=0.002); least of all muscle and muscle with fibrotic foci (Mahalanobis distance square=13.97, F=3.43, p=0.004).
These findings suggest that scar histology, as a reflection of a woman's individual reparative regeneration, takes its origins long before the onset of pregnancy and has extremely diverse risk factors. The predominance of one of the tissues (muscular or fibrotic) is associated with the formation of menstrual function, the presence of a connective tissue defect outside of known genetically determined syndromes (undifferentiated connective tissue dysplasia), reproductive experience (number of births), the interval between the previous CS and the current pregnancy. The correlation of estimated fetal weight and amniotic fluid index with uterine scar histology after CS indicates the association of the latter with the functional state of the fetal placental unit. The correlation between the minimal thickness of the scar and the histology of the myometrium was confirmed. There was a correlation between the scar histology and the resistance index of the right uterine artery (D), which reflects the known histofunctional asymmetry of the uterine blood supply.
Clearly, the determinants of scar histology after CS are not limited to postoperative uterine surgery and postoperative inflammation. Recent studies of the immunohistochemical characteristics of the operated uterus confirm this [7]. However, the presented differentiation was not universal. Similar discrimination of women with not attempted (antenatal CS) and attempted (intrapartum CS) vaginal delivery after CS for the latter was unsuccessful. Differentiation criteria for women with three histological scar variants were established only for women with not attempted vaginal delivery after CS (antenatal CS). In addition, a pooled discriminant analysis selected only the set of pre-gestational criteria (Wilks' Lambda=0.06, F (12.52) =14.18, p<0.001) (Table 3). Criteria that were previously specified for the entire cohort, including estimated fetal weight, amniotic fluid index, minimal scar thickness, and left uterine artery (S) resistance index, were excluded.
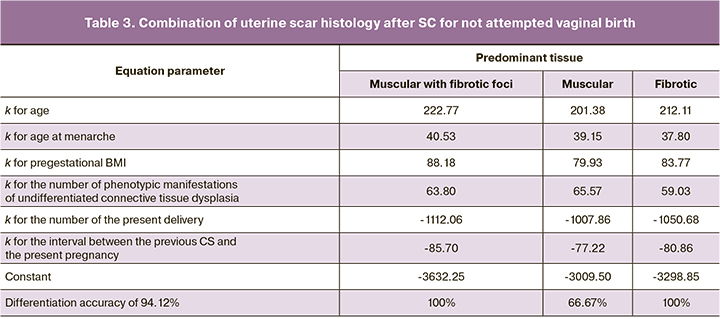
Individual criteria for significant differentiation were established for the predominance of muscular or fibrotic tissue in not attempted (antepartum CS) and attempted (intrapartum CS) vaginal birth (Table 4).
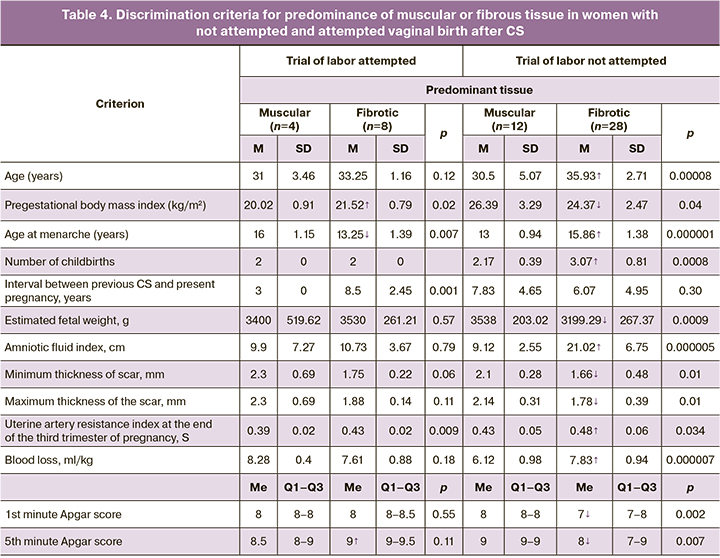
Common discriminators (p<0.05) of predominance of fibrotic tissue versus muscular tissue for intrapartum and antenatal CS were pre-gestational body weight index, age of menarche, left uterine artery resistance index (S), and fifth minute Apgar score. Exclusively for intrapartum CS, the predominance of fibrous tissue was associated with a lower age at menarche, a higher pre-gestational BMI and fifth minute Apgar score. Only in antenatal CS, the prevalence of fibrosis was determined by the same predictors, but with diametrically opposite directions of differences: greater age at menarche, lower pre-gestational body weight index and the fifth minute Apgar score. The interval between the previous CS and the present pregnancy had differential significance only for intrapartum CS (p=0.001). The direction of the differences in risk factors for fibrotic versus muscular predominance in the uterine scar after CS in women with not attempted (antenatal CS) and attempted (intrapartum CS) vaginal birth are indicated by arrows in Table 5.
Interestingly, in women with not attempted vaginal birth (antenatal CS), the predominance of fibrotic tissue predetermined all selected factors, except for the interval between the previous CS and the present pregnancy. The explanation for this is the heterogeneity of the indications for the present CS, most of which did not have scar roots, and were not associated with scar dehiscence and a history of more than two previous CS.
Thus, the factor of postoperative inflammation is indisputable and of paramount importance. However, it is obvious that myometrial reparation regeneration is subject to a spectrum of factors beyond postoperative inflammation or uterine surgery. The prevalence of a latent course of infectious and inflammatory complications after CS prevents risk assessment for subsequent birth.
These findings suggest that the histological differentiation of the scar is determined by an interrelated set of factors, different in time (pre-gestational, gestational, and postpartum), or belonging maternal or fetal factors.
The histology of the uterine scar after CS reflects a continuum from birth (presence of undifferentiated connective tissue dysplasia), to the formation of menstrual function (age of menarche), reproductive experience, including CS, up to the present pregnancy and its progress (amniotic fluid index, estimated fetal weight, uterine artery resistance index), further to determine the factors specificity of the postpartum maternal (blood loss) and newborn (Apgar score) factors.
Established factors (age at menarche, maternal age at delivery, number of births, number of phenotypic manifestations of undifferentiated connective tissue dysplasia, gestational age at delivery, amniotic fluid index, estimated fetal weight, uterine artery resistance index before delivery, scar thickness (minimum and maximum) are confounders of uterine scar histology. They may be considered intervening factors for myometrial regeneration and for indications or contraindications for trial of labor after CS.
Conclusion
The proportion of contemporary women who insist on natural childbirth after a history of one CS is 61.77%, after 2 CS is 27.94% and more than 2 is 10.29%. Not attempted vaginal birth after CS was more common for women with a history of two CS than one CS (OR =6.0; 95% CI 2.89–12.46).
With one uterine scar, the predominance of muscle tissue (almost no fibrotic component) is possible for a history of one CS and no more. The predominance of muscle tissue with fibrotic foci was not a criterion for differentiating women with a history of two CS from one CS (p=0.13), whereas the predominance of fibrotic tissue was (OR =5.39). Women with a history of more than two CS, contrary to stereotypes, were not characterized by a more unfavorable histological picture. The predominance of muscle tissue with fibrotic foci was detected significantly more frequently compared with one CS (p=0.003) and two CS (p=0.0003), and the predominance of fibrous tissue was not detected at all, refuting the fear about the guaranteed poor quality of myometrial regeneration after repeated excision of the scar compared with one CS.
In not attempted and attempted vaginal birth after CS, a comparable prevalence of muscle (p=0.94), muscle fibrotic foci (p=0.35), or fibrotic tissue (p=0.55) was found. The determinants of the three variants of uterine scar histology after CS were not limited to uterine surgery. Highly informative (p<0.05) clinical and anamnestic pre-gestational, gestational, and postpartum (maternal and fetal) criteria for differentiating the three histological variants of the myometrium after CS as a manifestation of its individual reparation were identified. This differentiation was possible exclusively for women with not attempted vaginal delivery (with antenatal CS), for whom the set of criteria had exclusively pre-gestational origins (Wilks' Lambda=0.06, p<0.001), including age, age at menarche, pre-gestational body mass index, number of phenotypic manifestations of undifferentiated connective tissue dysplasia, number of present delivery, interval between previous CS and present pregnancy. Individual criteria significantly distinguishing the predominance of muscular or fibrotic tissue in not attempted (antenatal CS) and attempted (intrapartum CS) vaginal births were (p<0.05) pre-gestational BMI, age at menarche, left uterine artery resistance index (S), and the fifth minute Apgar score. The differences in most of these criteria between attempted and not attempted vaginal births were observed to be multidirectional.
Myometrial regeneration after CS characterizes the woman's body as a whole, which explains its correlation with the age at menarche, the number of years lived, and the experience of childbirth. The established confounders are interconnected with the tissue structures of the myometrium. There is an obvious parallel between the histology of scar tissue and the factors that form in the dynamics of pregnancy, but its pathogenesis is not completely clear. Greater blood loss in predominantly fibrous tissue, despite extremely low vascularization of the scar tissue vascularization, can be explained by concomitant risk factors (large fetus, pre-eclampsia, etc.). The negative role of the predominance of fibrotic tissue in neonatal condition (1st and fifth minute Apgar score) cannot be overlooked. However, histology of the myometrium is not shown to be an argument justifying or challenging post-factum attempts at vaginal delivery. There is a clear need to consider the histology of the uterine scar after CS as summarizing pre-gestational, gestational, and intrapartum factors beyond those that are surgically determined.
References
- Вученович Ю.Д., Новикова В.А., Радзинский В.Е. Успех попытки родов через естественные родовые пути после двух кесаревых сечений. Каковы шансы? Российский вестник акушера-гинеколога. 2020; 20(5): 61-7. [Vuchenovich Yu.D., Novikova V.A., Radzinsky V.E. Successful vaginal delivery after two caesarean sections. What are the chances? Rossiiskii vestnik akushera-ginekologa/ Russian Bulletin of Obstetrician-Gynecologist. 2020; 20(5): 61-7. (in Russian)]. https://dx.doi.org/10.17116/rosakush20202005161.
- Краснопольский В.И., ред. Кесарево сечение. Проблемы абдоминального акушерства. Руководство для врачей. 3-е изд. М.: СИМК; 2018. [Krasnopolsky V.I., ed. Cesarean section. Problems of abdominal obstetrics. A guide for doctors. 3rd ed. M.: Special Publishing House of Medical Books (SIMK); 2018. 222p. (in Russian)].
- Ищенко А.И., Давыдов А.И., Александров Л.С., Пашков В.М., Ищенко А.А., Хохлова И.Д., Джибладзе Т.А., Горбенко О.Ю., Брюнин Д.В., Пташинская В.А., Тарасенко Ю.Н., Таирова М.Б. Несостоятельность рубца на матке после кесарева сечения. Выбор метода хирургического вмешательства. Вопросы гинекологии, акушерства и перинатологии. 2018, 17(4): 51-9. [Ishchenko A.I., Davydov A.I., Aleksandrov L.S., Pashkov V.M., Ishchenko A.A., Khokhlova I.D., Dzhibladze T.A., Gorbenko O.Yu., Bryunin D.V., Ptashinskaya V.A., Tarasenko Yu.N., Tairova M.B. Uterine scar incompetency of the after cesarean section. Choice of surgical intervention method. Issues of Gynecology, Obstetrics and Perinatology. 2018; 17(4): 51-9. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2018-4-51-59.
- Zietek M., Szczuko M., Celewicz Z. Morphological estimation of incomplete uterine scar rupture (dehiscence) in post-cesarean deliveries. Immunohistochemical studies. Ginekol. Pol. 2020; 91(11): 685-92. https://dx.doi.org/10.5603/GP.2020.0115.
- Vervoort A.J., Uittenbogaard L.B., Hehenkamp W.J., Brölmann H.A., Mol B.W., Huirne J.A. Why do niches develop in Caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum. Reprod. 2015; 30(12): 2695-702. https://dx.doi.org/10.1093/humrep/dev240.
- Ножницева О.Н., Семенов И.А., Беженарь В.Ф. Рубец на матке после операции кесарева сечения и оптимальный алгоритм диагностики его состояния. Лучевая диагностика и терапия. 2019; 2: 85-90. [Nozhnitseva O.N., Semenov I.A., Bezhenar V.F. The scar on the uterus after a cesarean section and the optimal algorithm for diagnostics. Diagnostic radiology and radiotherapy. 2019; 2: 85-90. (in Russian)]. https://dx.doi.org/10.22328/2079-5343-2019-10-2-85-90.
- Ziętek M., Świątkowska-Freund M., Celewicz Z., Szczuko M. Uterine cesarean scar tissue - an immunohistochemical study. J. Reprod. Med. Gynecol. Obstet. 2021; 6: 081. https://dx.doi.org/10.24966/RMGO-2574/100081.
- Kamel R., Eissa T., Sharaf M., Negm S., Thilaganathan B. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of Cesarean section. Ultrasound Obstet. Gynecol. 2021; 57(3): 466-70. https://dx.doi.org/10.1002/uog.22053.
- Fan Y., Sun J., Zhang Q., Lai D. Transplantation of human amniotic epithelial cells promotes morphological and functional regeneration in a rat uterine scar model. Stem Cell Res. Ther. 2021; 12(1): 207. https://dx.doi.org/10.1186/s13287-021-02260-6.
- Пекарев О.Г., Майбородин И.В., Пекарева Е.О., Поздняков И.М., Брега Е.С. Применение стволовых клеток для улучшения репаративных свойств рубца миометрия. Доктор.Ру. 2017; 3: 20-5. [Pekarev O.G., Maiborodin I.V., Pekareva Ye.O., Pozdnyakov I.M., Brega Ye.S. Using Stem Cells to Improve Myometrial Scar Repair. Doctor.Ru. 2017; 3(132): 20-5. (in Russian)].
- Министерство здравоохранения Российской Федерации. Кесарево сечение. Показания, методы обезболивания, хирургическая техника, антибиотикопрофилактика, ведение послеоперационного периода. Клинические рекомендации (протокол лечения). М.; 2014. 44c. [Ministry of Healthcare of the Russian Federation. Cesarean section. Indications, methods of anesthesia, surgical technique, antibiotic prophylaxis, management of the postoperative period. Clinical recommendations (treatment protocol). Moscow; 2014. 44p. (in Russian)].
- Кан Н.Е., Шмаков Р.Г., Кесова М.И., Тютюнник В.Л., Баев О.Р., Пекарев О.Г., Тетруашвили Н.К., Клименченко Н.И. Самопроизвольное родоразрешение пациенток с рубцом на матке после операции кесарева сечения. Клинический протокол. Акушерство и гинекология. 2016; 12(Приложение): 12-9. [Kan N.E., Shmakov R.G., Kesova M.I., Tyutyunnik V.L., Baev O.R., Pekarev O.G., Tetruashvili N.K., Klimenchenko N.I. Spontaneous delivery is women with a uterine scar after cesarean section. Clinical protocol. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2016; 12: 12-9. (in Russian)].
Received 30.12.2021
Accepted 31.03.2022
About the Authors
Yulia D. Vuchenovich, PhD, Associate Professor at the Department of Obstetrics and Gynecology with a course in Perinatology, Medical Institute, RUDN University,117198, Russia, Moscow, Miklukho-Maclay str., 6; Head of the Department of Pathology of Pregnant Women, N.E. Bauman City Clinical Hospital No. 29,
Moscow Department of Healthcare, 111020, Russia, Moscow, Gospitalnaya sqr., 28, +7(499)137-48-81, vuchrd15@mail.ru, https://orcid.org/0000-0002-7152-4560
Vladislava A. Novikova, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology with a course in Perinatology, Medical Institute, RUDN University,
117198, Russia, Moscow, Miklukho-Maclay str., 6; obstetrician-gynecologist, H-Clinic, 1270838, Russia, Moscow, 8 March str., 6A, bld. 1, +7(499)137-48-81,
vladislavan@mail.ru, https://orcid.org/0000-0002-6109-7331
Victor E. Radzinsky, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology with a course in Perinatology, Medical Institute, RUDN University, 117198, Russia, Moscow, Miklukho-Maclay str., 6, +7(499)137-48-81, kafedra-aig@mail.ru, https://orcid.org/0000-0003-4956-0466
Mikhail I. Vasilchenko, Dr. Med. Sci, Deputy Chief Physician for Surgery, M.P. Konchalovsky City Clinical Hospital, Moscow Department of Healthcare,
124617, Russia, Moscow, Zelenograd, Kashtanovaya alley, 2, bld. 1, +7(499)735-25-97, vasilhenko@mail.ru, https://orcid.org/0000-0002-7942-5145
Nadezhda V. Trykina, Head of the Department of Anatomic Pathology, M.P. Konchalovsky City Clinical Hospital, Moscow Department of Healthcare,
124617, Russia, Moscow, Zelenograd, Kashtanovaya alley, 2, bld. 1, +7(499)734-59-70, n.tryckina@yandex.ru, https://orcid.org/0000-0003-3443-423X
Nadezhda M. Startseva, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology with a course in Perinatology, Medical Institute, RUDN University,
117198, Russia, Moscow, Miklukho-Maclay str., 6, +7(499)137-48-81, n.startseva@yahoo.com, https://orcid.org/0000-0001-5795-2393
Irina A. Yarotskaya, PhD, Chief Physician, M.P. Konchalovsky City Clinical Hospital, Moscow Department of Healthcare, 124617, Russia, Moscow, Zelenograd,
Kashtanovaya alley, 2, bld. 1, +7(499)735-64-29, Yrotskayia@zdrav.mos.ru, https://orcid.org/0000-0002-8739-2267
Corresponding author: Yulia D. Vuchenovich, vuchrd15@mail.ru
Authors' contributions: Vuchenovich Y.D. – conception and design of the study, data collection and analysis, statistical analysis, manuscript drafting; Novikova V.A. – conception and design of the study, data collection and analysis, statistical analysis, manuscript editing; Radzinsky V.E. – conception and design of the study; Vasilchenko M.I. – conception and design of the study, manuscript editing; Trykina N.V., Startseva N.M. – data collection and analysis; Yarotskaya I.A. – conception and design of the study, and analysis.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of RUDN University (Ref. No. 5 of 18.10.2018).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Vuchenovich Yu.D., Novikova V.A., Radzinsky V.E., Vasilchenko M.I., Trykina N.V., Yarotskaya I.A. Histological determinants of trial of labor after cesarean delivery.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 5: 128-139 (in Russian)
https://dx.doi.org/10.18565/aig.2022.5.128-139



