The role of vasoactive intestinal polypeptide in the development of gestational complications in women with repeat cesarean sections
Sebyakina T.A., Ivanova O.Yu, Ishunina T.A.
Background: Current obstetric and gynecological practice is characterized by increased caesarean section (CS) rates and CS-related prenatal and perinatal complications. The uterine scar is a potential risk factor for preterm birth, uterine rupture at the site of previous SC scar, preterm placental abruption, increased risk of placental insufficiency and other placenta-associated complications.
Objective: Given the fact that vasoactive intestinal polypeptide (VIP) plays an important role in regulation of myometrial contractility and uteroplacental blood flow, the aim of the study was to investigate correlation between VIP expression in the myometrium and prenatal complications in women with repeat CSs.
Material and methods: The study was carried out in the Obstetric Emergency Hospital in Kursk. Complex medical examination of 21 women after repeat СS at 37–41 weeks of pregnancy was performed including general and obstetric anamnesis; obstetric examination; ultrasound and Doppler ultrasound; immunohistochemical staining of biopsy material of uterine tissues, using polyclonal antibodies that recognize VIP; pathomorphological examination of the placenta; analysis of сomplete blood count and calculation of the Garkavi index.
Results: Negative correlation was found between VIP expression in the myometrium and the concentration of leukocytes (p=0.012), and positive correlation was found between VIP expression in the myometrium and the percentage of lymphocytes and the Garkavi index (p=0.031). In women with low expression of VIP, decreased placental mass (p = 0.0009), abnormal placental function, calcium deposits, foci of fibrosis and pseudo-infarction that manifested placental insufficiency, were found.
Conclusion: Low expression of VIP in the myometrium is associated with pelvic inflammatory disease, increased concentration of leukocytes, reduced level of lymphocytes and high rate of placental insufficiency, possibly due to immunological disorders associated with VIP deficiency. High expression of VIP in the myometrium is associated with preeclampsia and is considered to be a compensatory mechanism that can improve uterine and placental blood flows.
Authors' contributions: Sebyakina T.A., Ishunina T.A. – the concept and design of the study, statistical data processing; Sebyakina T.A. – material collection and processing; Sebyakina T.A., Ivanova O.Yu., Ishunina T.A. – article writing;
Ivanova O.Yu., Ishunina T.A. – article editing.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was carried out without any sponsorship.
Ethical Approval: The study was approved by the Regional Ethics Committee.
Patient Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Sebyakina T.A., Ivanova O.Yu, Ishunina T.A. The role of vasoactive intestinal polypeptide
in the development of gestational complications in women with repeat cesarean sections.
Akusherstvo i Gynecologia/Obstetrics and Gynecology (in Russian). 2024; 12: 42-49 (in Russian)
https://dx.doi.org/10.18565/aig.2024.202
Keywords
Cesarean delivery (CS) rate remains high in the Russian Federation and reaches up to 30.3% [1]. In 61.1% of women who previously underwent CS, complications of pregnancy include placenta previa, placenta accreta, cesarean scar defect (isthmocele), cesarean scar pregnancy (CSP), placental abruption, chronic placental insufficiency (PI) and PI-related chronic fetal hypoxia. At the same time, in every third pregnant woman with uterine scar, placental disorders and impaired hemodynamics of mother-placenta-fetus system are found [2].
Placental insufficiency is a condition characterized by morphofunctional changes in the placenta, significantly reducing the physiological exchange between mother and fetus, that leads to fetal growth restriction and/or hypoxia [3, 4]. In pregnant women with uterine scars from the previous C-sections, the rate of PI is high. Moreover, in babies born from women with uterine scars, moderate asphyxia was reported 2 times more often. Mild asphyxia and the signs of perinatal damage of the central nervous system of hypoxic genesis were found 1.4 times more often in babies born from women in this group [5].
There is information in literature about the important role of neuropeptides in regulation of uteroplacental blood flow and contraction of uterine smooth muscle cells (also known as myocytes). Vasoactive intestinal polypeptide (VIP) is one of the most common neuropeptides in the uterus, where it functions as a neurotransmitter with immunoregulatory properties [6–8]. VIP is localized in sensory and parasympathetic C-fibers originating from neurons in the paracervical ganglia [9–11]. In the myometrium, VIPergic fibers are located between smooth muscle cells parallel to their axis or occupy perivascular spaces [10, 12]. VIP induces the relaxant effect in smooth muscle cells of the myometrium and is considered the most powerful vasodilator among all neuropeptides in the uterus [13, 14]. Due to this, VIP induces an increase in uterine blood flow, that is especially important in regulation of the uteroplacental blood flow in late pregnancy [13, 14]. In addition, VIPergic fibers participate in the inflammatory response and transmit sensory information about potential irritants from the uterus to the central nervous system [10].
The concentration of VIP in myometrium decreases during pregnancy. However, the concentration of VIP significantly increases during delivery [15]. In patients with repeat CS, VIP expression is reduced, that probably influences relaxation of the inner uterine compartment, compromising the formation of the lower segment and the maturation of the cervix [16]. In women, whose pregnancies were complicated by preeclampsia, VIP plasma levels increased by 3 times, and was considered to be a powerful compensatory mechanism to restore various organs perfusion, including the uterus and placenta [17]. Recently, the interest of researchers in the role of this neuropeptide in the pathogenesis of gynecological diseases has increased. In particular, increased VIP expression in the endometrium in women with endometriosis, and to a greater extent, in patients with chronic pelvic pain was found was reported [18]. Moreover, in women with severe pain syndrome against the background of endometriosis and adenomyosis, VIPergic fibers density during office hysteroscopy was higher compared with women who experienced less pain intensity [19]. It has been shown that VIPergic sensory C fibers are involved in occurrence of pain syndrome, as well as the conditions that are characterized by impaired sensitivity – allodynia, hyperalgesia and peripheral sensitization. It is suggested that inflammatory mediators released in the endometrium activate VIPergic sensory C-fiber nerve terminals, inducing neurogenic inflammation [11]. Given the fact the VIP plays an important role in regulation of myometrial contractility and uteroplacental blood flow, as well as participates in inflammatory processes in the female reproductive system, the purpose of this study was to investigate correlation between VIP expression in the myometrium and prenatal complications in women with repeat CSs.
Materials and methods
Сomplex examination of 21 women, who underwent repeat cesarean delivery at 37–41 weeks of pregnancy, was performed in the Obstetric Emergency Hospital, the Department of Obstetrics and Gynecology of Kursk State Medical University, Ministry of Health of Russia. The study was conducted in compliance with ethical norms after getting approval of the Regional Ethics Committee. The women with multiple pregnancy, severe extragenital pathology, or aged over 40 years were not included in the study. General and special obstetric examination was performed in accordance with the current clinical guidelines “Singleton birth, cesarean delivery” approved by the Ministry of Health of the Russian Federation on June 24, 2021. Assessment of the fetal condition and detection of vascular abnormalities in the fetoplacental complex were performed by Doppler ultrasound with color flow mapping, using Toshiba ultrasound device with convex transducer (100–150 Hz).
Voluntary informed consent for intraoperative myometrial biopsy was obtained from the patients. During abdominal delivery, the fragments of 1–2 cm of the uterine wall were removed through the surgical incision at the site of scar tissue, fixed in 10% neutral buffered formalin, embedded in paraffin using standard technique, and stained with VIP polyclonal antibodies (NIBR), that specifically recognize the amino acid sequence of this neuropeptide [20, 21]. After deparaffinization and heating in a warm water bath, 4 μm- thick sections were treated with VIP antibody diluted at 1:500 and incubated for 1 h at room temperature. Biotin-free polymer detection system HiDef Detection HRP (Cell Marque, USA) was used to visualize the primary antibodies. Microphotographs of the preparations were taken using Leica CME 3-2 microscope (Germany) and a Micromed MVV 5000 digital camera (China). VIP staining intensity in the myometrium was assessed in arbitrary units of measurement using semi-quantitative method. (Table 1. Figure. +++ (3) – high; ++ (2) – moderate; + (1) – low; +- (0.5) – very low [22]. It is possible that sections showing the uneven distribution of immunoreactive material can have intermediate values, for example, +/++ or +/+- (Table 1). This method is acceptable for assessment of immunohistochemical (IHC) staining intensity due to lack of other techniques capable to correctly recognize the color spectrum of the chromogen (Figure) [22, 23].
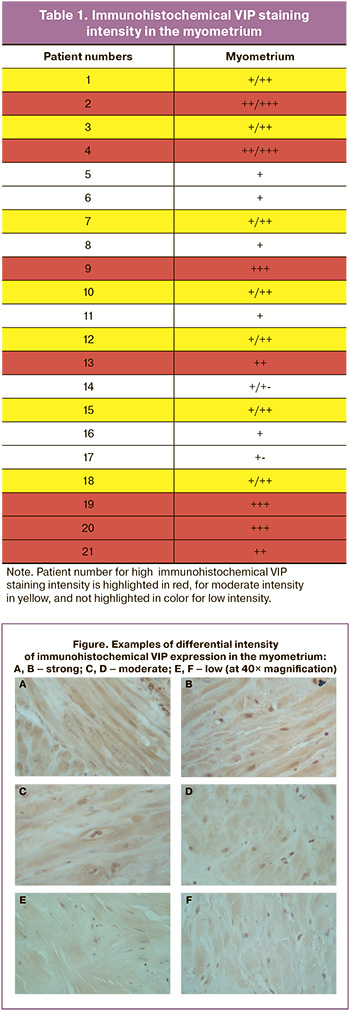
The Garkavi index (the lymphocytes-to-segmented neutrophils ratio) in the peripheral blood was determined as an objective indicator of the intensity of inflammatory processes [24]. Assessment of the fetoplacental complex included pathomorphological examination of the placentas.
Statistical analysis
Statistical data processing was performed using software programs MS Excel 2007 (Microsoft, USA) and Statistica 13.3 (StatSoft, USA). According to Shapiro-Wilk test, distribution of the parameters differed from the normal distribution. Therefore, to identify intergroup differences, the Kruskal–Wallis nonparametric test and the Dunn's test for multiple comparisons with Bonferroni correction of 0.017 were used. The Spearman’s correlation was used to test the strength of correlation between the variables. The results were considered to be significant at p≤0.05.
Results
According to assessment results of VIP-immunoreactive material staining intensity in the myometrium, the women were divided into 3 groups: with high (group I), moderate (group II) and low (group II) VIP expression (Table 1). According to the Kruskal–Wallis test, the differences in VIP staining intensity between the three groups of women were significant (χ2(2)=18.82; р<0.001).
The age of patients enrolled in the study was 21–39 years. The age of women in group I was 29.71 (3.45) years, in group II – 32.4 (1.9) years, in group III – 30.42 (7.27) years.
Extragenital diseases (including their combinations) complicating pregnancy were identified in 14/21 (66.6%) patients. Among them, cardiovascular diseases were diagnosed in 28.5% of women, varicose veins in 19%, gastrointestinal diseases in 9.5%, eye diseases in 14.3%, urinary system diseases in 9.5% of women. At the same time, no significant intergroup differences in the structure of extragenital diseases were identified.
Assessment of gynecological anamnesis showed that in 18/21 (85.7%) patients, different reproductive system diseases were diagnosed, most of which – 14/21 (67%) were of inflammatory genesis (endomyometritis, salpingo-oophoritis, vulvovaginitis) with significant predominance of endomyometritis and salpingo-oophoritis in the groups of women with moderate or low immunohistochemical VIP expression. So, in group III, endometritis was in 3/21 (14.3%) women. Salpingo-oophoritis was in 6/21 (28.6%) patients, while the women in group 1 had no this pathology. Vulvovaginitis was diagnosed in 9/21 (42.8%) patients in group III, and in 3/21 (14/3%) women in group I.
Examination of patients for sexually transmitted infections showed the absence of acute infection, however, there were single cases of carriage of Chlamydia trachomatis and Human papillomavirus in the groups with medium and low VIP expression.
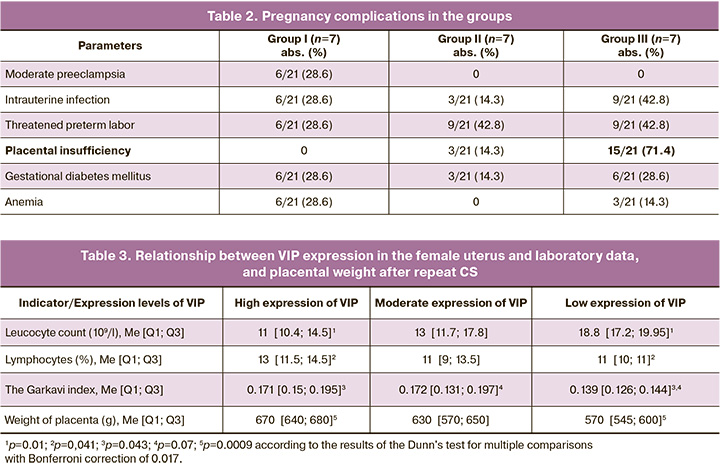
Most women in the study – 19/21 (90.5%) had various pregnancy complications. There was threatened preterm labor, placental insufficiency and intrauterine infection in the vast majority of cases. It should be noted than in 15/21 (71.4%) women with low VIP expression, the signs of placental insufficiency were detected most often compared with other groups (Table 2). At the same time, preeclampsia was found only in patients with high VIP expression – 6/21 (28.6%). The structure of gestational complications and the frequency of occurrence in the groups is represented in Table 2.
Clinical data indicate close association of VIP expression with inflammatory diseases of the reproductive organs, placental insufficiency and termination of early pregnancy. Consequently, the next stage of the study was investigation of correlations between VIP expression and various indicators of complete blood count. In the whole cohort of patients, significant negative relationship was found between serum concentration of leukocytes and immunohistochemical VIP expression (r=-0.540, p=0.0115), and significant positive relationship was found between immunohistochemical VIP expression with lymphocytes (r=0.472, p=0.031) and the Garkavi index (the lymphocytes-to-segmented neutrophils ratio) (r=0.471, p=0.031).
According to the Kruskal–Wallis test, there were significant differences in leucocyte levels between the three groups of women (χ2(2)=6.52; р=0.038). In addition, the maximum differences were found between group I and group III (р=0.01) (Table 3). It should be noted that statistical trend analysis showed significantly higher value of the Garkavi index (χ2(2)=4.91; р=0.086) in group I compared with group III (р=0.043).
Thus, in group III, reduced VIP expression was associated with high leukocyte counts, but lower lymphocyte counts and lower values of the Harkavi index.
There were significant differences in placental weight between the groups of women with different levels of VIP expression (χ2(2) = 10.92, p=0.004). In patients with high VIP expression it was significantly higher compared with women with low VIP expression (р=0.0009) (Table 3).
Villi of mature type predominated in most of the examined placentas. However, intermediate villi with weak development of compensatory mechanisms and deficiency of terminal branches were found in 9/21 (42.8%) histological samples only in the group with low VIP expression. Abnormal placental zones, calcium deposits, fibrosis, and pseudoinfarctions in the placenta were detected in 12/21 (57.1%) women in the group with low VIP expression, in 28.6% women in the group with moderate VIP expression, and in 3/21 (14.3%) women with high VIP expression. The signs of extraplacental amnionitis were diagnosed in 6/21 (28.6%) women with moderate VIP expression, and in 3/21 (14.3%) patients with low VIP expression.
Discussion
This study demonstrates for the first time significant negative relationship between VIP expression in the myometrium in women with repeat CS and leukocyte count. It was found that reduction of VIP concentration is associated with high leukocyte count and the presence of inflammatory gynecological diseases against the background of low lymphocyte count, indicating failure of the specific link of the immune response. Moreover, in patients with low VIP expression, pregnancy was more often complicated by placental insufficiency compared with other groups, that can be explained by insufficient activity in VIP in terms of providing appropriate uteroplacental blood flow. High frequency of miscarriages in women in this group can be associated with loss of the inhibitory effect of VIP on myometrial contractility and impaired implantation.
Moreover, it was found that in women with low VIP expression, there was significant reduction in placental weight, as well as the presence of abnormal zones, calcium deposits, fibrotic foci, and pseudoinfarctions in the placenta. Reduction in placental weight and the number of vessels in villi, up to their absence, the presence of fibrotic foci, infarctions, and increase in syncytial knots are manifestations of PI and fetal growth restriction [25]. Insufficient vascularization of the placental tissue in PI and fetal growth restriction are associated with villous immaturity and reduction in proliferative activity of cytotrophoblast [26].
According to literature data, initially the functions of VIP as the neurotransmitter were limited to its inhibitory effect on uterine contractility and vasodilatory effect, enabling to increase myometrial blood flow. However, the studies in recent years reported on extended functions of VIP in the uterus, indicating its important role as the anti-inflammatory neuroimmunomodulator during implantation.
For successful implantation, it is necessary to ensure immunologically calm environment in the uterus and maternal tolerance, that becomes possible in case of shift in cytokine balance Th1/Th2 towards Th2 and activation of CD4+CD25+FoxP3+ regulatory T cells against the background of high concentration of progesterone [27–31]. It is noteworthy that at the implantation site, both VIP receptors are expressed, promoting increased Th2 cytokine production, increased population of CD4+CD25+FoxP3+ regulatory T cells, relaxation of smooth muscle cells and vasodilation, that in the aggregate promote decidualization and “calming” of the uterus at embryo implantation [27]. At the same time, maternal lymphocytes, and to a lesser extent, trophoblast and decidual cells of the endometrial stroma, function as the sources of VIP secretion. VIP deficiency is associated with recurrent pregnancy losses (spontaneous abortions). In this case, proinflammatory reactions of Th1-type and natural killer cells are activated, and lead to damage in embryonic tissues and subsequent resorption. Reduction in population of CD4+CD25+FoxP3+ regulatory T cells and in the number of VIP receptors type 1 has been reported [32]. Thus, in women with recurrent miscarriages, the number of VIP-producing cells in the endometrium are reduced, that leads to disruption of immune balance and termination of pregnancy. These studies initiated a new direction in research on feto-maternal tolerance, which emphasizes the role of neuropeptides that mediate both immune and neuroeffector mechanisms. These neuropeptides primarily include VIP, that was investigated in our study. It should be noted that positive correlation between VIP expression intensity in the myometrium and endometrium was found (r=0.692, p=0.00051). Therefore, high frequency of pregnancy terminations and fetoplacental insufficiency in women in group III with very low VIP expression can be explained by immunological disorders associated with VIP deficiency in the endometrium. This hypothesis is confirmed by decrease in lymphocyte count and the Harkavi index, that reflect the functioning of the specific link in the immune mechanism.
In women in group I, high VIP expression in the myometrium is associated with high frequency of preeclampsia, that confirms earlier observed 3-fold increase of plasma levels of VIP in women with gestational proteinuric hypertension [17]. However, our study examined VIP expression in the myometrium, that is more important in making conclusion about the compensatory significance of increased VIP expression in terms of restoration of uterine and placental blood flows.
Conclusion
Thus, this study showed that in women with repeat CS, low VIP expression in the myometrium is associated with increased leukocyte concentration, reduced lymphocyte count, and high frequency of PI, that may be caused by immunological disorders associated with VIP deficiency. The results of this study suggest thorough investigation of VIP as a potential therapeutic target in correction of recurrent implantation failure in women and prediction of placental insufficiency.
High VIP expression in the myometrium is associated with preeclampsia and is considered to be a compensatory mechanism that can improve uterine and placental blood flows.
References
- Лисицына О.И., Шмаков Р.Г. «Ниши» рубца на матке после кесарева сечения: диагностика, лечение и исходы. Акушерство и гинекология. 2019; 9: 24-31. [Lisitsyna O.I., Shmakov R.G. Niches of the uterine scar after cesarean section: diagnosis, treatment, and outcomes. Obstetrics and Gynecology. 2019; (9): 24-31. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.9.24-31.
- Камилова М.Я., Юнусова С.Х., Узакова У.Д. Плацентарная недостаточность у беременных женщин с рубцом на матке. Sciences of Europe. 2017; 16(16): 27-9. [Kamilova M.Ya., Yunusova S.Kh., Uzakova U.D. The placental insufficientcy in pregnant woman with uteri scar. Sciences of Europe. 2017; 16(16): 27-9. (in Russian)].
- Гаспарян С.А., Орфанова И.А., Ахмедова С.М., Василенко И.А. Новые аспекты патогенеза плацентарной недостаточности. Медицинский алфавит. 2023; 19: 44-8. [Gasparyan S.A., Orfanova I.A., Akhmedova S.M., Vasilenko I.A. New aspects of the pathogenesis of placental insufficiency. Medical Alphabet. 2023; (19): 44-8. (in Russian)]. https://dx.doi.org/10.33667/2078-5631-2023-19-44-48.
- Савельева Г.М., Сухих Г.Т., Серов В.Н., Радзинский В.Е. Акушерство. Национальное руководство. 2-е изд. М.: ГЭОТАР-Медиа; 2019. 1080с. [Savelyeva G.M., Sukhikh G.T., Serov V.N., Radzinsky V.E. Obstetrics. National guide. 2nd ed. Moscow: GEOTAR-Media; 2019. 1080p. (in Russian)].
- Полянин Д.В., Михельсон А.А., Мелкозерова О.А., Лукьянова К.Д. Дискуссионные вопросы несостоятельности рубца на матке в эру эпидемии кесарева сечения. Уральский медицинский журнал. 2019; 5: 17-23. [Polyanin D.V., Mikhel’son А.А., Melkozerova О.А., Luk’yanova K.D.Discussion issues of incompetent uterine scar in the era of the caesarian section epidemic. Ural Medical Journal. 2019; (5): 17-23. (in Russian)].https://dx.doi.org/10.25694/URMJ.2019.05.30.
- Ramhorst R., Grasso E., Vota D., Gori S., Hauk V., Paparini D. et al. From decidualization to pregnancy progression: an overview of immune and metabolic effects of VIP. Am. J. Reprod. Immunol. 2022; 88(4): e13601.https://dx.doi.org/10.1111/aji.13601.
- Grasso E., Gori S., Paparini D., Soczewski E., Fernández L., Gallino L. et al. VIP induces the decidualization program and conditions the immunoregulation of the implantation process. Mol. Cell. Endocrinol. 2018; 460: 63-72. https://dx.doi.org/10.1016/j.mce.2017.07.006.
- Di Tommaso S., Cavallotti C., Malvasi A., Vergara D., Rizzello A., De Nuccio F. et al. A qualitative and quantitative study of the innervation of the human non pregnant uterus. Curr. Protein Pept. Sci. 2017; 18(2): 140-8. https://dx.doi.org/10.2174/1389203717666160330105341.
- Kelm Junior A.R., Lancellotti C.L., Donadio N., Auge A.P., Lima S.M., Aoki T. et al. Nerve fibers in uterosacral ligaments of women with deep infiltrating endometriosis. J. Reprod. Immunol. 2008; 79(1): 93-9.https://dx.doi.org10.1016/j.jri.2008.08.004.
- Pinsard M., Mouchet N., Dion L., Bessede T., Bertrand M., Darai E. et al. Anatomic and functional mapping of human uterine innervation. Fertil. Steril. 2022; 117(6): 1279-88. https://dx.doi.org/10.1016/j.fertnstert.2022.02.013.
- Tokushige N., Markham R., Russell P., Fraser I.S. Different types of small nerve fibers in eutopic endometrium and myometrium in women with endometriosis. Fertil. Steril. 2007; 88(4): 795-803. https://dx.doi.org/10.1016/j.fertnstert.2006.12.078.
- Bryman I., Norström A., Lindblom B., Dahlström A. Histochemical localization of vasoactive intestinal polypeptide and its influence on contractile activity in the non-pregnant and pregnant human cervix. Gynecol. Obstet. Invest. 1989; 28(2): 57-61. https://dx.doi.org/10.1159/000293515.
- Ottesen B., Gerstenberg T., Ulrichsen H., Manthorpe T., Fahrenkrug J., Wagner G. Vasoactive intestinal polypeptide (VIP) increases vaginal blood flow and inhibits uterine smooth muscle activity in women. Eur. J. Clin. Invest. 1983; 13(4):321-4. https://dx.doi.org/10.1111/j.1365-2362.1983.tb00107.x.
- Hansen V., Maigaard S., Allen J., Forman A. Effects of vasoactive intestinal polypeptide and substance P on human intramyometrial arteries and stem villous arteries in term pregnancy. Placenta. 1988; 9(5): 501-6.https://dx.doi.org/10.1016/0143-4004(88)90022-7.
- Ottesen B., Ulrichsen H., Fahrenkrug J., Larsen J.J., Wagner G., Schierup L. et al. Vasoactive intestinal polypeptide and the female genital tract: relationship to reproductive phase and delivery. Am. J. Obstet. Gynecol. 1982; 143(4): 414-20. https://dx.doi.org/10.1016/0002-9378(82)90083-7.
- Malvasi A., Tinelli A., Cavallotti C., Bettocchi S., Di Renzo G.C., Stark M. Substance P (SP) and vasoactive intestinal polypeptide (VIP) in the lower uterine segment in first and repeated cesarean sections. Peptides. 2010; 31(11): 2052-9. https://dx.doi.org/10.1016/j.peptides.2010.07.025.
- Holst N., Oian P., Aune B., Jenssen T.G., Burhol P.G. Increased plasma levels of vasoactive intestinal polypeptide in pre-eclampsia. Br. J. Obstet. Gynaecol. 1991; 98(8): 803-6. https://dx.doi.org/10.1111/j.1471-0528.1991.tb13486.x.
- Bourlev V., Moberg C., Ilyasova N., Davey E., Kunovac Kallak T.,Olovsson M. Vasoactive intestinal peptide is upregulated in women with endometriosis and chronic pelvic pain. Am. J. Reprod. Immunol. 2018; 80(3): e12857. https://dx.doi.org/10.1111/aji.12857.
- Di Spiezio Sardo A., Florio P., Fernandez L.M., Guerra G., Spinelli M.,Di Carlo C. et al. The potential role of endometrial nerve fibers in the pathogenesis of pain during endometrial biopsy at office hysteroscopy. Reprod. Sci. 2015; 22(1): 124-31. https://dx.doi.org/10.1177/1933719114534536.
- Van der Woude P.F., Goudsmit E., Wierda M., Purba J.S., Hofman M.A., Bogte H. et al. No vasopressin cell loss in the human hypothalamus in aging and Alzheimer's disease. Neurobiol. Aging. 1995; 16(1): 11-8.https://dx.doi.org/10.1016/0197-4580(95)80003-a.
- Hogenboom R., Kalsbeek M.J., Korpel N.L., de Goede P., Koenen M., Buijs R.M. et al. Loss of arginine vasopressin- and vasoactive intestinal polypeptide-containing neurons and glial cells in the suprachiasmatic nucleus of individuals with type 2 diabetes. Diabetologia. 2019; 62(11): 2088-93.https://dx.doi.org/10.1007/s00125-019-4953-7.
- Ishunina T.A., Swaab D.F. Estrogen receptor α splice variant TADDI in the human supraoptic nucleus: an effect on neuronal size and changes in pneumonia. Neuro Endocrinol. Lett. 2021; 42(2): 128-32.
- Ishunina T.A., Bogolepova I.N., Swaab D.F. Increased neuronal nuclear and perikaryal size in the medial mamillary nucleus of vascular dementia and Alzheimer's disease patients: relation to nuclear estrogen receptor α. Dement. Geriatr. Cogn. Disord. 2019; 47(4-6): 274-80.https://dx.doi.org/10.1159/000500244.
- Михальченко Д.В., Македонова Ю.А., Афанасьева О.Ю. Иммунологический анализ крови как диагностический фактор психоэмоционального стресса на стоматологическом приеме. Вестник Волгоградского государственного медицинского университета. 2020; 3: 70-4. [Mikhalchenko D.V., Makedonova Yu.A., Afanasyeva O.Yu. Immunological blood analysis as a diagnostic factor of psychoemotional stress at a dental appointment. Bulletin of Volgograd State Medical University. 2020; (3): 70-4. (in Russian)].https://dx.doi.org/10.19163/1994-9480-2020-3(75)-70-74.
- Ляпин В.М., Туманова У.Н., Щеголев А.И. Синцитиальные узелки в ворсинах плаценты при преэклампсии. Современные проблемы науки и образования. 2015; 4: 499. [Lyapin V.M., Tumanova U.N., Shchegolev A.I. Syncytial knots of placental villi at preeclampsia. Modern Problems of Science and Education. 2015; (4): 499. (in Russian). https://dx.doi.org/10.17513/spno.21421.
- Ревина Д.Б., Балацкий А.В., Ларина Е.Б., Мамедов Н.Н., Самоходская Л.М., Панина О.Б. Плацента-ассоциированные осложнения беременности:влияние полиморфизма rs4065 гена урокиназы. Вопросы гинекологии, акушерства и перинатологии. 2021; 20(1): 5-10. [Revina D.B.,Balatskiy A.V., Larina E.B., Mamedov N.N., Samokhodskaya L.M.,Panina O.B. Placental-related disorders of pregnancy: the influence of RS4065 polymorphism of the urokinase gene. Gynecology, Obstetrics andPerinatology. 2021; 20(1): 5-10. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2021-1-5-10.
- Roca V., Calafat M., Larocca L., Ramhorst R., Farina M., Franchi A.M. et al. Potential immunomodulatory role of VIP in the implantation sites of prediabetic nonobese diabetic mice. Reproduction. 2009; 138(4): 733-42.https://dx.doi.org/10.1530/REP-09-0171.
- Ramhorst R., Calo G., Paparini D., Vota D., Hauk V., Gallino L. et al. Control of the inflammatory response during pregnancy: potential role of VIP as a regulatory peptide. Ann. N. Y. Acad. Sci. 2019; 1437(1): 15-21. https://dx.doi.org/10.1111/nyas.13632.
- Gallino L., Hauk V., Fernández L., Soczewski E., Gori S., Grasso E. et al. VIP promotes recruitment of tregs to the uterine–placental interface during the peri-implantation period to sustain a tolerogenic microenvironment. Front. Immunol. 2020; 10: 2907. https://dx.doi.org/10.3389/fimmu.2019.02907.
- Paparini D.E., Choudhury R.H., Vota D.M., Karolczak-Bayatti M., Finn-Sell S., Grasso E.N. et al. Vasoactive intestinal peptide shapes first-trimester placenta trophoblast, vascular, and immune cell cooperation. Br. J. Pharmacol. 2019; 176(7): 964-80. https://dx.doi.org/10.1111/bph.14609.
- Paparini D.E., Grasso E., Fernandez L.D.C., Merech F., Weingrill-Barbano R., Correa-Silva S. et al. Decidual factors and vasoactive intestinal peptide guide monocytes to higher migration, efferocytosis and wound healing in term human pregnancy. Acta Physiol. (Oxf.). 2021; 232(1): e13579.https://dx.doi.org/10.1111/apha.13579.
- Fraccaroli L., Grasso E., Hauk V., Cortelezzi M., Calo G., Pérez Leirós C. et al. Defects in the vasoactive intestinal peptide (VIP)/VPAC system during early stages of the placental-maternal leucocyte interaction impair the maternal tolerogenic response. Clin. Exp. Immunol. 2012; 170(3): 310-20.https://dx.doi.org/10.1111/j.1365-2249.2012.04668.x.
Received 09.08.2024
Accepted 28.11.2024
About the Authors
Tatyana A. Sebyakina, PhD, Teaching Assistant at the Department of Obstetrics and Gynecology, Kursk State Medical University, Ministry of Health of Russia, 305041, Russia, Kursk, K. Marks str., 3, +7(920)262-20-11, doctor_gyn11@mail.ru, 0009- https://orcid.org/0007-6706-549XOksana Yu. Ivanova, Dr. Med. Sci., Head of the Department of Obstetrics and Gynecology, Kursk State Medical University, Ministry of Health of Russia, 305041, Russia, Kursk, K. Marks str., 3, +7(905)041-82-89, ivonavao1@mail.ru, https://orcid.org/0000-0003-2350-1740
Tatjana A. Ishunina, Dr. Med. Sci., Professor at the Department of Histology, Embryology, Cytology, Kursk State Medical University, Ministry of Health of Russia, 305041, Russia, Kursk, K. Marks str., 3, +7(960)689-19-79, ishunina@gmail.com, https://orcid.org/0000-0002-2743-7515
Corresponding author: Tatyana A. Sebyakina, doctor_gyn11@mail.ru



