Plasma levels of cell adhesion molecules in normal pregnancy and preeclampsia
Shelekhin A.P., Baev O.R., Andreev Yu.V., Sadekova A.A., Krasnyi A.M.
Objective: The objective of this study was to determine the levels of soluble forms of cell adhesion molecules (sVCAM-1, sICAM-1, sE-selectin, and sP-selectin) in the maternal blood of women with preeclampsia (PE) and compare them to levels in women with normal pregnancies, taking into account the severity and phenotype of PE.
Materials and methods: A prospective case-control study was conducted involving peripheral blood plasma samples collected from women with normal pregnancies (n=30) and preeclampsia (n=50). Multiplex assay was used to determine the concentrations of sVCAM-1, sICAM-1, sE-selectin, and sP-selectin.
Results: The concentration of sVCAM-1 in peripheral blood was significantly higher in women with preeclampsia than in those with uncomplicated pregnancies (p<0.001). Furthermore, sVCAM-1 levels were significantly higher in severe than in moderate preeclampsia (p=0.014). In addition, sVCAM-1 levels were higher in patients with early-onset than in those with late-onset preeclampsia. However, no statistically significant differences were observed in the levels of sICAM-1, sE-selectin, or sP-selectin.
Conclusion: Our study revealed increased sVCAM-1 levels in the peripheral blood of pregnant women with preeclampsia. This elevation correlates with the severity of the disease, suggesting the involvement of this molecule in the development of preeclampsia. The differences in sVCAM-1 levels between early- and late-onset preeclampsia suggest different pathogenic mechanisms underlying these two conditions.
Authors' contributions: Shelekhin A.P., Baev O.R., Andreev Y.V., Sadekova A.A., Krasnyi A.M. – study design, obtaining data for analysis, review of relevant literature, statistical analysis, drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Shelekhin A.P., Baev O.R., Andreev Yu.V., Sadekova A.A., Krasnyi A.M. Plasma levels of cell adhesion molecules in normal pregnancy and preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (11): 71-77 (in Russian)
https://dx.doi.org/10.18565/aig.2023.200
Keywords
Preeclampsia (PE) is a life-threatening and unpredictable complication of pregnancy that involves various organs and systems of the human body in a pathological process. In clinical practice, PE can be classified into early (<34 weeks of gestation) and late (>34 weeks of gestation) based on the time of manifestation as well as moderate and severe forms determined by blood pressure, clinical data, and degree of proteinuria [1]. The distinction between early and late forms of PE is based on the timing of manifestation, hemodynamic features, and impact on fetal growth. However, determining the severity of PE is often a subjective clinical assessment, which can make it challenging to differentiate it from less severe forms of the disorder [2]. Research on PE has revealed that severe early-onset PE is associated with adverse maternal and perinatal outcomes as well as an increased risk of future cardiovascular disease in both women and children [3, 4].
Although PE has been the subject of extensive research, it remains an enigma and a "disease of theories" even today [5]. The main mechanism involved in the pathogenesis of the disease is believed to be uteroplacental ischemia, resulting from shallow trophoblast invasion and impaired remodeling of the uterine spiral arteries [6]. Placental ischemia leads to damage to syncytiotrophoblasts and impaired placental barrier function, causing the release of proinflammatory cytokines, antiangiogenic agents, foreign fetal antigens, and cell-free fetal DNA into the maternal bloodstream [6, 8]. Consequently, these factors affect the maternal vascular endothelium, resulting in the development of a systemic inflammatory response and endothelial dysfunction.
Invasion of trophoblast cells into the spiral uterine arteries occurs with the participation of immune reactions in inflammatory orientation. An important factor in the inflammatory process is the adhesion of leukocytes to endothelial cells and their subsequent migration into perivascular tissue. The adhesion of leukocytes to the endothelium is mediated by the interaction between adhesion molecules and their ligands located on the surface of these cells. Three families of cell adhesion molecules play roles in these interactions: selectins, integrins, and members of the immunoglobulin gene superfamily. Selectins are distinguished according to their locations in different cells. Three types of selectins have been identified: P-selectins located on platelets, E-selectins on the endothelium, and L-selectins on leukocytes. Integrins are composed of α- and β-subunits and play a role in many processes in the body. The immunoglobulin gene superfamily includes a number of molecules, such as intercellular adhesion molecules (ICAM-1), type 2 (ICAM-2), type 3 (ICAM-3), and vascular cell adhesion molecules (VCAM-1).
Selectins are involved in the initial binding of leukocytes to activated endothelial cells, whereas integrins and the immunoglobulin gene superfamily regulate adhesion followed by leukocyte transmigration. Damage to molecules in one of the adhesion links can lead to impaired placentation. Adhesion molecules have membrane and soluble forms. When activated by cytokines or endotoxins, cell adhesion molecules are released from the endothelium, platelets, and leukocytes. The breakage of the bonds between the membrane and extracellular domains occurs via proteolysis. Proteolytic cleavage of transmembrane substrates near the cell surface leads to release of soluble ectodomains (shedding). Critical in mediating the cleavage of membrane forms of cell adhesion molecules are zinc-dependent endopeptidases, including matrix metalloproteinases (MMPS) membrane-type matrix metalloproteinases (MT)-MMPS, and members of the disintegrin and metalloproteinase (ADAM) family [9]. ADAMs (a disintegrin and metalloproteinase) are a family of transmembrane and secreted proteins with a length of approximately 750 amino acids that play important roles in regulating cell phenotype via their effects on cell adhesion, migration, proteolysis, and signaling.
Several studies have indicated an increase in the soluble forms of cell adhesion molecules in the peripheral blood of PE [10–12]. These molecules are considered potential diagnostic and prognostic markers of PE and could enhance the management of patients with this diagnosis. However, previous studies on these molecules have provided ambiguous and contradictory data [12, 13], highlighting the need for further research.
The objective of this study was to determine the levels of soluble forms of cell adhesion molecules (sVCAM-1, sICAM-1, sE-selectin, and sP-selectin) in the maternal blood of women with preeclampsia and compare them to those in women with normal pregnancies, taking into account the severity and phenotype of PE.
Materials and methods
We conducted a case-control study of 80 pregnant women. All of these were observed in the V.I. Kulakov NMRC for OG&P of the Ministry of Health of the Russian Federation from 2019 to 2023.
The inclusion criteria for the study (control group) were age of patients 18–45 years, singleton pregnancy, informed consent to participate in the study, uncomplicated pregnancy, and labor. The inclusion criteria (study group) were age of patients 18–45 years, singleton pregnancy, informed consent to participate in the study, and pregnancy complicated by PE.
Non-inclusion criteria (for all groups) were multiple pregnancies, moderate to severe somatic comorbidities, history of cancer, autoimmune diseases, malformations, acute phase and exacerbation of chronic infectious diseases, refusal to participate in the study.
The patients were divided into two groups. The study group comprised 50 pregnant women with PE. The severity of PE was moderate in 39/50 (78%) patients and severe in 11/50 (22%). Early-onset PE occurred in 19/50 (38%) patients, and late-onset in 31/50 (62%) patients. In patients with early-onset disease, severe PE developed in 9/19 (47.3%) patients and moderate PE in 10/19 (52.6%) patients. In patients with late-onset disease, severe PE was observed in the of 2/31 (6.5%) patients and moderate PE in 29/31 (93.5%) patients.
Early-onset PE was diagnosed when it manifested before 34 weeks of gestation and late-onset PE was diagnosed after 34 weeks. The criteria for moderate PE were an increase in blood pressure (BP) above 140/90 mmHg (up to 160/110) first detected after 20 weeks of pregnancy, accompanied by proteinuria >0.3 g/L (up to 5 g/day or 3 g/L in two portions of urine). Severe PE was diagnosed when systolic BP ≥160 mmHg and/or diastolic BP ≥110 mmHg increased in combination with proteinuria ≥5 g/day or ≥3 g/L in two urine portions or signs of multi-organ failure.
Maternal venous whole blood (5 ml) was drawn into tubes containing ethylenediaminetetraacetate (EDTA) and processed within 30 min of sampling. They were then centrifuged and stored in the biobank of the V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia. The Luminex Performance Assay Human Adhesion Molecule Multiplex Kit (USA) and multiplex assays were used to determine the plasma levels of sICAM-1, sVCAM-1, sE-selectin, and sP-selectin. The adhesion molecule concentrations were calculated using a Luminex 100/200 dual-laser flow sorting and detection analyzer.
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P.
Statistical analysis
Statistical analysis was performed using Stattech statistical software (Russia). The normality of the distribution was tested using the Shapiro–Wilk test (when the number of subjects was < 50) or the Kolmogorov–Smirnov test (when the number of subjects was > 50). Variables that did not meet normality assumptions were reported as median (Me) and interquartile range (Q1; Q3). Continuous variables were compared using the nonparametric Mann–Whitney test. Categorical variables are summarized using counts (n) and percentages. Differences in categorical variables were assessed using Pearson's chi-square test. The diagnostic and prognostic significance of the studied indicators was evaluated using ROC analysis. The scatter plots in the figures are presented as the 5th, 25th, 50th, 75th, and 95th percentiles. The separating value of the studied indicators at the cutoff point was determined by the highest value of the Youden index. Differences were considered statistically significant at p<0.05.
Results
The characteristics of the study participants and their newborns are presented in table. The groups were similar in terms of age and body mass index. There were no differences in the frequency of smoking and PE in previous pregnancies. In the PE group, chronic arterial hypertension preceded PE in 11/50 (22%) patients. Gestational age at delivery, birth weight, and length were significantly lower in the PE group (p<0.001, p<0.001, p<0.001, p<0.001, and p<0.001, respectively), which was associated with higher rates of preterm birth in patients with PE.
sP-selectin
The plasma concentrations of sP-selectin in women with healthy pregnancies and those with PE were not significantly different (p=0.551). The sP-selectin concentration was 3566.0 (3236.50; 4587.875) pg/mL in the control group and 3831.50 (3370.50; 4353.750) pg/mL in women with PE.
sE-selectin
When comparing plasma sE-selectin concentrations in women with healthy pregnancies and those with PE, no statistically significant differences were found (p=0.077). In the control group, the concentration of sE-selectin was 2476.750 (2079.750; 3414.250) pg/mL. In PE, the sE-selectin concentration was 2939.00 (2456.375; 3564.125) pg/mL.
sICAM-1
Analysis of plasma sICAM-1 concentrations in women with healthy pregnancies and those with PE showed no statistically significant differences (p=0.548). The sICAM-1 concentration in the control group was 310.50 (190.50; 526.750) pg/mL, and in women with PE it was 378.50 (245.250; 610.250) pg/mL.
sVCAM-1
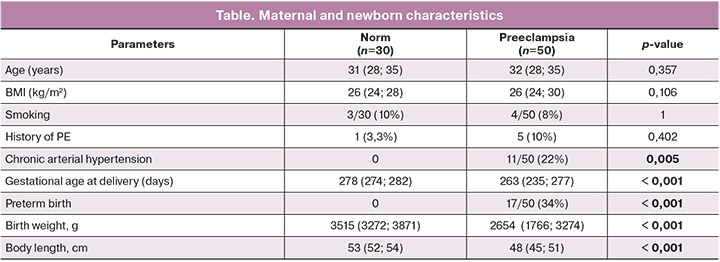
In the group with healthy pregnancies, the sVCAM-1 concentration was 492.0 (393.0; 586.125) pg/mL. In PE, the concentration of sVCAM-1 in the maternal blood was 987.0 (772.250; 1239, 375) pg/mL (p<0.001) (Fig. 1).
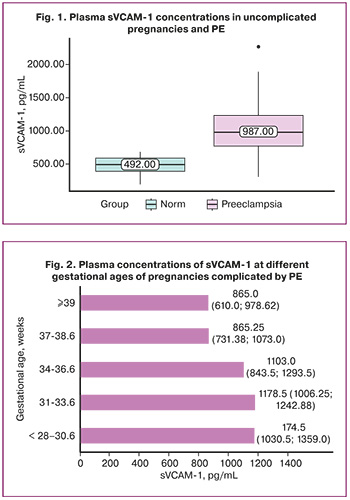
The distribution of sVCAM-1 levels according to gestational age in PE is shown in Figure 2.
To identify the significance of sVCAM-1 in determining the severity of PE, we compared the plasma concentrations of sVCAM-1 in women with moderate PE (39/50) and severe PE (11/50). In moderate PE, the plasma concentration of sVCAM-1 was 940.0 (713.750; 1181.50) pg/mL. A statistically significant increase in sVCAM-1 concentration was found in severe PE, 1174.50 (1065.250; 1318.50) pg/mL (p=0.014) (Fig. 3).
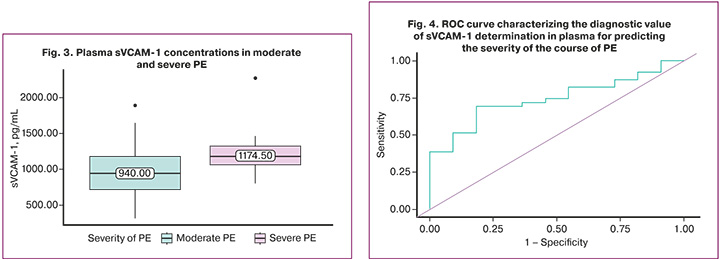
ROC analysis was performed to determine the level to assess the severity of PE. The area under the ROC curve was 0.744±0.092, with a 95% CI 0.562–0.925. The resulting model was statistically significant (p=0.014). The threshold value of sVCAM-1 above which severe PE was diagnosed was 1062.0 (Fig. 4). The sensitivity and specificity of the model were 69.2 and 81.8%, respectively. Positive and negative predictive values were 93.1% and 42.9 %, respectively.
The concentration of sVCAM-1 differed in PE at different time points of manifestation. In late-onset PE, the median sVCAM-1 concentration was 931.00 (713.75; 1130.00) pg/mL. In early-onset PE, the sVCAM-1 concentration was 1151.00 (973.75; 1263.00) pg/mL. This difference was statistically significant (p=0.027).
To exclude the confounding effect of PE severity on the assessment of sVCAM-1 level dependence on phenotype, we compared sVCAM-1 levels between women with moderate and severe PE in the early-onset PE group. In severe PE, sVCAM-1 levels were higher, but the differences were not significant: 1034.25 (906.75; 1227.38) pg/mL and 1174.50 (1068.0; 1372.0) pg/mL, (p=0.253) (Fig. 5).
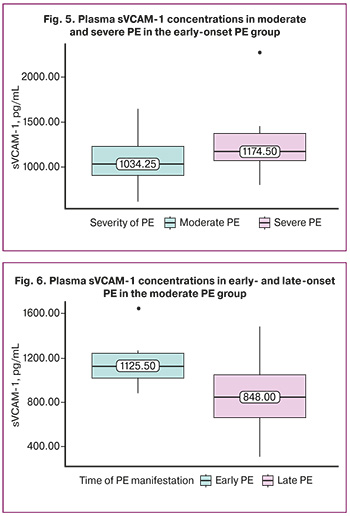
Comparison of sVCAM-1 levels in moderate PE according to phenotype (early and late-onset PE) revealed that sVCAM-1 concentration was significantly higher at 1125.5 (1015.88; 1242.88) pg/mL in moderate early-onset PE than 848.0 (661.12; 1048.25) pg/mL in moderate late PE (p=0.018) (Fig. 6).
Discussion
The information available in the literature on studies investigating the concentration of cell adhesion molecules in the maternal blood in preeclampsia is mixed and conflicting. Typically, serum was collected and an enzyme-linked immunosorbent assay was used to determine the adhesion molecules. In our study, we collected blood plasma and determined the content of adhesion molecules using a multiplex method, which is expected to yield more reliable results.
Our study revealed a significant increase in sVCAM-1 levels in pregnant women with preeclampsia. The cell adhesion molecule VCAM-1 is present in activated endothelial cells and plays a crucial role in attracting leukocytes to the site of inflammation. VCAM-1 mediates adhesion of lymphocytes, monocytes, and eosinophils to the endothelium. During pathological processes, the soluble form of VCAM-1 is released and circulates in the blood stream. The expression of the soluble form of VCAM-1 (sVCAM-1) is typically induced by pro-inflammatory cytokines in the endothelium of blood vessels to attract, adhere, and transmigrate monocytes and lymphocytes. In preeclampsia, the structure of the membrane form of VCAM-1 is disrupted, leading to formation of its soluble form, sVCAM-1.
One of the mechanisms in trophoblast invasion involves the interaction of membrane VCAM-1 with integrins located on the surface of leukocytes. Therefore, VCAM-1 promotes trophoblast invasion by retaining it on the endothelial surface [14]. In turn, sVCAM-1 blocks the interaction between VCAM-1 and integrins, potentially reducing placental invasion and pregnancy complications. Thus, VCAM-1 and sVCAM-1 play crucial roles in the regulation of placental invasion, and consequently, in pregnancy development [15]. Elevated sVCAM-1 levels may reduce trophoblast invasion, leading to concurrent vascular injury. Additionally, sVCAM-1 may serve as an indicator of systemic inflammatory responses and endothelial dysfunction in preeclampsia [16].
Our results are partially aligned with those of previous studies on the content of cell adhesion molecules in preeclampsia. Chaiworapongsa et al. (2002) investigated the levels of sL-selectin, sE-selectin, sP-selectin, sVCAM-1, sICAM-1, and sPECAM-1 in peripheral blood during normal pregnancy and preeclampsia. These findings indicated a significant increase in maternal plasma sP-selectin concentration during normal pregnancy, a decrease in sL-selectin, and no changes in sE-selectin, sVCAM-1, sICAM-1, and sPECAM-1. In preeclampsia, there was a notable increase in sP-selectin, sE-selectin, and sVCAM-1 levels, along with a decrease in sL-selectin concentrations. The concentrations of sICAM-1 and sPECAM-1 remained unchanged [12].
Additionally, an increase in sVCAM-1 levels in preeclampsia has been reported in studies conducted by Docheva et al. (2018) [17] and Pasaribu et al. [18]. However, our study did not show an increase in sE-selectin and sP-selectin levels in preeclampsia. Farzadnia et al. [13] demonstrated an increase in the concentration of not only sVCAM-1 but also sICAM-1 in preeclampsia. Nevertheless, our results did not show an increase in sICAM-1 levels in preeclampsia.
We observed a significant difference in sVCAM-1 levels between patients with moderate and severe preeclampsia. This suggests that preeclampsia progression is associated with an increase in sVCAM-1 levels. Similar findings regarding sVCAM-1 concentrations were presented by Kim et al., where concentrations of sVCAM-1, sICAM-1, and sE-selectin in peripheral blood serum were studied in normal pregnancy and in moderate and severe preeclampsia. However, their study revealed a statistically significant increase in sICAM-1 and sE-selectin levels in severe preeclampsia compared to normal pregnancies, which contradicts our results [19]. Elevated sVCAM-1 concentrations may signal both impaired placentation and increased endothelial dysfunction that accompanies preeclampsia.
The comparison of sVCAM-1 levels depending on the preeclampsia phenotype revealed significant differences, characterized by higher levels of this molecule in early-onset preeclampsia. Considering the potential influence of preeclampsia severity on the study results, we conducted a separate analysis of early- and late-onset moderate preeclampsia, confirming the observed dependence. However, the analysis within the early-onset preeclampsia group showed no significant differences in severity. Therefore, it can be assumed that in early-onset preeclampsia, the distinction between moderate and severe degrees is conditional as the level of endothelial dysfunction is consistently high.
While we did not find any studies comparing the concentrations of soluble forms of cell adhesion molecules based on preeclampsia phenotype, Liu et al. conducted a study showing a decrease in the content of the membrane form of VCAM-1 in placental tissue in early-onset preeclampsia compared with normal pregnancy [20]. Considering the inverse correlation between VCAM-1 and sVCAM-1, the observed dependence aligns with the current concept of the pathogenesis of endothelial dysfunction in preeclampsia.
Simultaneously, the detected increase in sVCAM-1 levels in the early manifestation of preeclampsia indicates that this phenotype has a higher likelihood of unfavorable maternal and perinatal outcomes due to a more pronounced course of endothelial dysfunction.
Conclusion
In conclusion, our study demonstrated a significant increase in peripheral blood levels of sVCAM-1 in pregnant women with preeclampsia, which was directly correlated with disease severity, suggesting the involvement of this molecule in the development of preeclampsia. The identified differences in sVCAM-1 levels in early- and late-onset preeclampsia confirmed the specific pathogenic features of this complication.
References
- Министерство здравлоохранения Российской Федерации. Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде. Клинические рекомендации. М.; 2021. [Ministry of Health of the Russian Federation. Preeclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders during pregnancy, childbirth and the postpartum period. Clinical guidelines. Moscow; 2021 (in Russian)].
- Roberts J.M., Rich-Edwards J.W., McElrath T.F., Garmire L., Myatt L. Subtypes of preeclampsia: recognition and determining clinical usefulness. Hypertension. 2021; 77(5): 1430-41. https://dx.doi.org/10.1161/HYPERTENSIONAHA.120.14781.
- Шелехин А.П., Баев О.Р., Красный А.М. Сравнение течения и исходов беременностей, осложненных гипертензивными расстройствами. Акушерство и гинекология. 2023; 1: 41-7. [Shelekhin A.P., Baev O.R., Krasnyi A.M. Comparison of the course and outcomes of pregnancies complicated by hypertensive disorders. Obstetrics and Gynecology. 2023;(1): 41-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.248.
- Madazli R., Yuksel M.A., Imamoglu M., Tuten A., Oncul M., Aydin B., Demirayak G. Comparison of clinical and perinatal outcomes in early- and late-onset preeclampsia. Arch. Gynecol. Obstet. 2014;290(1):53-7. https://dx.doi.org/10.1007/s00404-014-3176-x.
- Robillard P.-Y., Dekker G., Scioscia M., Saito S. Progress in the understanding of the pathophysiology of immunologic maladaptation related to early-onset preeclampsia and metabolic syndrome related to late-onset preeclampsia. Am. J. Obstet. Gynecol. 2022;226(2):S867-75. https://dx.doi.org/10.1016/j.ajog.2021.11.019.
- Redman C.W.G., Staff A.C., Roberts J.M. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am. J. Obstet. Gynecol. 2022;226(2):S907-27. https://dx.doi.org/10.1016/j.ajog.2020.09.047.
- Шелехин А.П., Баев О.Р., Садекова А.А., Кокоева Д.Н., Красный А.М. Особенности содержания Е-кадгерина в плазме крови и ткани плаценты при преэклампсии. Акушерство и гинекология. 2023; 3: 36-40. [Shelekhin A.P., Baev O.R., Sadekova A.A., Kokoeva D.N., Krasnyi A.M. The content of different forms of E-cadherin in blood plasma and placental tissue in preeclampsia. Obstetrics and Gynecology. 2023;(3): 36-40. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.282.
- Baev O.R., Karapetian A.O., Nizyaeva N.V., Sadekova А.А., Krasniy A.M. Content of free fetal DNA in maternal blood and expression of DNA recognition receptors ZBP-1 in placental tissue in preeclampsia and preterm labor. Bull. Exp. Biol. Med. 2019;168(1):145-9. https://dx.doi.org/10.1007/s10517-019-04665-z.
- Essick E., Sithu S., Dean W., D’Souza S. Pervanadate-induced shedding of the intercellular adhesion molecule (ICAM)-1 ectodomain is mediated by membrane type-1 matrix metalloproteinase (MT1-MMP). Mol. Cell Biochem. 2008;314(1-2):151-9. https://dx.doi.org/10.1007/s11010-008-9776-7.
- Зиганшина М.М., Кречетова Л.В., Ванько Л.В., Культербаева М.А., Соколян А.В., Сухих Г.Т. Динамика растворимых форм молекул клеточной адгезии при преэклампсии. Акушерство и гинекология. 2011;2:42-8. [Ziganshina M.M., Krechetova L.V., Vanko L.V., Kulterbayeva M.A., Sokolyan A.V., Sukhikh G.T. Time course of changes in the soluble forms of cell adhesion, molecules in preeclampsia. Obstetrics and Gynecology. 2011;(2):42-8 (in Russian)].
- Сидорова И.С., Никитина Н.А. Особенности патогенеза эндотелиоза при преэклампсии. Акушерство и гинекология. 2015;1:72-8. [Sidorova I.S., Nikitina N.A. Pathogenesis of endotheliosis in preeclampsia. Obstetrics and Gynecology. 2015;(1):72-8 (in Russian)].
- Chaiworapongsa T., Romero R., Yoshimatsu J., Espinoza J., Kim Y.M., Park K. et al. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J. Matern. Neonatal. Med. 2002;12(1):19-27. https://dx.doi.org/10.1080/jmf.12.1.19.27.
- Farzadnia M., Ayatollahi H., Hasan-Zade M., Rahimi H.R. A comparative study of vascular cell adhesion molecule-1 and high-sensitive C-reactive protein in normal and preeclamptic pregnancies. Interv. Med. Appl. Sci. 2013;5(1):26-30. https://dx.doi.org/10.1556/imas.5.2013.1.5.
- Adu-Gyamfi E.A., Czika A., Gorleku P.N., Ullah A., Panhwar Z., Ruan L.-L. et al. The involvement of cell adhesion molecules, tight junctions, and gap junctions in human placentation. Reprod. Sci. 2021;28(2):305-20. https://dx.doi.org/10.1007/s43032-020-00364-7.
- Haram K., Mortensen J.H., Myking O., Magann E.F., Morrison J.C. The Role of oxidative stress, adhesion molecules and antioxidants in preeclampsia. Curr. Hypertens. Rev. 2019;15(2):105-12. doi:10.2174/1573402115666190119163942.
- Kornacki J., Wirstlein P., Wender-Ozegowska E. Markers of endothelial injury and dysfunction in early- and late-onset preeclampsia. Life (Basel). 2020;10(10):239. https://dx.doi.org/10.3390/life10100239.
- Docheva N., Romero R., Chaemsaithong P., Tarca A.L., Bhatti G., Pacora P. et al. The profiles of soluble adhesion molecules in the “great obstetrical syndromes”. J. Matern. Neonatal. Med. 2019;32(13):2113-36. https://dx.doi.org/10.1080/14767058.2018.1427058.
- Pasaribu H.P., Hariman H., Roeshadi R.H., Koh S.C. Soluble vascular cell adhesion molecule-1 and magnesium sulfate with nifedipine treatment in Indonesian women with severe pre-eclampsia. Interv. Med. Appl. Sci. 2016;8(3):97-102. https://dx.doi.org/10.1556/1646.8.2016.3.4.
- Kim S.Y., Ryu H.M., Yang J.H., Kim M.Y., Ahn H.K., Lim H.J. et al. Maternal serum levels of VCAM-1, ICAM-1 and E-selectin in preeclampsia. J. Korean Med. Sci. 2004;19(5):688-92. https://dx.doi.org/10.3346/jkms.2004.19.5.688.
- Liu B., Liu L., Cui S., Qi Y., Wang T. Expression and significance of microRNA-126 and VCAM-1 in placental tissues of women with early-onset preeclampsia. J. Obstet. Gynaecol. Res. 2021; 47(6): 2042-50. https://dx.doi.org/10.1111/jog.14732.
Received 18.08.2023
Accepted 24.10.2023
About the Authors
Artemiy P. Shelekhin, postgraduate student, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), dr.shelekhin@gmail.com, https://orcid.org/0000-0002-7682-13298, 119991, Russia, Moscow, Trubetskaya str., 8-2.Oleg R. Baev, Dr. Med. Sci., Professor, Head of the 1st maternity ward, Academician V.I. Kulakov National Medical Research Сenter for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia; Professor at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), o_baev@oparina4.ru, https://orcid.org/0000-0001-8572-1971,
117997, Russia, Moscow, Ac. Oparina str., 4.
Yuliy V. Andreev, PhD, Senior Researcher at the Department of Biotechnologies and Transfusiology, N.V. Sklifosovsky Research Institute for Emergency Medicine, AndreevUV@sklif.mos.ru, https://orcid.org/0000-0001-8151-940X, 129090, Russia, Moscow, B. Sukharevskaya sqr., 3.
Alsu A. Sadekova, PhD, Senior Researcher at the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Сenter for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-22-72, sialsad@gmail.com
https://orcid.org/0000-0003-4726-7477, 117997, Russia, Moscow, Ac. Oparina str., 4.
Alexey M. Krasnyi, PhD, Head of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Сenter for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, a_krasnyi@oparina4.ru, https://orcid.org/0000-0001-7883-2702, 117997, Russia, Moscow, Ac. Oparina str., 4.



