Clinical and pathogenetic rationale for two-stage prevention of preeclampsia in high-risk women using an insulin sensitizer for preconception preparation
Objective: To demonstrate the effectiveness of a two-step approach for the prevention of pre-eclampsia in high-risk women, consisting of pregestational use of the insulin sensitizer metformin (ISM) followed by low-dose acetylsalicylic acid (LDAA) during pregnancy. Materials and methods: At the preconception stage, women at high risk of preeclampsia were divided into groups according to the prevention method: group 1, 77 patients who received two-stage prophylaxis (ISM at the preconception stage and LDAA during pregnancy); group 2, 75 patients who received LDAA monotherapy at the gestational stage; group 3, 72 patients who received metformin only preconceptionally; and group 4, 73 patients who refused prevention. Thirty women with physiological gestation served as the controls. At 11–14, 18–21, and 30–34 weeks, hormonal-metabolic, pro-inflammatory, and endothelial-hemostasiological patterns were assessed. Based on the primary outcome, the incidence of pre-eclampsia, the size of the effect of the preventive intervention was calculated. Results: Clinical and laboratory parallels proved the advantage of a two-stage approach to the prevention of preeclampsia: the incidence of preeclampsia in group 1 was 3.8 times lower (RR 0.26 [0.14; 0.49], RRR 73.7% [50.9; 85.9], ARR 36.3% [22.6; 50.1], NNT 3 [2; 4], χ2=21.58, p<0.001), compared with the group without prevention, and with ISM or LDAA alone, only 2.7 (RR 0.37 [0.21; 0.63], RRR 63.4% [36.9; 78.8], ARR 31.3% [16, 8; 45.8], NNT 4 [2; 6], χ2=14.47, p<0.001) and 1.6 times (RR 0.62 [0.41; 0.94], RRR 37.8 % [6.0; 58.8], ARR 18.7% [3.1; 34.2], NNT 6 [3; 32], χ2=4.62, p=0.03), respectively. In group 1, the incidence of severe preeclampsia was reduced by 3.1 and 3.6 times, early preeclampsia by 1.6 and 1.6 times, relative to groups 2 and 3. The effectiveness of the staged approach was confirmed by the influence of preeclampsia prevention methods on the intensity of compensation for insulin resistance and the associated proinflammatory and prothrombogenic patterns, markers of alteration, and remodeling of the vascular endothelium. The lack of effectiveness of the standard use of LDAA is associated with the lack of a periconceptual influence on key events in early pregnancy. Conclusion: A new strategy for the prevention of preeclampsia is represented by a staged approach that provides a reduction in cardiovascular risk by developing metabolic resistance in women at high risk of preeclampsia from the stage of pregnancy planning and is implemented as a promising, highly effective, pathogenetically substantiated method of prevention aimed at improving obstetric outcomes and stabilizing cardiovascular disease. the vascular continuum later in life. Authors’ contributions: Zumorina E.M., Azamatov A.R., Chekalovets A.L., Borisova A.I., Golodnova A.M. – data collection and analysis; Lipatov I.S., Tezikov Yu.V. – conception and design of the study, review of relevant literature, drafting of the manuscript; Tyutyunnik V.L., Kan N.E. – data interpretation, editing of the manuscript. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of Samara State Medical University, Ministry of Health of Russia. Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Tezikov Yu.V., Lipatov I.S., Zumorina E.M., Azamatov A.R., Tyutyunnik V.L., Kan N.E., Chekalovets A.L., Borisova A.I., Golodnova A.M. Clinical and pathogenetic rationale for two-stage prevention of preeclampsia in high-risk women using an insulin sensitizer for preconception preparation. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (9): 60-71 (in Russian) https://dx.doi.org/10.18565/aig.2023.139Tezikov Yu.V., Lipatov I.S., Zumorina E.M., Azamatov A.R., Tyutyunnik V.L., Kan N.E., Chekalovets A.L., Borisova A.I., Golodnova A.M.
Keywords
The implementation of the WHO mission to enhance the prevention and treatment of hypertensive disorders of pregnancy is most effectively addressed through preventive and personalized medicine [1]. However, existing methods of preventing preeclampsia, as recommended by international federations and professional societies, tend to have a late onset when critical gestational restructuring processes are already underway, triggering the pathogenic events of preeclampsia [2]. Current clinical guidelines and results from multicenter studies, including randomized, double-blind, placebo-controlled trials, provide insufficient confidence in the recommendation for the use of low-dose acetylsalicylic acid (LDAA) during pregnancy. Furthermore, the effectiveness of LDAA has yielded conflicting results [3, 4]. Several factors can also influence the effectiveness of ASA in individual women, such as age, body weight, concurrent somatic pathologies, use of other medications, and potential development of aspirin resistance [5]. Similarly, conflicting data emerged from a 2017 Cochrane review of calcium supplementation, which indicated a reduced risk of preeclampsia in trials involving 15,730 women. However, the reduction in the incidence of preeclampsia was modest (8%) in the largest trial, and the confidence interval of the relative risk included the possibility of no effect [6]. Given the low effectiveness of the existing prevention methods, there is a need to search for new strategies to prevent preeclampsia. It is important to note that despite clear evidence of more favorable outcomes in healthy women, there has been a lack of targeted, pathogenetically based prevention for women at high risk of preeclampsia during the pre-pregnancy stage. Furthermore, the interventions during pregnancy mainly focus on specific corrective measures for pathophysiological mechanisms already in progress, such as inflammation, oxidative stress, and increased platelet aggregation [3, 4].
The increasing incidence of preeclampsia is closely associated with a higher risk of future manifestations of metabolic syndrome (MS), type 2 diabetes mellitus (DM), essential arterial hypertension, and its complications. This highlights the key role of pathological insulin resistance (IR) in endothelial destabilization and the development of these conditions [7]. The well-established link between preeclampsia and high cardiovascular risk explains the similarity in causes of death between women with a history of preeclampsia and those with MS. Meta-analyses involving millions of women with preeclampsia and MS demonstrate common causes of death, including strokes, heart attacks, thromboembolism, vascular dementia, and end-stage renal failure [8, 9]. These findings confirm the association between preeclampsia and diseases and complications that are part of the insulin-resistant continuum across different stages of life [10].
Based on the current understanding, preeclampsia can be considered a manifestation of metabolic crisis within a distinct gestational clinical-pathogenetic variant of MS. Pathological insulin resistance and hyperinsulinemia play crucial roles in fetal development and serve as the main mechanisms of energy-plastic support for fetal growth. These mechanisms become depleted due to factors like heredity, epigenetics, environment, lifestyle, and nutrition, leading to associated inflammatory, oxidative, and prothrombotic conditions [11, 12].
Therefore, to prevent preeclampsia, the goal is to reduce cardiovascular risk by enhancing metabolic stability and counteracting the formation of pathological insulin signaling pathways and intracellular energy dysfunction. This should be initiated from the preconception stage to establish a positive "metabolic memory.” In this regard, metformin, an insulin sensitizer with proven pleiotropic effects such as vasoprotection, antioxidant properties, anti-inflammatory activity, and hypolipidemic effects, holds promise [13, 14].
This study aimed to substantiate the effectiveness of a two-step approach for the prevention of preeclampsia in high-risk women, consisting of pregestational use of ISM followed by LDAA during pregnancy.
Materials and methods
The study involved 297 women at high risk for developing preeclampsia, who were divided according to the method of prevention. Group 1 included 77 patients who received two-stage prophylaxis: ISM at the pre-pregnancy stage and LDAA at the gestational stage. Group 2 included 75 patients who received LDAA only during the gestational period. Group 3 included 72 patients who received ISM only during the pre-pregnancy stage. Group 4 included 73 patients who refused the preventive therapy. Group 5 (control group) included 30 healthy women with healthy pregnancies. The inclusion criteria were as follows: personal and/or family history of preeclampsia, age >35 years, body mass index of 18.5–24.9 kg/m2; waist circumference <80 cm, blood pressure <130 and 85 mmHg, and presence of prediabetes. The diagnosis of prediabetes in women with independent high-risk factors for preeclampsia, the presence of which justified the prescription of ISM as a preventive agent at the pregestational stage, was made based on assessment of the level of glycated hemoglobin (6.0–6.4%), the presence of impaired glucose tolerance and/or impaired fasting glycemia, and use of the validated FINDRISK [Finnish Diabetes Risk Score (FINDRISK] questionnaire (≥12 points indicative of prediabetes) [15]. Non-inclusion criteria were severe extragenital comorbidities, including type 1 and type 2 diabetes, history of infertility, congenital malformations of the maternal genital organs, infectious diseases, polycystic ovary syndrome, mental illness, and the presence of contraindications to ISM. Exclusion criteria were violation of the individual protocol, including adverse events when taking ISM, pregnancy as a result of assisted reproductive technologies, and multiple pregnancies.
Two-stage prevention of preeclampsia included: at the preconception stage, taking orally ISM 500 mg twice daily for 4–6 months, lifestyle modification (clinical recommendations “Type 2 diabetes in adults,” 2022 [16]) and at the gestational stage, taking oral LDAA at a dosage of 150 mg at night, from the 12th to the 36th week (according to current recommendations [17]). The use of LDAA only during pregnancy and ISM before pregnancy in women in groups 2 and 3, respectively, was similar to that in group 1.
All patients were tested for hormonal-metabolic parameters (venous plasma glucose, insulin, HOMA-IR, leptin, cortisol, placental lactogen (PL), total cholesterol (TC), triglycerides (TG), high-density lipoproteins (HDL), TG/HDL, and uric acid), pro-inflammatory (tumor necrosis factor (TNF-α), and endothelial-hemostasiological (circulating endothelial cells (CEC), aggregogram, fibronectin (FN)) patterns as well as markers of placental angiogenesis (PGF) and decidualization (PAMG-1). During the examination, the ultrasound system "Voluson E6" GE Healthcare (GE, USA), analyzer Architect c4000, Architect i1000 SR (Abbotte, USA), Sysmex XN-1000 (Sysmex Corporation, Japan), and ALAT-2 (LLC SPF Biola, Russia) were used. Additionally, patient evaluation included 24-h blood pressure monitoring (BP-Lab system (Petr Telegin, Russia), accumulation and distribution of fat, such as subcutaneous fat thickness (SCF) and preperitoneal fat thickness (PPF). The abdominal wall fat index (AFI) is the ratio of preperitoneal to subcutaneous fat thickness (PPF/SCF), with a value >1.0 indicates visceral fat deposition. In addition, the examination included a subjective assessment of sleep characteristics (Ya.I. Levin questionnaire (1995)), nocturia, and periods of gestational sleep apnea (SOMNOcheck (Weinmann, Germany)).
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics 25 HC IMAGO 5.0 program (IBM, USA). The normality of the distribution was tested using the Shapiro-Wilk test. Numerical variables were not normally distributed and were reported as the median (Me) and interquartile range from Q1 to Q3 (25–75% quartiles). The Kruskal–Wallis test was used to compare numerical data between groups, followed by pairwise comparison using the Mann – Whitney U-test with Bonferroni correction (critical level of significance when comparing four groups, p<0.008; five groups, p<0.005). Pearson’s χ² was used to compare categorical variables, and Pearson's χ² with Yates correction was used for the 2×2 contingency tables. The Wilcoxon signed-rank test was used to compare the paired groups (changes during pregnancy). To evaluate the effectiveness of prevention, the following indicators were calculated: RR, relative risk; RRR, ARR, relative and absolute risk reduction; and NNT, the number of pregnant women who need to undergo prevention to prevent an unfavorable outcome in one patient. A 95% confidence interval (95% CI) was calculated [18, 19].
Results and discussion
In women from the control groups (n=297), high-risk factors for preeclampsia were presented as follows: a personal history of preeclampsia was present in 56.9% (169/297), a family history of preeclampsia was present in 34% (101/297); primiparas of late reproductive age accounted for 9.1% (27/297); there were no intergroup differences in high-risk factors (p>0.05). Targeted selection of women into the high-risk group for preeclampsia according to the “prediabetes” criterion [16] showed its presence in more than 90% of women with these high-risk factors for preeclampsia.
The primary endpoint of this study was the incidence of preeclampsia. The overall frequency of preeclampsia in the groups with different approaches to prevention was 13.0% (10/77) in group 1, 30.7% (23/75) in group 2, and 18.1% (13/77) in group 2. 72) in group 3, which was significantly lower than that in women in group 4 who categorically refused prophylaxis (49.3% [36/73], p1-4, p3-4<0.001, p2-4=0.03 (Table 1). However, it should be noted that the incidence of preeclampsia in patients in group 1 was significantly lower than that in women in group 2 (p=0.015), which is an important argument reflecting the advantage of the proposed two-stage prevention in high-risk women, including preconception preparation ISM and LDAA use during pregnancy, compared with standard LDAA prophylaxis. At the same time, we identified a clear trend towards a decrease in preeclampsia incidence in group 3 women with targeted preconception use of ISM, compared with the standard use of LDAA. Interesting data were obtained by analyzing the incidence of preeclampsia, depending on its duration and severity. It is important to note that there was a statistically significant decrease in severe preeclampsia only in groups 1 (p=0.01) and 2 (p=0.03) with pre-pregnancy use of ISM, with only a trend towards a decrease in the indicator in group 2 with standard use of LDAA, 2 times compared to group 4 (p>0.05). The incidence of moderate preeclampsia in pregnant women in group 4 was 31.5%, which was significantly higher than that in patients who received staged (p=0.01) or only pre-pregnancy (p=0.03) prophylaxis (3 and 2.3 times). Statistically significant reduction in both early severe preeclampsia (p = 0.03) and early preeclampsia in general (7.4 and 4.6 times, p=0.01) in groups 1 and 3, accordingly, in the absence of significant differences between the groups 2 and 4, this is explained by the preconception stage of ISM prevention, as well as the presence of a two-stage approach to prevention.
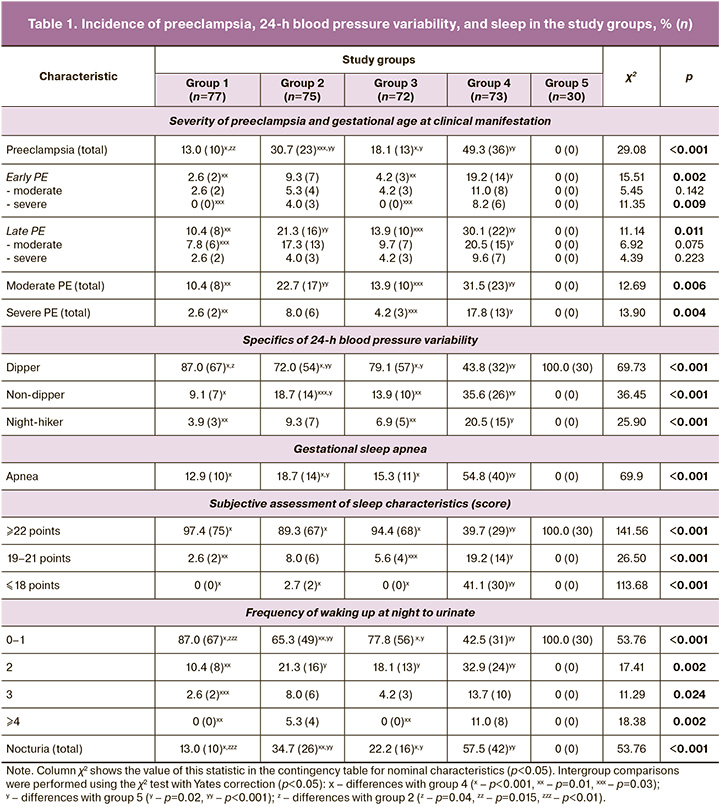
We analyzed the leading diagnostic criteria for preeclampsia, blood pressure, and proteinuria (Table 2). Patients in group 4 had higher systolic blood pressure, diastolic blood pressure, and mean blood pressure, as well as proteinuria in a single urine sample and 24-h urine protein loss (p<0.001). It should be noted that women who took ISM (groups 1 and 3) showed a tendency to have lower blood pressure and proteinuria than women in group 2 without the preconception stage of prevention.
Currently, the features of blood pressure in preeclampsia are being studied in detail, particularly the 24-h variability in blood pressure [20]. Analysis of this parameter showed that pathological types of blood pressure, night-picker (increased blood pressure at night), and non-dipper (decrease in blood pressure at night <10%) had the highest frequency in group 4. At the same time, owing to the preventive therapy in women in groups 1, 2, and 3, these types were recorded significantly less frequently (p<0.05) (Table 1). Moreover, the use of two-stage prevention of preeclampsia in high-risk women led to a significantly higher frequency of normal blood pressure variability than standard LDAA prevention (p=0.04).
In the contemporary literature, there is evidence of an important role of obstructive sleep apnea syndrome in the development of preeclampsia [21]. In this regard, we analyzed the quality of breathing and subjective characteristics of sleep (Ya.I. Levin’s questionnaire) (Table 1). Gestational sleep apnea (an analog of obstructive sleep apnea associated with pregnancy) was recorded in more than half of the pregnant women in group 4 (54.8%, 40/73), which was significantly higher than that in groups 1, 2, and 3 (4.2, 2.9 and 3.6 times, respectively; p<0.001).
Women with preeclampsia also experience insomnia, characterized by poor nighttime sleep quality, negative dreams, and increased sleepiness during the day. It is important to note that among women in group 4 without preventive treatment, 41.1% had a result of ≤18 points on the Ya.I. Levin Questionnaire confirmed the presence of insomnia. At the same time, none of the patients in the ISM prevention group had serious sleep disorders.
We analyzed the frequency of nocturia, a multifactorial symptom associated with a number of pathological conditions accompanied by IR, an independent risk factor for poor quality of night sleep that worsens the course of pregnancy, a marker of hormonal and metabolic imbalance, disorders of energy supply, metabolism of the urinary system due to endothelial and mitochondrial dysfunction of the urogenital tract, and all systems regulating it [22]. Awakening from sleep is associated with an increase in heart rate and blood pressure, which are the main determinants of oxygen consumption by the myocardium, severity of vascular stress, endocrine regulation, and activation of the coagulation system [23]. In pregnant women in group 1 with staged preventive measures, there were significant differences from group 4 without preventive treatment (p>0.001) in the manifestations of nocturia of varying severity (from 2 to 4 or more awakenings at night to urinate); however, there were no differences with the control (p>0.05) (Table 1). A similar situation was observed when comparing groups 2 and 3 with group 4, but there were no statistically significant differences with the control only for nocturia with pronounced manifestations (3 and 4 or more nocturnal awakenings). These results indicate the advantage of two-stage prevention with pre-pregnancy use of ISM in leveling early symptoms of endothelial and mitochondrial dysfunction, such as nocturia.
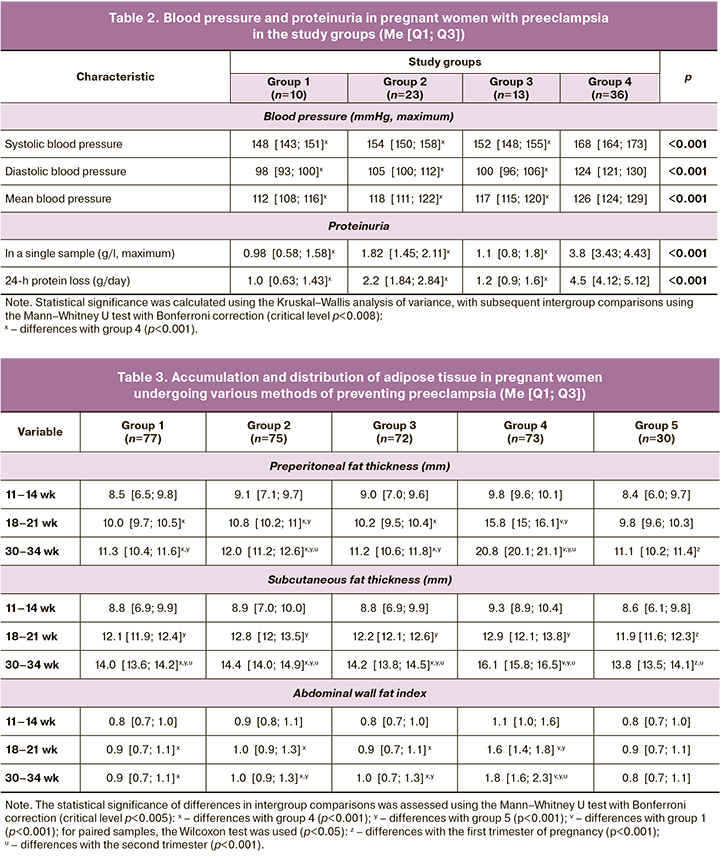
Considering the role of pathological IR and hyperinsulinemia in the development of preeclampsia [12], the involvement of visceral adipose tissue in the mechanisms of development of this pregnancy complication seems important. The dynamics of the accumulation and distribution of adipose tissue in pregnant women at a high risk of preeclampsia are presented in Table 3.
Starting from the second trimester, there were statistically significantly higher values of SCF, PPF, and AFI in patients who refused preventive measures than in patients in groups 1, 2, and 3 (p1-4, p2-4, p3-4<0.001). In addition, considering the pronounced beneficial effect of ISM on carbohydrate and lipid metabolism, the prolonged protective effect of pregestational ISM prophylaxis causes lower rates of adipose tissue accumulation in patients in groups 1 and 3, in contrast to women in group 2 with standard prevention of LDAA, reaching statistical differences by the third trimester (p<0.001). We searched for a rationale for the clinical effectiveness of a staged approach to prevent a high risk of preeclampsia in order to study the influence of various prevention options on the mechanisms of preeclampsia formation based on the results of laboratory monitoring of pregnant women (Table 4, figure).
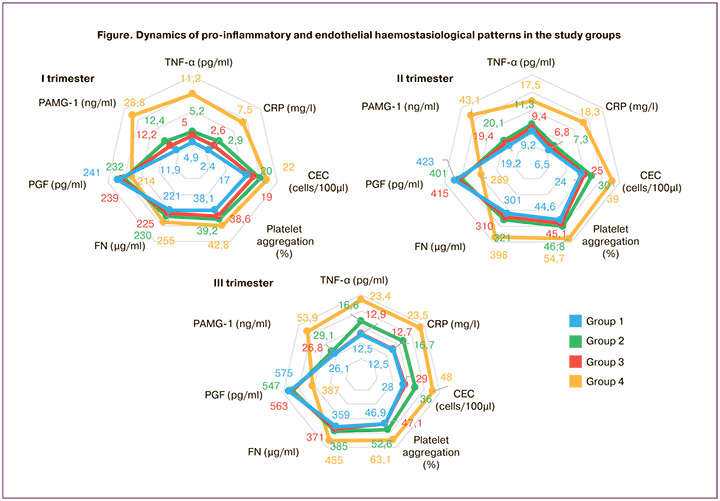
Dynamic assessment of pathogenetically significant markers showed that targeted pre-pregnancy care using ISM within the framework of the proposed method of preventing preeclampsia made it possible to maintain the level of basal glycemia at all stages of gestation <5.1 mmol/l in group 1 (4.1 [3.3; 4. 5]) and group 3 (4.3 [3.8; 4.6]), p1-5, p3-5>0.05. However, in group 2 (4.8 [4.3; 5.0]) and group 4 (5.0 [4.7; 5.5]), there was a tendency for this indicator to increase during gestation compared with the control (4.3 [3.4; 4.5]), reaching in group 4 significant differences by the third trimester (p4-5<0.001). Similar dynamics were observed when the insulin and HOMA-IR levels were analyzed. There were no significant differences between the control group and groups 1 and 3; the highest values of insulin and HOMA-IR were found in patients in group 4 without preventive measures (p<0.001 for all groups at all time points). Significant differences in women in group 2 compared to the controls started in the third trimester (p2-5<0.01) (Table 3). The identified patterns indicate a significant contribution of pregestational insulin sensitization, which consists of creating a positive periconceptional “heritage effect” necessary for adaptation to gestational metabolic changes due to more intense compensation of IR and hyperinsulinemia.
It is widely known that the most powerful inducer of IR during pregnancy is PL, which has lipolytic properties aimed at switching maternal metabolism towards increasing lipid consumption and enhancing IR [11]. It is important to note that, due to preventive therapy for preeclampsia, in patients of groups 1, 2, and 3, the PL indicators at all stages of the study did not have significant differences with the results of the examination of women with physiological gestation (p1-5, p2-5, p3-5>0.05), while in women without preventive therapy, the highest values of the indicator were significantly higher than the values in all groups (p<0.001). Leptin also participates in the formation of pathological IR and, consequently, arterial hypertension during preeclampsia [12]. According to the data obtained, leptin levels in groups 1, 2, and 3 were significantly lower than those in group 4 (p1-4, p2-4, p3-4<0.001 at all time points); however, only in group 1 this indicator did not significantly exceed the level of the control group in the third trimester (p1-5>0.05), which indicates a decrease in leptin resistance and lipotoxicity in patients at high risk of preeclampsia against the background of two-stage prophylaxis. The dynamics of cortisol, which also plays an important role in the development of pathological IR and hyperinsulinemia in the preclinical stage of preeclampsia, had similar patterns.
Lipid profile disorders (increased TC, LDL, and TG and decreased HDL) are one of the main markers of the dysmetabolic link in the pathogenesis of preeclampsia [11]. The results of the examination of group 4 confirmed the formation of atherogenic changes in the lipid profile of women with preeclampsia. At the same time, pregnant women in groups 1, 2, and 3 showed a statistically significant decrease in the levels of TG and TG/HDL, and an increase in HDL, compared with women without preventive measures (Table 3). The greatest effect of preventive therapy was achieved when using a two-stage prevention method, which was confirmed by significantly higher physiological levels of lipid profile indicators when compared with group 2 (p<0.001 by the end of gestation). It has been proven that disorders of purine metabolism are associated with the development of pathological IR, including preeclampsia [12]. According to the results, the hyperuricemic state was significantly more frequent in group 4 patients, while in patients in groups 1 and 3, the uricemia values were similar to those of the controls (p>0.05), which was due to prevention using ISM. During the use of standard LDAA prophylaxis, there was a statistically significant increase in uric acid levels compared with groups 1 and 5 (p1-2, p2-5<0.001).
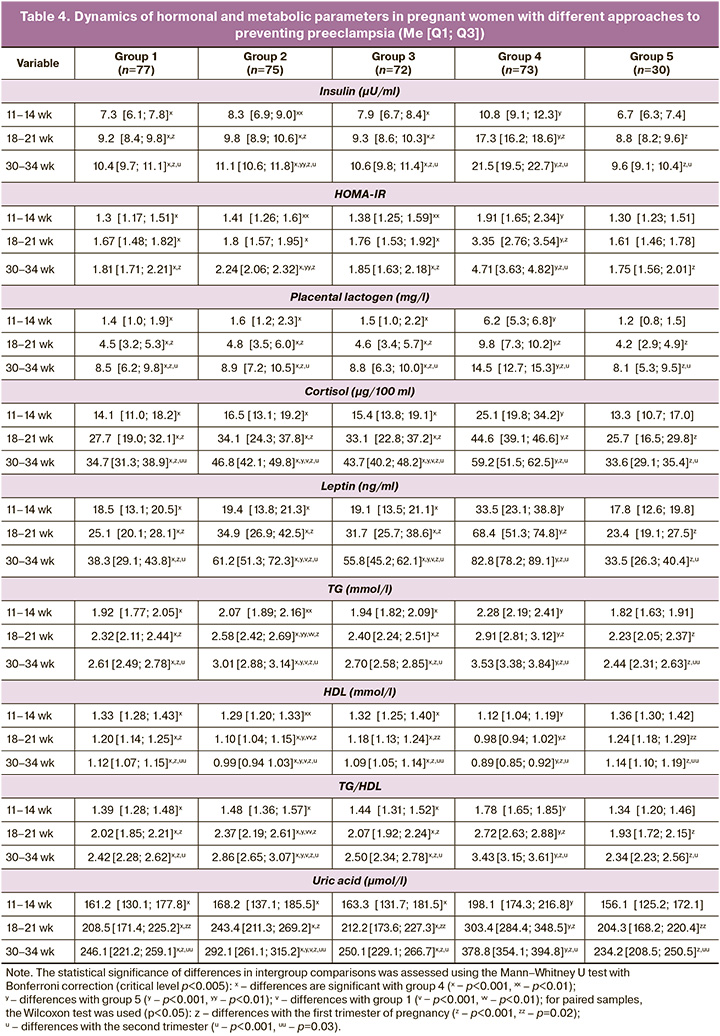
Many studies have confirmed the formation of proinflammatory changes during preeclampsia. In recent years, TNF-α has been shown to be an important mediator of IR and hyperinsulinemia [12]. The use of ISM at the pregestational stage has a pronounced protective effect, inhibiting excessive growth of TNF-α. At the same time, by the 3rd trimester there was a significant increase in TNF-α in group 2 compared to group 1 and controls (p1-2<0.01, p2-5<0.001) (Figure).
Endothelial dysfunction is considered a pathogenic precursor to the formation of arterial hypertension in preeclampsia and is strongly associated with pathological IR and hyperinsulinemia [24]. Important markers of endothelial dysfunction include CECs, FN, and platelet aggregation activity. Analysis of the number of CECs in pregnant women in groups 1, 2, and 3 did not show statistically significant differences from the control group in the first trimester (15 [12; 18] cells/100 μl), p1-5, p2-5, p3- 5>0.05, and in pregnant women in group 1, the absence of differences persisted throughout pregnancy.
The CEC levels detected in group 4 had the highest values, and were pathogenetically associated with a high frequency of preeclampsia (Figure). Similar trends were observed when analyzing the levels of FN released into the plasma when endothelial cells were damaged, which led to a significant pro-coagulation shift [13]. The aggregation activity of platelets with collagen increased during pregnancy [25]. Against the background of the use of different options for the prevention of preeclampsia in patients in groups 1, 2, and 3, the level of platelet aggregation remained significantly lower than in pregnant women who refused prophylaxis (p1-4, p2-4, p3-4<0.001 – at 18–21 and 30–34 weeks), and had no differences from the control (37.4 [34.5; 41.1] %, 42.1 [40.8; 45.3] %, 46.2 [42.1;48.1] %, respectively, I, II, and III trimesters), p1-5, p2-5, p3-5>0.05.
The formation of a proangiogenic state associated with the stable development of the embryo(feto)placental complex and the protection of the vascular endothelium from excessive activation and alteration during pregnancy has been well studied [26, 27]. This was confirmed by PGF dynamics (figure). When using preventive therapy in women in groups 1, 2, and 3, PGF levels remained higher than those in group 4, and in women with two-stage prevention receiving pregestational ISM and LDAA during pregnancy, the most similar indicators to the controls were observed (248 [224; 272], 431 [393;478], 591 [531;638] pg/ml, respectively; I, II, III trimesters), p1-5, p2-5, and p3-5>0.05. An important peptide that modulates pathological IR and plays a role in preeclampsia is PAMG-1 [12]. In all women with preeclampsia prevention, the level of PAMG-1 was significantly lower than that in patients in group 4 (p<0.001), which corresponded to a less pronounced increase in IR, hyperinsulinemia, and a lower frequency of preeclampsia.
Consequently, the basis for reducing cardiovascular risk in women with a high probability of developing preeclampsia is the effects of ISM, proven in numerous studies, realized through the activation of adenosine monophosphate-activated protein kinase, a key enzyme in energy homeostasis and cellular metabolism, a basic protective factor of the cardiovascular system [28], and subsequent inhibition of the nutrient-sensory signaling pathway of the mammalian target of rapamycin (mTOR), which disinhibits senescent cells with an exhausted division resource, limits their hyperfunction, including proinflammatory and hypersecretion, and reduces resistance to mitogen signals including insulin [29]. The optimization of intracellular signaling pathways achieved by prevention using ISM at the periconceptional stage, potentiated by the influence of the gestational preventive agent LDAA, contributes to the fact that gestational IR and hyperinsulinemia increase due to the action of placental contractile factors, retain their physiological nature, and do not initiate the mechanisms of gestational hypertension and multiple organ failure through the formation of inflammatory-oxidative-thrombogenic status. Objectification of the size of the effect of preventive intervention was carried out by calculating the indicators RR, RRR, ARR, and NNT with [95% CI]. In groups with different preventive approaches, the indicators were distributed accordingly as follows: group 1, 0.26 [0.14; 0.49], 73.7% [50.9; 85.9], 36.3% [22.6; 50.1], 3 [2; 4] (χ2=21.58, p<0.001); Group 3: 0.37 [0.21; 0.63], 63.4% [36.9; 78.8], 31.3% [16.8; 45.8], 4 [2; 6] (χ2=14.47, p<0.001); Group 2 – 0.62 [0.41; 0.94], 37.8% [6.0; 58.8], 18.7% [3.1; 34.2], 6 [3; 32] (χ2=4.62, p=0.03), which indicates a higher effectiveness of the two-stage method of preventing preeclampsia in high-risk women using ISM at the preconception and LDAA at the gestational stages.
Conclusion
Currently, the importance of pre-pregnancy care for high-risk pregnant women, aimed at promoting healthy gestational restructuring and reducing the early onset and severity of pregnancy complications, is widely acknowledged.
This study evaluated the effectiveness of a two-stage prevention approach for preeclampsia, involving the use of ISM before pregnancy and LDAA during pregnancy. Clinical and laboratory evaluations in high-risk pregnant women with preeclampsia have revealed the benefits of this staged approach, which exploits a positive "metabolic memory effect" through epigenetic mechanisms. This approach enhances compensatory mechanisms against insulin resistance (IR) and hyperinsulinemia, minimizes markers of vascular endothelial damage and remodeling, and is supported by the significant effect sizes of preventive actions. Restricting preventive measures to pre-pregnancy ISM use alone does not ensure adequate gestational adaptation throughout pregnancy, thereby limiting its clinical effectiveness. Likewise, the effectiveness of LDAA as a standalone prevention method is diminished owing to its lack of influence during the critical early stages of gestation that shape the subsequent course of pregnancy. The advantage of the two-stage prevention approach lies in the use of monotherapy at each stage, employing safe drugs with well-established pharmacological properties and proven biological effects.
In summary, a new strategy for the prevention of preeclampsia involves a staged approach that focuses on reducing cardiovascular risk by promoting metabolic resilience in women at high risk of developing preeclampsia from the preconception stage. This approach represents a promising and highly effective means of prevention, based on the underlying pathogenesis, aimed at improving obstetric outcomes and maintaining cardiovascular well-being in the subsequent stages of life.
References
- Ходжаева З.С., Ошхунова М.С., Муминова К.Т., Горина К.А., Холин А.М. Прогнозирование и ранняя диагностика преэклампсии: научные перспективы и клинические возможности. Акушерство и гинекология. 2022; 12: 57-65. [Khodzhaeva Z.S., Oshkhunova M.S., Muminova K.T., Gorina K.A., Kholin A.M. Prediction and early diagnosis of preeclampsia: scientific perspectives and clinical opportunities. Obstetrics and Gynecology. 2022; (12): 57-65. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.218.
- Серов В.Н., Нестерова Л.А. Особенности современного акушерства. Акушерство и гинекология. 2022; 3: 5-11. [Serov V.N., Nesterova L.A. Features of modern obstetrics. Obstetrics and Gynecology. 2022; (3): 5-11.(in Russian)]. https://dx.doi.org/10.18565/aig.2022.3.5-11.
- Roberge S., Bujold E., Nicolaides K.H. Aspirin for the prevention of preterm and term preeclampsia: systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2018; 218(3): 287-93. https://dx.doi.org/10.1016/j.ajog.2017.11.561.
- Rolnik D.L., Wright D., Poon L.C, O'Gorman N., Syngelaki A., de Paco Matallana C. et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N. Engl. J. Med. 2017; 377(7): 613-22.https://dx.doi.org/10.1056/NEJMoa1704559.
- Khan H., Kanny O., Syed M.H., Qadura M. Aspirin resistance in vascular disease: a review highlighting the critical need for improved point-of-care testing and personalized therapy. Int. J. Mol. Sci. 2022; 23(19): 11317.https://dx.doi.org/10.3390/ijms231911317.
- Hofmeyr G.J., Lawrie T.A., Atallah Á.N., Torloni M.R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev. 2018; 10(10): CD001059.https://dx.doi.org/10.1002/14651858.cd001059.pub3.
- Липатов И.С., Тезиков Ю.В., Тютюнник В.Л., Кан Н.Е., Кузьмина А.И.,Зуморина Э.М., Якушева А.О. Роль инсулинорезистентности в механизмах адаптации и формировании патологии послеродового и раннего неонатального периодов. Акушерство и гинекология. 2022; 2: 28-36. [Lipatov I.S., Tezikov Yu.V., Tyutyunnik V.L., Kan N.E.,Kuzmina A.I., Zumorina E.M., Yakusheva A.O. Role of insulin resistance in the mechanisms of adaptation and development of disease in postpartum and early neonatal periods. Obstetrics and Gynecology. 2022; (2): 28-36. (in Russian)].https://dx.doi.org/10.18565/aig.2022.2.28-36.
- Wu P., Haththotuwa R., Kwok C.S., Babu A., Kotronias R.A., Rushton C. et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes. 2017; 10(2): e003497.https://dx.doi.org/10.1161/CIRCOUTCOMES.116.003497.
- Testo A.A., McBride C., Bernstein I.M., Dumas J.A. Preeclampsia and its relationship to pathological brain aging. Front. Physiol. 2022; 13: 979547. https://dx.doi.org/10.3389/fphys.2022.979547.
- Godoy-Matos A.F., Silva Júnior W.S., Valerio C.M. NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020; 12: 60. https://dx.doi.org/10.1186/s13098-020-00570-y.
- Липатов И.С., Тезиков Ю.В., Азаматов А.Р., Шмаков Р.Г. Общность клинических проявлений преэклампсии и метаболического синдрома: поиск обоснования. Акушерство и гинекология. 2021; 3: 81-9. [Lipatov I.S., Tezikov Yu.V., Azamatov A.R., Shmakov R.G. Identity of preeclampsia and metabolic syndrome clinical manifestations: searching for substantiation. Obstetrics and Gynecology. 2021; (3): 81-9. (in Russian)].https://dx.doi.org/10.18565/aig.2021.3.81-89.
- Тезиков Ю.В., Липатов И.С., Азаматов А.Р., Тютюнник В.Л., Кан Н.Е., Зуморина Э.М., Кузьмина А.И. Формирование преэклампсии с позиции отдельного гестационного клинико-патогенетического варианта синдрома инсулинорезистентности. Акушерство и гинекология. 2022; 4: 64-74. [Tezikov Yu.V., Lipatov I.S., Azamatov A.R., Tyutyunnik V.L., Kan N.E., Zumorina E.M., Kuzmina A.I. Preeclampsia as a separate gestational clinical and pathogenetic form of insulin resistance syndrome. Obstetrics and Gynecology. 2022; (4): 64-74. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.4.64-74.
- Herman R., Kravos N.A., Jensterle M., Janež A., Dolžan V. Metformin and insulin resistance: a review of the underlying mechanisms behind changes in GLUT4-mediated glucose transport. Int. J. Mol. Sci. 2022; 23(3): 1264.https://dx.doi.org/10.3390/ijms23031264.
- Boddepalli C.S., Gutlapalli S.D., Lavu V.K., Abdelwahab Mohamed Abdelwahab R., Huang R., Potla S. et al. The effectiveness and safety of metformin compared to sulfonylureas in diabetic nephropathy: a systematic review. Cureus. 2022; 14(12): e32286. https://dx.doi.org/10.7759/cureus.32286.
- Milovanovic S., Silenzi A., Kheiraoui F., Ventriglia G., Boccia S., Poscia A. Detecting persons at risk for diabetes mellitus type 2 using FINDRISC: results from a community pharmacy-based study. Eur. J. Public Health. 2018; 28(6): 1127-32. https://dx.doi.org/10.1093/eurpub/cky009.
- Министерство здравоохранения Российской Федерации. Сахарный диабет 2 типа у взрослых. Клинические рекомендации. М.; 2022. [Ministry of Health of Russian Federation. National clinical guidelines «Type 2 diabetes in adults». Moscow; 2022. (in Russian)]. Available at: https://cr.minzdrav.gov.ru/schema/290_2
- Министерство здравоохранения Российской Федерации. Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде. Клинические рекомендации. М.; 2021. [Ministry of Health of Russian Federation. National clinical guidelines «Pre-eclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders during pregnancy, childbirth and postpartum period». Moscow; 2021. (in Russian)]. Available at: https://roag-portal.ru/recommendations_obstetrics
- Ланг Т., Альтман Д. Основы описания статистического анализа в статьях, публикуемых в биомедицинских журналах. Руководство «Статистический анализ и методы в публикуемой литературе (САМПЛ)». Медицинские технологии. Оценка и выбор. 2014; 1: 11-6. [Lang T., Altman D. Basic description of statistical analysis in articles published in biomedical journals. The leadership of the «Statistical analyses and methods in the published literature (SAMPL)». Medical Technologies. Evaluation and Selection. 2014; (1): 11-6. (in Russian)].
- de Moel-Mandel C. Understanding and communicating epidemiological measures of risk and benefit. Fam. Pract. 2023; 40(2): 423-5.https://dx.doi.org/10.1093/fampra/cmac117.
- Altikardes Z.A., Kayikli A., Korkmaz H., Erdal H., Baba A.F., Fak A.S. A novel method for dipper/non-dipper pattern classification in hypertensive and non-diabetic patients. Technol. Health Care. 2019; 27(Suppl. 1): 47-57.https://dx.doi.org/10.3233/THC-199006.
- Калачин К.А., Пырегов А.В., Шмаков Р.Г. Гестационное сонное апноэ. Связь беременности и преэклампсии с синдромом обструктивного апноэ сна. Альманах клинической медицины. 2019; 47(3): 266-75. [Kalachin K.A., Pyregov A.V., Shmakov R.G. Gestational sleep apnea. The relationship of pregnancy and preeclampsia with obstructive sleep apnea syndrome. > Almanac of Clinical Medicine. 2019; 47(3): 266-75. (in Russian)].https://dx.doi.org/10.18786/2072-0505-2019-47-031.
- Gordon D.J., Emeruwa C.J., Weiss J.P. Management strategies for nocturia. Curr. Urol. Rep. 2019; 20(11): 75. https://dx.doi.org/10.1007/s11934-019-0940-2.
- Lombardo R., Tubaro A., Burkhard F. Nocturia: the complex role of the heart, kidneys, and bladder. Eur. Urol. Focus. 2020; 6(3): 534-6.https://dx.doi.org/10.1016/j.euf.2019.07.007.
- Сухих Г.Т., Силачев Д.Н., Горюнов К.В., Волочаева М.В., Шмаков Р.Г. Роль дисфункции стволовых клеток в развитии больших акушерских синдромов. Акушерство и гинекология. 2018; 7: 5-11. [Sukhikh G.T., Silachev D.N., Goryunov K.V., Volochaeva M.V., Shmakov R.G. Role of stem cell dysfunctionin the development of great obstetrical syndromes. Obstetrics and Gynecology. 2018; (7): 5-11. (in Russian.)]. https://dx.doi.org/10.18565/aig.2018.7.5-11.
- Макацария А.Д., Бицадзе В.О., Акиньшина С.В. Тяжелые формы преэклампсии как проявление тромботической микроангиопатии. Акушерство и гинекология. 2017; 4: 21-6. [Makatsaria A.D., Bitsadze V.O., Akinshina S.V. Severe forms of preeclampsia as a manifestation of thrombotic microangiopathy. Obstetrics and Gynecology. 2017; (4): 21-6. (in Russian)].https://dx.doi.org/10.18565/aig.2017.4.21-6.
- Игнатко И.В., Флорова В.С., Кузнецов А.С., Кузина Е.Ю. Роль биохимических маркеров в стратификации риска развития преэклампсии: взгляд клинициста. Архив акушерства и гинекологии им. В.Ф. Снегирева. 2017; 4(4): 181-6. [Ignatko I.V., Florova V.S., Kuznetsov A.S.,Kuzina E.Yu. The role of biochemical markers in stratifying the risk of developing preeclampsia: a clinician's perspective. V.F. Snegirev Archives of Obstetrics and Gynecology. 2017; 4(4): 181-6. (in Russian)].https://dx.doi.org/10.18821/2313-8726-2017-4-4-181-186.
- Липатов И.С., Тезиков Ю.В., Мартынова Н.В., Мингалиева Л.К., Гогель Л.Ю., Белоконева Т.С., Калинкина О.Б., Жернакова Е.В., Юсупова Р.Р. Универсальный подход к профилактике синдрома патологической беременности. Наука и инновации в медицине. 2017; 1(5): 13-23. [Lipatov I.S., Tezikov Yu.V., Marty'nova N.V., Mingalieva L.K., Gogel' L.Yu., Belokoneva T.S. et al. Universal approach to the prevention of the syndrome of pathological pregnancy. Science and Innovation in Medicine. 2017; 1(5): 13-23. (in Russian)].
- Hasanvand A. The role of AMPK-dependent pathways in cellular and molecular mechanisms of metformin: a new perspective for treatment and prevention of diseases. Inflammopharmacology. 2022; 30(3): 775-88.https://dx.doi.org/10.1007/s10787-022-00980-6.
- Triggle C.R., Mohammed I., Bshesh K., Marei I., Ye K., Ding H. et al. Metformin: Is it a drug for all reasons and diseases? Metabolism. 2022; 133: 155223.https://dx.doi.org/10.1016/j.metabol.2022.155223.
Received 31.05.2023
Accepted 29.08.2023
About the Authors
Yurii V. Tezikov, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, +7(846)958-24-18, yra.75@inbox.ru, https://orcid.org/0000-0002-8946-501X, Researcher ID: С-6187-2018, SPIN-код: 2896-6986, Author ID: 161372, Scopus Author ID: 6603787595, 443099, Russia, Samara, Chapaevskaya str., 89.Igor S. Lipatov, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, +7(846)958-24-18, i.lipatoff2012@yandex.ru, https://orcid.org/0000-0001-7277-7431, Researcher ID: С-5060-2018, SPIN-код: 9625-2947,
Author ID: 161371, Scopus Author ID: 6603787595, 443099, Russia, Samara, Chapaevskaya str., 89
Ellina M. Zumorina, Obstetrician-Gynecologist at the Perinatal Center, Seredavin Samara Regional Clinical Hospital, +7(846)958-24-18, ellina.zumorina@yandex.ru,
https://orcid.org/0000-0002-0140-5566, SPIN-код: 9924-2273, Author ID: 1105503, 443095, Russia, Samara, Tashkentskaya str., 159.
Amir R. Azamatov, PhD, Obstetrician-Gynecologist at the Perinatal Center, Seredavin Samara Regional Clinical Hospital, +7(846)958-24-18, azamatov.amir@yandex.ru,
https://orcid.org/0000-0003-0372-6889, SPIN-код: 9261-9264, Author ID: 1013819, 443095, Russia, Samara, Tashkentskaya str., 159.
Victor L. Tyutyunnik, Dr. Med. Sci., Professor, Leading Researcher at the Department of Research Administration, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(903)969-50-41, tioutiounnik@mail.ru, https://orcid.org/0000-0002-5830-5099,
Researcher ID: B-2364-2015, SPIN-код: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, 117997, Russia, Moscow, Ac. Oparina str., 4.
Natalia E. Kan, Dr. Med. Sci., Professor, Deputy Director for Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(926)220-86-55, kan-med@mail.ru, https://orcid.org/0000-0001-5087-5946, Researcher ID: B-2370-2015, SPIN-код: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, 117997, Russia, Moscow, Ac. Oparina str., 4.
Anastasia L. Chekalovets, 6th year student at the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, +7(846)958-24-18, chekalovets1999@icloud.com, https://orcid.org/0009-0008-9777-8076, Researcher ID: IQU-6493-2023, SPIN-код: 7128-2648,
Authors ID: 1200421, 443099, Russia, Samara, Chapaevskaya str., 89.
Anastasia I. Borisova, 5th year student at the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, +7(846)958-24-18,
nsts.nvk@yandex.ru, https://orcid.org/0000-0003-4604-9099, SPIN-код: 6245-2050, Authors ID: 1146911, 443099, Russia, Samara, Chapaevskaya str., 89.
Anastasia M. Golodnova, 5th year student at the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, +7(846)958-24-18,
anastacia_gol1999@mail.ru, https://orcid.org/0009-0000-4312-1281, SPIN-код: 1338-6990, Authors ID: 1200403, 443099, Russia, Samara, Chapaevskaya str., 89.



