Systemic and placental hemodynamics in preeclampsia
Dolgushina V.F., Syundyukova E.G., Chulkov V.S., Ryabikina M.G., Kirsanov M.S., Chulkov Vl.S.
Objective: To examine the characteristics of systemic and placental hemodynamics in women with preeclampsia.
Materials and methods: This case-control study was based on a prospective cohort study that included 95 pregnant women. The women were divided into three groups: group 1 (n=29) consisted of women without preeclampsia, group 2 (n=32) consisted of pregnant women with moderate preeclampsia, and group 3 (n=34) consisted of pregnant women with severe preeclampsia. This study examined the results of assessing uteroplacental-fetal hemodynamics and fetal biometric parameters, brachial artery endothelium-dependent vasodilation during pregnancy, arterial stiffness indices (CAVI), and vascular age at 6–8 weeks postpartum.
Results: Women with preeclampsia had primary hemodynamic disorders associated with chronic arterial hypertension and a history of preeclampsia. Severe preeclampsia in the second trimester was linked to changes in uterine and fetal blood flow, as well as fetal growth restriction. After brachial artery compression, the percentage increase in brachial artery diameter in patients with severe preeclampsia was pathologically low (4.8%). In severe preeclampsia, the brachial artery diameter after decompression was negatively correlated with uterine and umbilical cord blood flow and severe asphyxia of the newborn, and positively correlated with gestational age at delivery and the newborn Apgar score. At 6–8 weeks postpartum, women with preeclampsia still had significantly higher blood pressure, CAVI, and vascular age than normotensive women. There were negative correlations between CAVI and Apgar score, while positive correlations were found with biological age, body mass index, baseline (first trimester of pregnancy), and current (6–8 weeks postpartum) blood pressure levels.
Conclusion: Characteristics of endothelial dysfunction have been proposed as potential early diagnostic markers for preeclampsia and its consequences. Identification of these markers may allow timely initiation of preventive and therapeutic measures.
Authors' contributions: Dolgushina V.F., Syundyukova E.G. – conception and design of the study; Syundyukova E.G., Chulkov V.S., Ryabikina M.G., Chulkov Vl.S. – data collection and analysis; Syundyukova E.G., Chulkov V.S., Ryabikina M.G., Kirsanov M.S., Chulkov Vl.S. – drafting of the manuscript; Dolgushina V.F., Kirsanov M.S. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the South Ural SMU, Ministry of Health of Russia (Ref. No: № 1 of 17.01.2020).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Dolgushina V.F., Syundyukova E.G., Chulkov V.S., Ryabikina M.G., Kirsanov M.S., Chulkov Vl.S. Systemic and placental hemodynamics in preeclampsia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (9): 63-72 (in Russian)
https://dx.doi.org/10.18565/aig.2024.109
Keywords
Preeclampsia remains a significant medical and social problem associated with high maternal and perinatal morbidity and mortality. According to global statistics, the incidence of hypertensive disorders during pregnancy is 2–8%. Effective methods of preeclampsia treatment have not yet been developed; the only method of medical care remains effective prevention and timely delivery [1, 2]. Women with a history of gestational hypertension have a significantly increased risk of cardiovascular, metabolic and mental disorders, kidney pathology, and their children have an increased risk of developing arterial hypertension, endocrine disorders and neurological deficits [3–5]. The main recognized mechanisms of preeclampsia development are insufficient remodeling of spiral arteries, endothelial dysfunction, hemostasis disorders, immune response, somatic diseases of the mother, and the influence of unfavorable environmental factors [2, 6]. The importance of the placenta and the substances it produces in the development of endothelial dysfunction in gestational hypertensive disorders is undeniable; however, it cannot be ruled out that it is the primary cardiovascular disorder in pregnant women that may lead to preeclampsia [7, 8]. Therefore, studying the characteristics of maternal and placental hemodynamics in arterial hypertension during pregnancy remains a priority and determines the significance of this study.
This study aimed to examine the characteristics of systemic and placental hemodynamics in women with preeclampsia.
Materials and methods
This case-control study was based on a prospective cohort study including 95 pregnant women who gave birth at the Clinic of the South Ural SMU, Ministry of Health of Russia (Chelyabinsk) in 2021. The inclusion criteria were observation at the antenatal clinic, availability of medical documentation, and signed consent to participate in the study. The exclusion criteria were pregnancy term of less than 22 weeks, oncological diseases in the last 5 years, tuberculosis, severe somatic pathology in the decompensation stage, mental disorders and mental illnesses, and drug addiction. Group 1 (control) included 29 women whose pregnancies were not complicated by preeclampsia (they did not receive preeclampsia prophylaxis), group 2 consisted of 32 pregnant women with moderate preeclampsia, and group 3 included 34 patients with severe preeclampsia. The outcomes of pregnancy and childbirth were studied using the results of questionnaires and the analysis of medical documentation (delivery case records). The information contained in the materials under consideration does not fall under the List of Information constituting a State Secret (Article 5 of the Law of the Russian Federation "On State Secrets"), does not belong to the list of information classified as a state secret, is approved by the Decree of the President of the Russian Federation of November 30, 1995, No. 1203, and is not subject to classification. This study was reviewed and approved by the Research Ethics Committee of the South Ural SMU, Ministry of Health of Russia (Ref. No: 1 of 17.01.2020). The diagnosis and classification of nosologies of obstetric pathology were established in accordance with the current clinical guidelines (https://cr.minzdrav.gov.ru/clin_recomend). Based on ultrasound fetal biometric parameters and Doppler ultrasound, a diagnosis of fetal growth restriction (FGR) was established, and blood flow velocity curves in the umbilical artery, uterine arteries, and pulsatility index (PI) were assessed. Diagnostic ultrasound systems M7 (Mindray, China), Aplio 500 (Toshiba, Japan), and Voluson E8 (GE, Austria) and obstetric monitor Sonicaid Team Care (Great Britain) with analysis by the short-term variation (STV) indicator were used. The study of the vasodilatory reserve of the brachial artery (flow-mediated dilation, FMD) or endothelium-dependent vasodilation (EDV) was performed according to the modified method of Celermajer et al. (1992) by ultrasound scanning of the brachial artery with a 7.5–12 MHz convex sensor. The brachial artery was located in the longitudinal section above the elbow bend by 2–5 cm, and a sphygmomanometer cuff was placed over the visualization site to generate a pressure of 50 mmHg above the systolic blood pressure (SBP). The diameter of the brachial artery was measured before and after a 5-minute occlusion of the vessel (D1 and D2, respectively). EDV was calculated as the percentage increase in the diameter of the brachial artery after decompression relative to the initial value using the formula: (D2-D1)/(D1/100); a normal response was considered to be a dilation of the brachial artery by 10% or more of the initial diameter [9].
Vascular wall stiffness was measured using volumetric sphygmography (VaSera VS-1500N; Fukuda Denshi, Japan). The women who took part in the study were given sphygmomanometer cuffs on both sides of the patient’s shoulders and ankles 6–8 weeks postpartum, electrocardiograph electrodes were placed on both sides of the wrists, and a phonocardiogram sensor was placed on the chest (second intercostal space from the sternum on the left). The vascular stiffness index (CAVI) was automatically calculated based on the cardio-ankle pulse wave velocity from the aortic valve to the right and left leg arteries: R-CAVI (on the right) and L-CAVI (on the left). The estimated vascular age of the arteries (in years) was determined simultaneously. Automatically obtained SBP and diastolic blood pressure (DBP) values for four limbs with subsequent calculation of the ankle-brachial index (ABI), which is the result of dividing the SBP at the ankle (measured slightly above the ankle) of the patient by the pressure recorded in the shoulder area: R-ABI (right) and L-ABI (left).
Statistical analysis
Statistical analysis was performed using SPSS Statistics for Windows version 17.0. Categorical variables are presented as frequencies (%) and continuous variables as medians and interquartile range (Me (Q1–Q3]) based on the nature of the data distribution. The Kruskal–Wallis test was used to compare continuous data between the three groups, followed by pairwise comparison of groups using the Mann–Whitney U test with Bonferroni adjustment. Categorical variables were compared using Pearson’s χ2 test and Fisher’s exact test (when the expected frequency of one or more cells was less than 5) with Bonferroni adjustment. Paired quantitative data were compared using the Wilcoxon’s test. Correlation analysis was conducted by calculating the Spearman's rank correlation coefficients. Differences were considered statistically significant at p<0.05.
Results
The age and marital status of women in the study groups were not statistically different (Table 1). A history of severe preeclampsia was associated with a reduced likelihood of full-term delivery and an increased prevalence of complications, including preeclampsia, preterm delivery, cesarean section, and low birth weight. The pattern of gynecological conditions was the same in all three groups. Overweight/obesity and chronic arterial hypertension were significantly more frequent in patients with preeclampsia. The frequency of recorded episodes of hypertension before pregnancy was also higher in women in groups 2 and 3 (group 1, 2/29 (6.9%); group 2, 13/32 (40.6%); and group 3, 15/34 (44.1%); p=0.003).
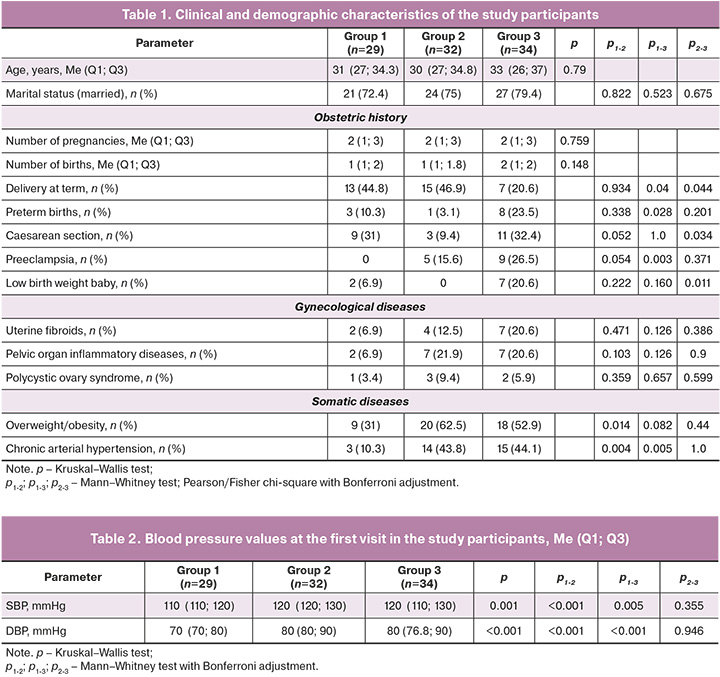
The SBP and DBP observed at the initial antenatal clinic visit (Table 2) were significantly elevated in pregnant women with preeclampsia compared with those in the control group.
Analysis of uteroplacental hemodynamics (Table 3) revealed that patients in group 3 demonstrated the earliest onset of blood flow disorders in the uterine arteries, occurring as early as the second trimester. These disorders were significantly more prevalent than in the other groups. Only women with severe preeclampsia in the second trimester had IGR and umbilical cord blood flow disorders. Three cases of severe preeclampsia were associated with placental disorders (IGR and reversed blood flow in the umbilical artery and venous duct) and required emergency cesarean section at an extremely early stage.
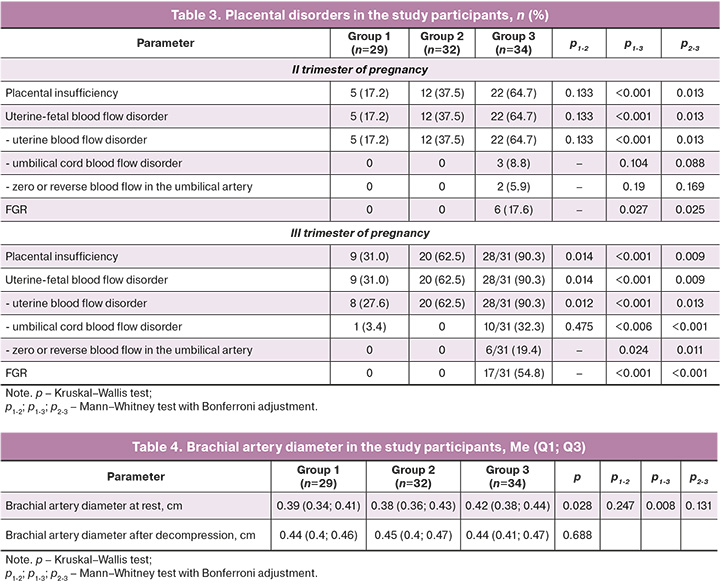
In the third trimester (Table 3), uterine blood flow disorders were most prevalent among women with preeclampsia, with a higher incidence of severe cases. Umbilical cord blood flow disorders were primarily diagnosed in patients with severe preeclampsia. The critical indicators of zero and reverse blood flow were exclusively identified in group 3. At 30 (28; 35) weeks IGR was identified in 20/34 patients (58.8%) with severe preeclampsia.
A Doppler evaluation of blood circulation prior to delivery demonstrated that the median PI index increased with increasing severity of preeclampsia in both the uterine arteries (group 1 – 0.66 (0.59; 0.73); group 2 – 0.75 (0.66; 1.11); group 3 – 1.22 (0.99; 1.49); p<0.001) and umbilical arteries (group 1 – 0.76 (0.67; 0.84); group 2 – 0.93 (0.69; 0.96); group 3 – 1.15 (0.84; 1.86); p<0.001).
Analysis of the EDV study results showed that the brachial artery PI values at rest did not differ between all three groups (group 1 – 37.6 (29.3; 46.4); group 2 – 42.14 (34.9; 51); group 3 – 37.25 (32.4; 45.7); p=0.21). After the brachial artery reactive hyperemia test, the brachial artery PI (36.7 (29.9; 51.1)) did not change in the control group (p1=0.782). In preeclampsia, a statistically significant (p2=0.008; p3=0.018) increase in PI in the brachial artery was revealed (group 2 – 46.47 (38.8; 54.5); group 3 – 42.45 (36.8; 48.8), while the PI values in preeclampsia were higher than those in the control group (p=0.035). The diameter of the brachial artery at rest (Table 4) in the women with severe preeclampsia was significantly larger than that in the control group. After the occlusion test, this indicator significantly increased dynamically in each groups (p1<0.001, p2<0.001, and p3=0.01), with no statistical differences between the groups.
The percentage increase in brachial artery diameter after the ischemic test in comparison to the baseline value at rest in the control group and with moderate preeclampsia was 12.8% and 18.4%, respectively. These values correspond to normative limits. In severe preeclampsia after cuff occlusion, the percentage increase in brachial artery diameter was pathologically low (4.8 %) compared to groups 1 and 2 (p1-3=0.01; p2-3=0.008).
The brachial artery diameter at rest in women with severe preeclampsia (Table 5) negatively correlated with the average PI in the uterine arteries. The brachial artery diameter after decompression in women with severe preeclampsia was negatively correlated with the average PI in the uterine arteries, PI in the umbilical artery, severe asphyxia of the newborn, positive linear correlations with gestational age at delivery, and newborn Apgar scores at the 1st and 5th minutes.
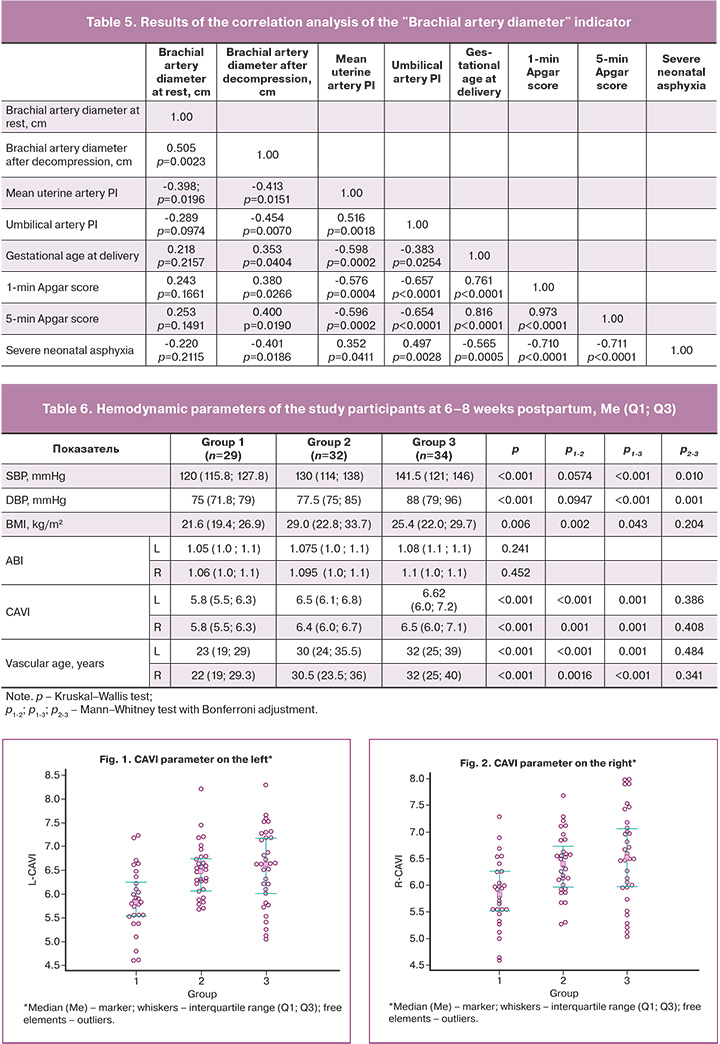
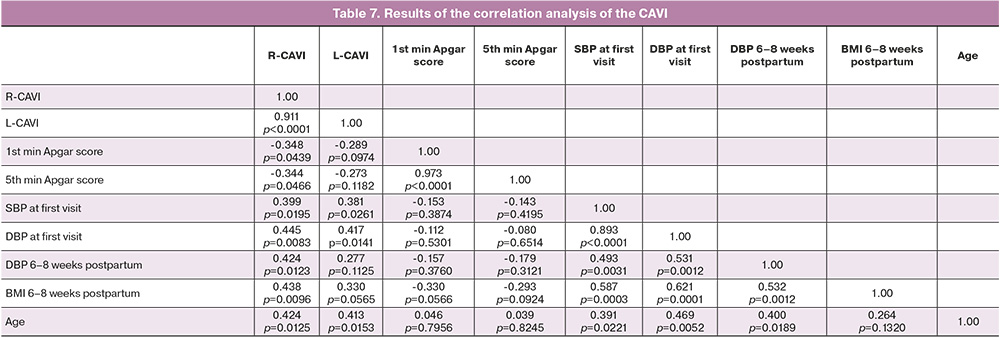
To examine the cardiovascular status of women who had experienced preeclampsia, a comprehensive assessment was conducted six–eight weeks postpartum. This assessment included evaluation of blood pressure, vascular stiffness indicators (CAVI), ankle-brachial index (ABI), and vascular age (Table 6, Figs. 1, 2).
The SBP and DBP (Table 6) in women with severe preeclampsia were significantly higher at six–eight weeks postpartum than in the other groups. A similar trend was observed in patients with moderate preeclampsia. The CAVI after preeclampsia was significantly higher than that in the control group. In the severe preeclampsia group, there was a tendency towards an increase in this parameter.
The CAVI in women with severe preeclampsia (Table 7) demonstrated inverse correlations with the newborn Apgar scores at the 1st and 5th minutes and positive correlations with biological age, body mass index (BMI), baseline SBP and DBP in the 1st trimester of pregnancy, and DBP at 6–8 weeks postpartum (at the time of the study). No significant differences were observed in ABI between the two groups (Table 6). In patients with a history of preeclampsia, the vascular age was found to align with the biological age, yet it was markedly elevated compared to the control group. Conversely, in women without arterial hypertension, vascular age was observed to be lower than biological age (p<0.001).
Discussion
According to modern concepts, preeclampsia is believed to be caused by placental ischemia. However, the initial state of maternal hemodynamics also plays a significant role in the development of this condition [7, 8]. Commonly recognized risk factors for preeclampsia are chronic arterial hypertension and a history of gestational hypertensive disorders [7, 8, 10]. The findings of this study support these known risk factors. Therefore, cardiovascular disorders may be the primary cause of placenta-associated complications, including hypertensive disorders.
The results of this study indicate that preeclampsia, particularly in severe cases, is linked to placental disorders that begin to manifest in the second trimester as changes in uterine blood flow. These disorders progress as the severity of hypertension increases. Severe preeclampsia is associated with changes in fetal blood flow, including critical changes and development of early intrauterine growth restriction (IGR). Abnormal fetal biometric parameters and Doppler ultrasound results in preeclampsia demonstrate a close pathogenetic relationship between maternal hemodynamic disorders, placental malperfusion, and fetal damage.
According to modern concepts, the inadequate invasion of cytotrophoblasts in preeclampsia leads to the presence of deep arterioles with preserved endothelial and muscle layers. This condition results in a decrease in vessel diameter and disruption of uteroplacental-fetal blood flow [7, 10]. Decreased placental perfusion in preeclampsia is characterized by oxidative stress and activation of inflammatory cytokines, antiangiogenic factors, and microparticles. These factors contribute to generalized endothelial dysfunction and the subsequent development of multiple organ failure [3, 7, 11–13].
To date, it has been established that signs of endothelial dysfunction are recorded long before the manifestation of preeclampsia and persist long after the end of pregnancy [14–16]. Therefore, additional laboratory and instrumental techniques have been proposed to assess endothelial functions for the early prediction of preeclampsia and to monitor the effectiveness of disease therapy. These techniques include assessing endothelin-1 and extracellular microvesicles of endothelial origin (E-EVs) (CD-144) [14], phosphatidylethanolamine PE 16:022:6, phosphatidylcholine PC 18:018:1c, sphingomyelins SM d24:0/18:1 and SM d22:1/20:4 [15], vascular endothelial growth factor (VEGF), endothelial microparticles CD32+CD40+ in blood serum [16], and abnormal concentrations of placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) [17]. However, most of these methods for studying endothelial function are invasive and expensive, making their use in clinical practice challenging.
Nonetheless, EDV indices in various authorial modifications have been proposed as reliable criteria for assessing endothelial dysfunction and as potentially effective noninvasive prognostic tests for the development of preeclampsia [17–20]. This method is both technically and financially affordable.
According to the results of the present study, after the ischemic test, the PI in the brachial artery increased in patients with preeclampsia, in contrast to that in the control group. This increase indicates a highly resistant pathological blood flow and systemic hemodynamic changes in this group of women. Interestingly, the diameter of the brachial artery at rest was significantly larger in women with severe preeclampsia than in the control group, possibly due to vascular remodeling as an early compensatory mechanism in response to arterial wall damage [21]. After compression, the percentage increase in the diameter of the brachial artery in severe preeclampsia was pathologically low, suggesting severe structural trauma to the arterial wall [20, 22]. Therefore, the percentage increase in the diameter of the brachial artery could potentially be used as an additional criterion for assessing preeclampsia severity.
Furthermore, we found negative correlations between the diameter of the brachial artery after decompression in severe preeclampsia and uterine and umbilical blood flow indices and cases of severe neonatal asphyxia, and positive correlations with the assessment of the newborn according to the Apgar scale and the term of delivery. These findings support the relationship between systemic maternal and placental hemodynamics and the possibility of predicting perinatal outcomes.To date, we have obtained convincing data on the influence of hypertensive disorders during pregnancy on the increased risk of chronic non-communicable diseases [3, 23–25]. Arterial hypertension damages the arterial wall due to mechanical stress and leads to endothelial dysfunction, inflammation, and oxidative stress, activating the renin-angiotensin-aldosterone system. This process also stimulates the production of collagen fibers and accelerates the degradation of elastin fibers. Vascular rigidity compensates for changes in blood pressure and creates a vicious cycle of inflammation and calcification, leading to arterial hypertension [26–28]. These mechanisms of vascular changes are similar to those observed during aging. After severe preeclampsia, signs of subclinical atherosclerosis, early vascular aging, and a high risk of cardiovascular diseases are observed [26, 29].
In our study, we examined several systemic cardiovascular characteristics in women 6–8 weeks postpartum. We found that patients with preeclampsia had significantly higher levels of endothelial dysfunction, arterial stiffness, CAVI, and vascular age than those with normotensive pregnancies did. Furthermore, we discovered negative correlations between CAVI and the newborn's Apgar score and positive correlations with passport age, BMI, baseline (first trimester of pregnancy), and current (6–8 weeks after delivery) blood pressure. These findings suggest a close relationship among endothelial damage, biological aging, arterial hypertension, metabolic disorders, and adverse obstetric outcomes.
Conclusion
Our results have significant implications for understanding the potential links between gestational hypertension and cardiovascular diseases. They help to identify women with preeclampsia as a high-risk group for cardiovascular pathology. This emphasizes the need for interdisciplinary recommendations to minimize the probability of developing chronic noncommunicable diseases and preeclampsia in the future. The characteristics of endothelial dysfunction are particularly valuable for the early detection and assessment of pathological changes in preeclampsia. This will enable the timely implementation of preventive measures and the development of suitable treatment plans.
References
- Robillard P.Y., Dekker G., Scioscia M., Bonsante F., Boukerrou M., Iacobelli S., Tran P.L. Preeclampsia in 2023: time for preventing early onset- and term preeclampsia: the paramount role of gestational weight gain. J. Reprod. Immunol. 2023; 158: 103968. https://dx.doi.org/10.1016/j.jri.2023.103968.
- Jung E., Romero R., Yeo L., Gomez-Lopez N., Chaemsaithong P., Jaovisidha A. et al. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022; 226(2S): S844-S866. https://dx.doi.org/10.1016/j.ajog.2021.11.1356.
- Долгушина В.Ф., Сюндюкова Е.Г., Чулков В.С., Рябикина М.Г. Отдаленные последствия перенесенных гипертензивных расстройств во время беременности. Акушерство и гинекология. 2021; 10: 14-20. [Dolgushina V.F., Syundyukova E.G., Chulkov V.S., Ryabikina M.G. Long-term outcomes of hypertensive disorders of pregnancy. Obstetrics and Gynecology. 2021; (10): 14-20. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.10.14-20.
- Benschop L., Duvekot J.J., Versmissen, J., van Broekhoven V., Steegers E.A.P., Roeters van Lennep J.E. Blood pressure profile 1 year after severe preeclampsia. Hypertension. 2018; 71(3): 491-8. https://dx.doi.org/10.1161/HYPERTENSIONAHA.117.10338.
- Riise H.K.R., Sulo G., Tell G.S., Igland J., Egeland G., Nygard O. et al. Hypertensive pregnancy disorders increase the risk of maternal cardiovascular disease after adjustment for cardiovascular risk factors. Int. J. Cardiol. 2019; 282: 81-7. https://dx.doi.org/10.1016/j.ijcard.2019.01.097.
- Staff A.C. The two-stage placental model of preeclampsia: an update. J. Reprod. Immunol. 2019; 134-135: 1-10. https://dx.doi.org/10.1016/j.jri.2019.07.004.
- Сюндюкова Е.Г., Чулков В.С., Рябикина М.Г. Преэклампсия: современное состояние проблемы. Доктор.Ру. 2021; 20(1): 11-6. [Syundyukova E.G., Chulkov V.S., Ryabikina M.G. Preeclampsia: the current state of the problem. Doctor.Ru. 2021; 20(1): 11-6. (in Russian)]. https://dx.doi.org/10.31550/1727-2378-2021-20-1-11-16.
- Foo F.L., Mahendru A.A., Masini G., Fraser A., Cacciatore S., MacIntyre D.A. et al. Association between prepregnancy cardiovascular function and subsequent preeclampsia or fetal growth restriction. Hypertension. 2018; 72(2): 442-50. https://dx.doi.org/10.1161/HYPERTENSIONAHA.118.11092.
- Сидоренко Б.А., Затейщиков Д.А. Дисфункция эндотелия в патогенезе атеросклероза и его осложнений. Кремлевская медицина. 1999; 2: 51-4. [Sidorenko B.A., Zateishchikov D.A. Endothelial dysfunction in the pathogenesis of atherosclerosis and its complications. Kremlin Medicine. 1999; (2): 51-4. (in Russian)].
- Jung E., Romero R., Yeo L., Gomez-Lopez N., Chaemsaithong P., Jaovisidha A. et al. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022; 226(2S): S844-S866. https://dx.doi.org/10.1016/j.ajog.2021.11.1356.
- Chaemsaithong P., Sahota D.S., Poon L.C. First trimester preeclampsia screening and prediction. Am. J. Obstet. Gynecol. 2022; 226(2S): S1071-S1097.e2. https://dx.doi.org/10.1016/j.ajog.2020.07.020.
- Ciobanu A., Wright A., Syngelaki A., Wright D., Akolekar R., Nicolaides K.H. Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet. Gynecol. 2019; 53(4): 465-72. https://dx.doi.org/10.1002/uog.20157.
- Panda S., Jante V., Das A., Shullai W., Sharma N., Basu R. et al. Unveiling preeclampsia prognosis: uterine artery doppler indices in low-risk pregnancies. Cureus. 2023; 15(9): e46060. https://dx.doi.org/10.7759/cureus.46060.
- Николаева М.Г., Терехина В.Ю., Кудинов А.В., Шахматов И.И., Момот А.П. Маркеры системного эндотелиоза при рецидиве ранней преэклампсии. Акушерство, гинекология и репродукция. 2023; 17(4): 433-42. [Nikolaeva M.G., Terekhina V.Yu., Kudinov A.V., Shakhmatov I.I., Momot A.P. Markers of systemic endotheliosis in early-onset preeclampsia relapse. Obstetrics, Gynecology and Reproduction. 2023; 17(4): 433-42. (in Russian)]. https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2023.437.
- Минаева Е.А., Стародубцева Н.Л., Шмаков Р.Г., Чаговец В.В., Токарева А.О., Новоселова А.В., Кукаев Е.Н., Франкевич В.Е. Потенциал липидома плазмы крови первого триместра беременности в группах с высоким риском. Акушерство и гинекология. 2023; 10: 108-18. [Minaeva E.A., Starodubtseva N.L., Shmakov R.G., Chagovets V.V., Tokareva A.O., Novoselova A.V., Kukaev E.N., Frankevich V.E. Potential of first trimester plasma lipidome in high-risk pregnancy groups. Obstetrics and Gynecology. 2023; (10): 108-18. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.229.
- Zelinka-Khobzey M.M., Tarasenko K.V., Mamontova T.V. Assessment of endothelial dysfunction in pregnant women with obesity and preeclampsia. Wiad. Lek. 2021; 74(8): 1905-9. https://dx.doi.org/10.36740WLek202108122.
- Liu S., Li W., Zhang J., Qi L., Dong Y., Fu L., Li Y. Clinical value of flow-mediated dilatation of brachial artery in hypertensive disorders complicating pregnancy. Clin. Hemorheol. Microcirc. 2022; 82(3): 265-74. https://dx.doi.org/10.3233/CH-221533.
- Kirollos S., Skilton M., Patel S., Arnott C. A systematic review of vascular structure and function in pre-eclampsia: non-invasive assessment and mechanistic links. Front. Cardiovasc. Med. 2019; 6: 166. https://dx.doi.org/10.3389/fcvm.2019.00166.
- Carranza-Lira S., Jaime-Barrera G., Rosales-Ortiz S., García-Espinosa M., Moreno-Álvarez O. Doppler de las arterias uterinas y braquial en mujeres sanas y con preeclampsia [Brachial and uterine arteries Doppler in healthy women and with preeclampsia]. Rev. Med. Inst. Mex. Seguro Soc. 2018; 56(4): 360-3. (in Spanish).
- Шаваева Р.Х., Мурашко А.В., Зуев В.М., Тимофеев С.А., Джибладзе Т.А. Прогнозирование состояния пациенток с преэклампсией на основании показателей эндотелийзависимой вазодилатации. РМЖ. Мать и дитя. 2021; 4(4): 317-21. [Shavaeva R.Kh., Murashko A.V., Zuev V.M., Timofeev S.A., Dzhibladze T.A. Predicting preeclampsia course based on endothelium-dependent dilation. Russian Journal of Woman and Child Health. 2021; 4(4): 317-21. (in Russian)]. https://dx.doi.org/10.32364/2618-8430-2021-4-4-317-321.
- Montalcini T., Gorgone G., Gazzaruso C., Garzaniti A., Pujia A. Large brachial artery diameter and diabetes in post-menopausal women. Nutr. Metab. Cardiovasc. Dis. 2011; 21(10): 830-4. https://dx.doi.org/10.1016/j.numecd.2010.02.009.
- Михайлова Ю.В., Шехтер М.С. Выраженность эндотелиальной дисфункции как объективный критерий степени тяжести преэклампсии. Медико-фармацевтический журнал "Пульс". 2023; 25(3): 84-9. [Mikhailova Yu.V., Shechter M.S. The severity of endothelial dysfunction as an objective criterion of the severity of preeclampsia. Medical and Pharmaceutical Journal "Pulse". 2023; 25(3): 84-9. (in Russian)]. https://dx.doi.org//10.26787/nydha-2686-6838-2023-25-3-84-89.
- Cho G.J., Jung U.S., Sim J.Y., Lee Y.J., Bae N.Y., Choi H.J. et al. Is preeclampsia itself a risk factor for the development of metabolic syndrome after delivery? Obstet. Gynecol. Sci. 2019; 62(4): 233-41. https://dx.doi.org/10.5468/ogs.2019.62.4.233.
- Werlang A., Paquin A., Coutinho T. The EVA study: early vascular aging in women with history of preeclampsia. J. Am. Heart Assoc. 2023; 12(8): e028116. https://dx.doi.org/10.1161/JAHA.122.028116.
- Khashan A.S., Evans M., Kublickas M., McCarthy F.P., Kenny L.C., Stenvinkel P. et al. Preeclampsia and risk of end stage kidney disease: a Swedish nationwide cohort study. PLoS Med. 2019; 16(7): e1002875. https://dx.doi.org/10.1371/journal.pmed.1002875.
- Фомина Е.С., Никифоров В.С. Артериальная жесткость и сосудистое старение: последствия артериальной гипертензии. Архивъ внутренней медицины. 2021; 11(3): 196-202. [Fomina E.S., Nikiforov V.S. Arterial stiffness and vascular aging: effects of hypertension. The Russian Archives of Internal Medicine. 2021; 11(3): 196-202. (in Russian)]. https://dx.doi.org/10.20514/2226-6704-2021-11-3-196-202.
- Anthoulakis C., Mamopoulos A., Rousso D., Karagiannis A., Athanasiadis A., Grimbizis G. et al. Arterial stiffness as a cardiovascular risk factor for the development of preeclampsia and pharmacopreventive options. Curr. Vasc. Pharmacol. 2022; 20(1): 52-61. https://dx.doi.org/10.2174/1570161119666211006114258.
- Kim H.L. Arterial stiffness and hypertension. Clin. Hypertens. 2023; 29(1): 31. https://dx.doi.org/10.1186/s40885-023-00258-1.
- Benschop L., Schelling S.J., Duvekot J.J., Roeters van Lennep J.E. Cardiovascular health and vascular age after severe preeclampsia: a cohort study. Atherosclerosis. 2020; 292: 136-42. https://dx.doi.org/10.1016/j.atherosclerosis.2019.11.023.
Received 27.04.2024
Accepted 05.09.2024
About the Authors
Valentina F. Dolgushina, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, South Ural State Medical University, Ministry of Health of Russia, 454091, Russia, Chelyabinsk, Vorovsky str., 64, +7(919)329-32-45, dolgushinavf@yandex.ru,. https://orcid.org/0000-0002-3929-7708Elena G. Syundyukova, Dr. Med. Sci., Associate Professor, Professor, South Ural State Medical University, Ministry of Health of Russia,
454091, Russia, Chelyabinsk, Vorovsky str., 64; Obstetrician-Gynecologist, Clinic of the South Ural State Medical University, Ministry of Health of Russia,
454091, Russia, Chelyabinsk, Cherkasskaya str., 2, +7(351)232-73-71, seg269@mail.ru, https://orcid.org/0000-0001-9535-1871
Vasily S. Chulkov, Dr. Med. Sci., Associate Professor, Professor at the Department of Internal Diseases, Novgorod State University, 173003, Russia, Veliky Novgorod,
Bolshaya St. Petersburg str., 4, +7(816)297-42-02, vschulkov@rambler.ru, https://orcid.org/0000-0002-0952-6856
Maria G. Ryabikina, obstetrician-gynecologist, Clinic of the South Ural State Medical University, Ministry of Health of Russia, 454091, Russia, Chelyabinsk, Cherkasskaya str., 2, +7(919)311-24-88, mryabikina@mail.ru, https://orcid.org/0000-0003-0943-0448
Mikhail S. Kirsanov, doctor of ultrasound diagnostics, Head of the Cabinet of Perinatal Giagnostics, Clinic of the South Ural State Medical University,
Ministry of Health of Russia, 454091, Russia, Chelyabinsk, Cherkasskaya str., 2, +7(904)812-42-05, kirsmikhail@gmail.com
Vladislas S. Chulkov, PhD, Associate Professor at the Department of Faculty Therapy, South Ural State Medical University, Ministry of Health of Russia,
454091, Russia, Chelyabinsk, Vorovsky str., 64, vlad.chulkov.1989@mail.ru, https://orcid.org/0000-0002-1948-8523
Corresponding author: Maria G. Ryabikina, mryabikina@mail.ru



