Pathogenetic rationale for the ineffectiveness of antihypertensive therapy based on vasoactive status analysis in patients with early-onset preeclampsia
Ziganshina M.M., Muminova K.T., Khodzhaeva Z.S., Baranov I.I., Sukhikh G.T.
The central-acting antihypertensive drug Dopegyt and the calcium channel blocker Cordaflex are commonly used in obstetric practice for managing hypertensive disorders during pregnancy. However, in early-onset preeclampsia (ePE), their efficacy is notably diminished because of its limited impact on endogenous nitric oxide (NO) production, a critical vasodilator.
Objective: 1) Investigate NO production and factors influencing vascular tone regulation in ePE patients undergoing different antihypertensive therapy regimens: Dopegyt monotherapy and two-drug therapy (Dopegyt+Cordaflex), and 2) examine the relationship between NO, maternal hemodynamic parameters, the fetal-placental unit, and factors affecting vascular tone regulation.
Materials and methods: This study included 49 patients at up to 340 weeks of gestation. The control group consisted of 16 pregnant women with healthy pregnancies. The study group included 33 pregnant women with PE, with 16 receiving antihypertensive monotherapy (Group 1) and 17 receiving two-drug antihypertensive therapy (Group 2). All pregnant women underwent: 1) 24-hour blood pressure monitoring to assess hemodynamic status and determine parameters characterizing changes in central aortic pressure, intracardiac hemodynamics, and arterial stiffness; 2) Doppler assessment of fetoplacental and uteroplacental blood flow; and 3) determination of blood factors regulating vascular tone, thrombosis, and markers of cardiovascular insufficiency using ELISA.
Results: Patients in the study group exhibited significant disruption in endogenous NO production, coupled with vasodilator/vasoconstrictor imbalance, as well as impaired maternal and fetal-placental unit hemodynamics, which were more pronounced with two-drug therapy. There was an association between NO levels and maternal hemodynamic parameters and concentrations of factors regulating vascular tone.
Conclusions: The findings of this study indicate that the lack of a NO-stimulating effect on the endothelium of Dopegyt monotherapy and Dopegyt in combination with Cordaflex in patients with ePE leads to significant hemodynamic disturbances, justifying the early delivery of these patients.
Authors' contributions: Ziganshina M.M., Khodzhaeva Z.S., Baranov I.I., Sukhikh G.T. – conception and design of the study; Muminova K.T., Khodzhaeva Z.S. – data collection and processing; Ziganshina M.M. – statistical analysis; Ziganshina M.M., Muminova K.T. – drafting of the manuscript; Khodzhaeva Z.S., Baranov I.I., Sukhikh G.T. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the State Task of the Ministry of Health of the Russian Federation № 121040600435-0 "Justification of personalized approaches to antihypertensive therapy in HDP and PE".
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No 5 of May 27, 2021)
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Ziganshina M.M., Muminova K.T., Khodzhaeva Z.S., Baranov I.I., Sukhikh G.T. Pathogenetic rationale for the ineffectiveness of antihypertensive therapy based on vasoactive status analysis in patients with early-onset preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (11): 60-70 (in Russian)
https://dx.doi.org/10.18565/aig.2023.231
Keywords
Preeclampsia (PE) has two distinct clinical phenotypes that differ in the time of presentation and pathogenesis. Early-onset PE (ePE), occurring before 34 weeks of gestation, has a severe course that leads to preterm delivery and adverse perinatal outcomes [1]. The primary factor in the pathogenesis of ePE is placental dysfunction. This dysfunction involves inadequate gestational remodeling of the uterine spiral arteries and impaired trophoblast invasion, resulting in the preservation of the musculoelastic elements of the spiral arteries and compromised fetoplacental blood flow [2]. These changes lead to placental tissue ischemia, initiating a sterile inflammation that releases mediators strongly inducing endothelial dysfunction [3, 4]. Morphological abnormalities within the fetoplacental vascular network, mainly due to the absence of a transition from the epithelial to the endothelial phenotype, cause persistent early arterial hypertension, which is difficult to address pharmacologically [3]. Ineffective antihypertensive therapy worsens the maternal condition, marked by a significant increase in total peripheral vascular resistance (TPVR), decreased cardiac output, and rising blood pressure (BP). Development of multiorgan failure can have critical consequences for both the mother and fetus, often necessitating early delivery, typically the primary strategy in managing ePE [5].
Antihypertensive therapy is a crucial component of the management of patients with PE. According to clinical guidelines [6], first-line therapy involves Dopegyt (active substance methyldopa), which is a central-acting antihypertensive drug. Dopegyt stimulates α-adrenoreceptors in medulla oblongata neurons, inhibiting the vasomotor center and reducing sympathetic impulses. Its antihypertensive effect involves decreasing the TPVR, cardiac output, and heart rate (HR). If Dopegyt proves ineffective, a shift to two-drug antihypertensive therapy includes the additional administration of Cordaflex (active substance nifedipine). Cordaflex is a long-acting selective blocker of calcium channels in vascular smooth muscle cells and cardiomyocytes. Its antihypertensive effect is related to reducing TPVR by relaxing the vascular tone, dilating the coronary and peripheral arteries, and decreasing myocardial contractility and cardiac afterload [7]. Fetal safety of these drugs is a key factor in choosing antihypertensive therapy during pregnancy [8, 9].
An important criterion for evaluating the efficacy of antihypertensive drugs is their effect on the synthesis and release of endogenous nitric oxide (NO) into the bloodstream. NO is a primary endothelial factor that facilitates vascular relaxation and serves as a messenger molecule that signals dysregulation of vascular tone. It participates in the inhibition of intercellular adhesion and inflammation, thereby suppressing thrombosis and maintaining normal blood flow. Additionally, it limits vascular wall remodeling. In microvessels, particularly capillaries, NO in conjunction with growth factors plays a vital role in stimulating angiogenesis [10]. Many antihypertensive drugs have been shown to stimulate NO production and bioavailability [11]. However, the impact of Dopegyt and Cordaflex in pregnant women with PE has not been studied. This study aimed to: 1) to investigate endogenous NO production and its related factors involved in the regulation of vascular tone in patients with ePE undergoing different antihypertensive therapy regimens – monotherapy with Dopegyt and Dopegyt+Cordaflex therapy; and 2) examine the influence of endogenous NO levels in the blood of ePE patients receiving varied antihypertensive therapy regimens on maternal and fetoplacental complex hemodynamic parameters.
Materials and methods
Study design and study selection criteria
This non-randomized controlled study was conducted at V.I. Kulakov NMRC for OG&P (hereinafter referred to as the Center) from January 2021 to June 2023. The study was conducted in accordance with the Declaration of Helsinki of the World Medical Association, revised by the 64th WMA General Assembly, Fortaleza, Brazil (2013). The study design and informed consent form were reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No. 5 of May 27, 2021). All patients signed an informed consent form to participate in this study. The study included 49 patients with clinical manifestations of PE up to 34 weeks of gestation. The control group consisted of 16 conventionally healthy pregnant women with healthy pregnancies. The study group included 33 pregnant women with PE, 16 of whom received antihypertensive monotherapy (Group 1) and 17 of whom received two-drug antihypertensive therapy (Group 2). The inclusion criteria were singleton pregnancy in a natural cycle, age of the pregnant woman 18–43 years. The inclusion criteria in the control group were physiologic course of pregnancy, in the study group: diagnosis of PE, according to the criteria set forth in the clinical recommendations of the Ministry of Health of the Russian Federation [6]. The non-inclusion criteria were acute and chronic infectious diseases in the exacerbation stage and severe extragenital pathology, including oncological and autoimmune diseases. The exclusion criteria were HELLP syndrome and fetal malformations. The patients were enrolled using a pair-matching method based on age, BMI, and gestational age. None of the patients included in the study followed any specific diet.
Antihypertensive therapy regimens
Antihypertensive therapy was administered to all patients in the study group. The therapy was prescribed at a BP≥140/90 mmHg, according to the clinical recommendations of the Ministry of Health of the Russian Federation. Drug dosage correction was performed according to 24-h blood pressure monitoring (24-h BPM) using a BPLab device (Petr Telegin, Nizhny Novgorod, Russia), which is recommended for use in pregnant women [12]. Dopegyt was used as the main antihypertensive drug at an initial dose of 750 mg/day, with an increase in resistant hypertension to 2000 mg/day. In cases of persistent hypertension that was not controlled by Dopegyt, Cordaflex was added at a starting dose of 20 mg/day, and if ineffective, the dose of Cordaflex was increased to 40 mg/day. Dopegyt at a dose of 2000 mg/day and Cordaflex at 40 mg/day constituted the second scheme of therapy (two-drug antihypertensive therapy).
Evaluation of maternal hemodynamic profile
Antihypertensive therapy was evaluated and corrected based on 24-h BPM data using BPLab (Peter Telegin, Nizhny Novgorod, Russia). Oscillograms obtained from the device were analyzed using Vasotens software (Russia). The registration of hemodynamic parameters and analysis of oscillograms were performed similarly to the procedure described in [13]. Several hemodynamic parameters characterizing changes in central pressure in the aorta, intracardiac hemodynamics, and arterial stiffness were determined. The list of recorded parameters is similar to that presented in [13, 14] and includes maximal aortal diastolic BP (max DADao); maximal aortal systolic BP (max SADao); minimal aortal diastolic BP (min DADao); minimal aortal systolic BP (min SADao); mean aortal diastolic BP (med DADao); and mean aortal systolic BP (med SADao)., Reflected Wave Transit Time (RWTT), Aortic Pulse Wave Velocity (PWVao), augmentation index (AIx), Ambulatory Arterial Stiffness Index (AASI) calculated as AASI=1-(slope of DAD-SAD), maximal rate of rise of arterial pressure (dP/dt)max, Pulse Pressure Amplification (PPA), Ejection Duration (ED), Subendocardial Viability Ratio (SEVR) [13].
Assessment of uteroplacental and fetoplacental blood flows
Doppler study of uteroplacental and fetoplacental blood flow was performed using a transabdominal transducer on expert class devices (GE Voluson E8 device (USA)) and included determination of the following blood flow parameters: pulsation index (PI) in uterine (UtA-PI), umbilical (UmA-PI), middle cerebral (MCA-PI) arteries, venous duct, and calculation of cerebro-placental ratio (CPR). When assessing blood flow in the umbilical artery, the pulsatility index and presence or absence of diastolic blood flow were evaluated. The venous duct was evaluated using color Doppler mapping in the transverse or sagittal plane of the fetal abdomen. Curve evaluation was classified as normal with a positive A-wave, or abnormal with an absent or negative A-wave.
Determination of blood factors regulating vascular tone, thrombosis and markers of cardiovascular insufficiency
The study of factors was performed in the peripheral blood serum of patients using ELISA commercial kits. Fasting peripheral blood samples were collected from the antecubital vein. Blood was transported to the Biobank Center, where it was processed according to the Biobank SOP. Blood serum was aliquoted and stored at -80°C until testing.
The commercial test systems used in this study included Biomedica (BI-20812, determination of C-type natriuretic propeptide, NT-proCNP; BI-20082H, determination of large endothelin-1, 1-38, large); R&D systems (KGE001, determination of total NO based on nitrate/nitrite analysis; DTSP10, determination of thrombospondin-1, TSP-1); RayBiotech, Inc. (EIA-ANGII-1, angiotensin II determination; ELH-ENOS, endothelial nitric oxide synthase, eNOS determination); Enzo (ADI-900-020A, determination of endothelin-1; ADI-900-004, determination of 6-Keto-prostaglandin F1α, 6-Keto-PGF1α); ImmunDiagnostic (K7860, determination of asymmetric dimethylarginine, ADMA); Cloud-Clone Corp. (SEA837Hu, determination of inducible nitric oxide synthase, iNOS2), and AssayPro (EA3303-1, determination of antithrombin III). Pretreatment of serum samples for the determination of total NO by nitrate/nitrite assay was performed according to the manufacturer’s instructions for the KGE001 kit. Each serum sample was filtered using a tube with Amicon Ultra 0.5ML 10 K 96PK filters for NO kit (Millipore), which allowed ultrafiltration and separation in diluted serum of the fraction of high molecular weight components with a molecular weight greater than 10,000 kDa from low molecular weight components less than 10,000 kDa. In the latter fraction, nitrate/nitrite determination was performed.
Statistical analysis
Statistical analysis and graphical presentation of the data were performed using MedCalc version 16.4 (MedCalc, Belgium). The normality of the distribution was tested using the Shapiro–Wilk test. Continuous variables are presented as median (Me) and maximum and minimum values (Q0 – 0th percentile; Q4 – 100th percentile). Intergroup differences when comparing three or more groups for continuous variables whose distribution differed from normal were calculated using the Kruskal–Wallis test with Bonferroni correction at p<0.025. For post-hoc comparisons, the Mann–Whitney U test was used at p<0.050. The null hypothesis was rejected when the p-value was less than 0.050. Correlation analysis was performed by calculating the Spearman's rank correlation coefficient; differences were considered significant at p<0.050.
Results
According to the data presented in Table 1, the patients in all groups were comparable in terms of the main confounding factors of the study, including age, BMI, and gestational age at the time of blood sampling. The parameters reflecting the mean BP, neonatal weight, and Apgar scores demonstrated consistent differences between patients in the study and control groups (Table 1).
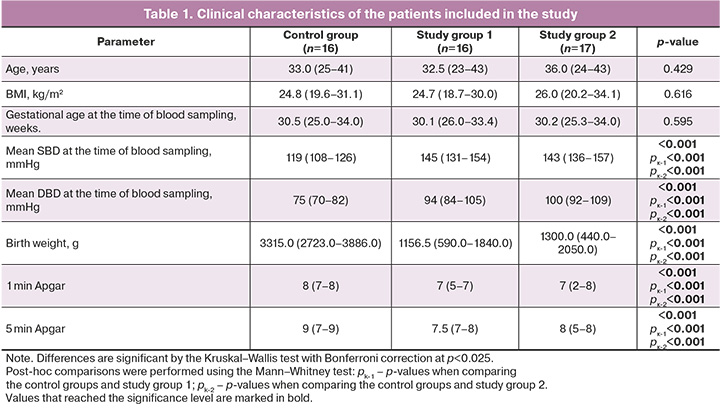
The analysis of hemodynamic data showed that all parameters reflecting 24-hour trends of BP changes were significantly higher in patients with PE receiving both schemes of antihypertensive therapy. The main parameters assessing arterial stiffness (PWVao and RWTT) did not differ significantly between the groups; however, PWVao, reflecting the estimated pulse wave velocity in the aorta, showed a tendency to increase at the borderline significance level. A post hoc comparison revealed a higher PWVao value in ePE patients receiving two-drug therapy. The augmentation index (AIx, pulse wave increment index), which is the most commonly used index in clinical practice to characterize arterial stiffness, was significantly higher in patients with PE receiving both antihypertensive therapy regimens. Alternative parameters of hemodynamic assessment, in particular, the index (dP/dt)max, indirectly reflecting myocardial contractility, total stiffness of the main arteries, and dynamic load, were significantly higher in patients with PE in all cases of observation. Significant intergroup differences were demonstrated for ED, which reflects the relative duration of the left ventricular ejection period; however, the specified level of significance was not reached in post-hoc comparisons (Table 2).
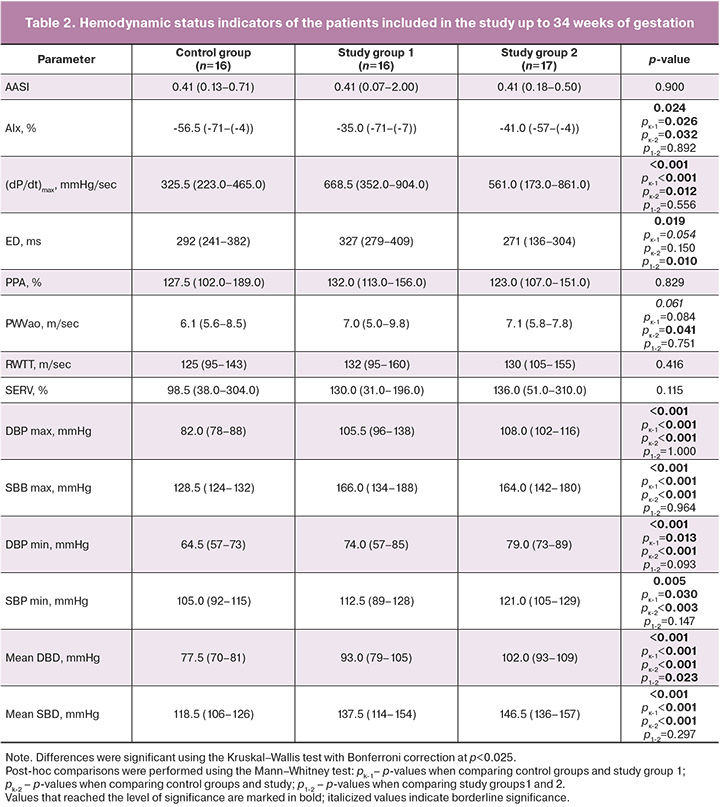
Evaluation of hemodynamic parameters in the maternal-placenta-fetus system (Table 3) showed an increase in the pulsatility index in the uterine arteries (significant) and umbilical arteries (borderline significant). A post-hoc comparison showed a significant increase in the UmA-PI in ePE patients receiving two-drug antihypertensive therapy. In contrast, the fetal middle cerebral artery pulsatility index and cerebroplacental ratio were significantly higher in patients with normal pregnancies.
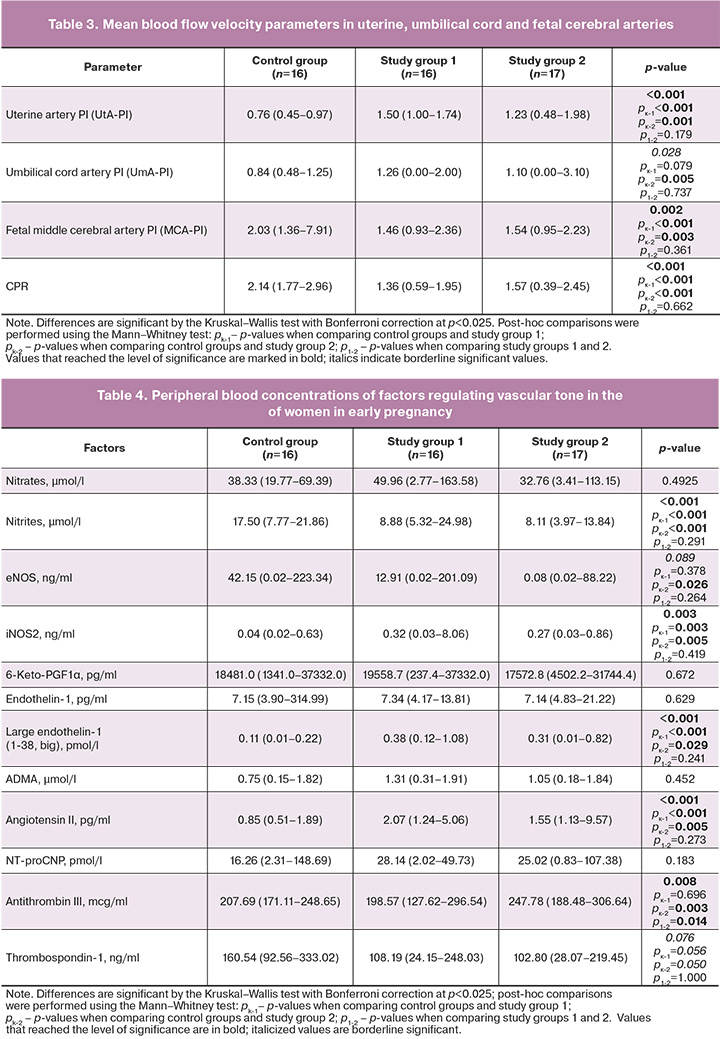
The analysis of the complex of factors regulating vascular tone, as well as their dependent parameters controlling thrombosis, indicates that the level of endogenous nitrite, a stable metabolite of NO, reflecting its content in the blood, is significantly reduced (2-fold) in patients with ePE receiving both monotherapy and two-drug antihypertensive therapy. In addition, the level of inducible nitric oxide synthase (iNOS2) was increased 8-fold in these patients. In the blood of patients with ePE receiving both schemes of antihypertensive therapy, a significantly higher level of factors with vasoconstrictor effect – large endothelin-1 (3 times) and angiotensin II (2 times) was found. The content of antithrombin III showed a decrease in the monotherapy group (post-hoc comparison did not confirm the significance of differences), and, on the contrary, a significant increase in the two-drug therapy group (Table 4). Notably, endothelial nitric oxide synthase (eNOS) content was high in patients with normal pregnancies, three times higher than the enzyme content in patients on monotherapy (differences not significant in post-hoc comparison), and 527 times higher than in patients with two-drug therapy (post-hoc comparison demonstrated significant differences with the control group). Thrombospondin-1 content, while apparently lower in patients with PE, did not reach the level of significance (Table 4). The heart failure markers NT-proCNP and the prostacyclin metabolite 6-Keto-PGF1α did not demonstrate significant differences in the intergroup analysis (Table 4).
Analysis of correlations in the combined sample of patients (i.e., obtained by combining all study groups, (n=49). This allowed us to reveal the dependence of several maternal hemodynamic parameters and factors regulating vascular tone on the quantitative content of nitrite, which reflects the content of endogenous NO in the blood (Table 5). Hemodynamic parameters reflecting trends in BP (SBP, DBP), arterial stiffness (AIx), and myocardial function ((dP/dt)max) showed reciprocal correlations with nitrite content (Table 5). That is, the higher the blood nitrite concentration (reflecting endogenous NO content), the lower the BP, stiffness, and maximum rate of the arterial pressure rise, which has logical explanations because NO is a potent vasodilator, causes relaxation of vascular smooth muscle, and reduces TPVR [5]. Notably, the calculation of correlations separately in each of the three groups did not demonstrate a similar systemic trend found in the pooled sample.
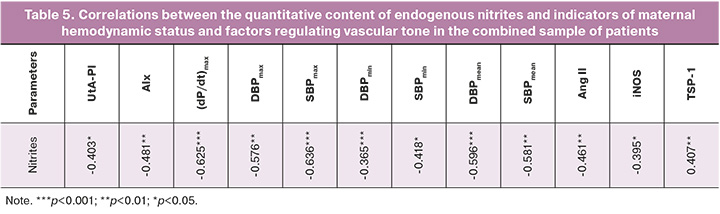
In the control group, only one reciprocal relationship (rs=-0.557; p=0.039) between nitrite content and the maximum CAD value was revealed. A strong direct relationship between similar parameters was found in study group 2 (rs=0.928; p=0.008). A direct correlation between nitrite content and thrombospondin-1 (rs=0.546; p=0.044) was observed in study group 1. A reciprocal relationship of medium strength between nitrite content and the uterine artery pulsatility index was also detected in the combined group. No significant correlations were found between the nitrate content and any of the studied hemodynamic parameters or blood factor concentrations.
Discussion
During a typical pregnancy, the increased volume of circulating blood stimulates the endothelium, prompting higher synthesis of molecules that regulate vascular tone. This contributes to systemic vasodilation and augments uteroplacental blood flow [15]. The ability of the endothelium to produce vasodilators is crucial for maintaining physiological blood pressure within the cardiovascular system (CVS) during pregnancy. Several studies have confirmed this phenomenon: elevated levels of molecules that regulate vascular tone and their metabolites, such as 6-Keto-PGF1α (a prostacyclin metabolite) and nitrites (metabolites of NO), in the blood and urine of pregnant women [5, 16, 17].
Conversely, hemodynamic disruption impairs endothelial cell mechanotransduction in preeclampsia. This is manifested by a decreased response to blood flow stimuli, reduced production of vasodilators, and increased biosynthesis of vasoconstriction-inducing factors [18]. Consequently, there is a loss of control over vascular tone, leading to the development of arterial hypertension (AH) and warranting corrective antihypertensive therapy.
Our results indicate that antihypertensive therapy did not rectify the imbalance between vasoconstrictor and vasodilator production observed in preeclampsia patients, irrespective of the antihypertensive therapy regimen employed. This imbalance is characterized by reduced nitrite levels, increased levels of the endothelin precursor (big endothelin-1), which has a lesser vasoconstrictive effect than endothelin-1, and elevated levels of angiotensin II in the blood of patients in both study groups (study groups 1 and 2). Notably, patients undergoing dual-drug antihypertensive therapy exhibited more pronounced manifestations of imbalance owing to a significant decrease in eNOS, the key enzyme involved in NO synthesis within the endothelium. This might be explained by the more severe hemodynamic status of the patients in study group 2, justifying the addition of an extra antihypertensive drug. These findings correlated with the hemodynamic status data (Table 2), where the mean diastolic blood pressure (DBP) values were significantly higher in study group 2 than in study group 1. Additionally, these patients displayed increased levels of antithrombin III, possibly influenced by higher doses of Dopegyt in study group 2.
Notably, although almost all velocity indices of blood flow in the fetoplacental system differed from the control in the study group patients, there was no significant difference between study groups 1 and 2. This suggests that antihypertensive therapy did not ameliorate the hemodynamic indices of the fetoplacental system regardless of the therapy regimen. This could be attributed to the inability of these drugs to influence NO and eNOS synthesis in the vessels of the fetoplacental system. Given that NO plays a pivotal role in regulating placental blood flow, its reduced production in preeclampsia results in vasoconstriction of the placental bed and abnormal placental perfusion [19]. This assumption is supported by the inverse relationship between nitrite content and pulsatility index of the uterine arteries.
Moreover, both study groups exhibited significantly increased iNOS2 levels in blood. iNOS2 activation primarily occurs in immune and glial cells during inflammatory responses accompanied by cytokine release. The primary function of iNOS is to induce cell death during inflammation by generating highly toxic doses of NO [20]. Previous studies involving a subset of patients from this study indicated a significantly heightened proinflammatory background in preeclampsia patients receiving both antihypertensive therapy schemes [21], suggesting a predisposing factor for increased iNOS production. Elevated concentrations of circulating angiotensin II exacerbate manifestations of inflammatory stress, serving as a potent stimulus for reactive oxygen species and proinflammatory cytokine production, leading to oxidative stress and adversely affecting blood vessels in arterial hypertension [22]. It is worth noting that high expression and activity of iNOS are essential for the expression of endothelial stress genes that protect against oxidative damage [23], suggesting a potential compensatory effect of high iNOS levels in protecting endothelial cells.
The equilibrium between NO and angiotensin II is pivotal in cardiovascular diseases, particularly in the pathogenesis of AH [24]. Activation of angiotensin type 1 receptors (AT1R) by angiotensin II inhibits eNOS expression and activity, underscoring its vasoconstrictive impact [22]. Based on this understanding, a reciprocal relationship between angiotensin II and the interconnected eNOS/NO system has been proposed. Our study revealed a moderate reciprocal correlation between angiotensin II and maternal blood NO in a pooled sample of preeclampsia patients and healthy pregnant women (Table 5), consistent with existing literature. High concentrations of angiotensin II in the study group coincided with suppressed eNOS/NO production and activity, whereas lower angiotensin II concentrations correlated with increased eNOS/NO production in the comparison group. However, similar relationships were not identified within individual groups, likely because of the limited statistical power caused by small sample sizes.
Overall, correlation analysis across the patient samples revealed systemic effects of NO on hemodynamics and suggested a balance between NO and vasoconstrictive factors. This balance implies the necessity of an optimal NO level for vasodilation, as low and excessive levels can cause vasodilation and cellular toxicity, respectively. Notably, the moderate inverse correlation between NO content and augmentation index AIx (Table 5) supports this premise. Similarly, the observed direct relationship between NO and thrombospondin, an NO antagonist that limits eNOS activity and signaling transmission [10], emphasizes the importance of maintaining a balance between NO production and its limiting factors.
This array of parameters signifies the "vasoactive status" of the patient, depicting the functional and synthetic characteristics of the endothelium and SSc vessels. This study highlights a critical deficiency in endogenous NO and an imbalance in the factors regulating vascular tone in preeclampsia patients receiving two recommended antihypertensive therapy regimens during pregnancy. Endogenous NO levels significantly influence maternal hemodynamics and several parameters that are pivotal in the early-onset of arterial hypertension during pregnancy.
Conclusion
The data obtained indicate a lack of NO-stimulating effect of antihypertensive drugs (Dopegyt and Dopegyt in combination with Cordaflex) in early-onset preeclampsia patients. This deficiency contributes to significant hemodynamic disturbances, leading to early delivery. Additionally, the addition of Cordaflex in more severe cases of arterial hypertension does not positively influence maternal or fetoplacental hemodynamics nor does it rectify the pronounced vasoconstrictor/vasodilator imbalance. Assessing 24-hour BPM-based hemodynamics, considering the presented parameters, and evaluating factors regulating vascular tone can depict the patient's "vasoactive status" and guide pathogenetically justified antihypertensive therapy selection. Future studies should focus on revising antihypertensive approaches in early-onset preeclampsia, emphasizing early detection of reduced endothelial NO production as a risk factor, and developing personalized NO-directed therapies incorporating NO-enriched diets, antioxidant-rich supplements, NOS substrates, and NO donors.
References
- Masini G., Foo L.F., Tay J., Wilkinson I. B., Valensise H., Gyselaers W., Lees C.C. Preeclampsia has two phenotypes which require different treatment strategies. Am. J. Obstet. Gynecol. 2022; 226: 1006-8. https://dx.doi.org/10.1016/j.ajog.2020.10.052.
- Ходжаева З.С., Холин А.М., Вихляева Е.М. Ранняя и поздняя преэклампсия: парадигмы патобиологии и клиническая практика. Акушерство и гинекология. 2013; 10: 4-11. [Khodzhaeva Z.S., Kholin A.M., Vikhlyaeva E.M. Early and late preeclampsia: Pathobiology paradigms and clinical practice. Obstetrics and Gynecology. 2013; (10): 4-11 (in Russian)]
- Opichka M.A., Rappelt M.W., Gutterman D.D., Grobe J.L., McIntosh J.J. Vascular dysfunction in preeclampsia. Cells. 2021; 10(11): 3055. https://dx.doi.org/10.3390/cells10113055.
- Ziganshina M.M., Yarotskaya E.L., Bovin N.V., Sulhikh G.T. Endothelial dysfunction as a consequence of endothelial glycocalyx damage: a role in the pathogenesis of preeclampsia. In: Lenasi H., ed. Endothelial dysfunction – Old concepts and new challenges. IntechOpen; 2018. https://dx.doi.org/10.5772/intechopen.75043.
- Osol G., Ko N.L., Mandalà M. Altered endothelial nitric oxide signaling as a paradigm for maternal vascular maladaptation in preeclampsia. Curr. Hypertens Rep. 2017; 19(10): 82. https://dx.doi.org/10.1007/s11906-017-0774-6.
- Министерство здравоохранения Российской Федерации. Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде. Клинические рекомендации. 2021. [Ministry of Health of the Russian Federation. Preeclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders during pregnancy, childbirth and the postpartum period. Clinical guidelines. 2021. (in Russian)].
- Толмачева Е.А. Лекарственные препараты в России. Справочник Видаль. Видаль-Рус; 2023; 1160 с. [Tolmacheva E.A. Medicines in Russia. Vidal Reference Book. Vidal-Rus; 2023; 1160p (in Russian)].
- Awaludin A., Rahayu C., Daud N.A.A., Zakiyah N. Antihypertensive medications for severe hypertension in pregnancy: a systematic review and meta-analysis. Healthcare (Basel). 2022; 10(2): 325. https://dx.doi.org/10.3390/healthcare10020325.
- Cífková R. Hypertension in pregnancy: a diagnostic and therapeutic overview. High Blood Press. Cardiovasc. Prev. 2023; 30(4): 289-303. https://dx.doi.org/10.1007/s40292-023-00582-5.
- Ghimire K., Altmann H.M., Straub A.C., Isenberg J.S. Nitric oxide: what's new to NO? Am. J. Physiol. Cell Physiol. 2017; 312(3): 254-62. https://dx.doi.org/10.1152/ajpcell.00315.2016.
- Hermann M., Flammer A., Lüscher T.F. Nitric oxide in hypertension. J. Clin. Hypertens (Greenwich). 2006; 8 (12 Suppl 4): 17-29. https://dx.doi.org/10.1111/j.1524-6175.2006.06032.x.
- Dorogova I.V., Panina, E.S. Comparison of the BPLab® sphygmomanometer for ambulatory blood pressure monitoring with mercury sphygmomanometry in pregnant women: Validation study according to the British Hypertension Society protocol. Vasc. Health Risk Manag. 2015; 11: 245-9. https://dx.doi.org/10.2147/VHRM.S82381.
- Муминова К.Т., Ходжаева З.С., Горина К.А., Шмаков Р.Г., Зиганшина М.М. Сравнительный анализ влияния двух схем гипотензивной терапии на гемодинамические показатели матери при преэклампсии с ранним и поздним началом. Акушерство и гинекология. 2023; 1: 55-66. [Muminova K.T., Khodzhaeva Z.S., Gorina K.A., Shmakov R.G., Ziganshina M.M. Comparative analysis of the effect of two antihypertensive therapy regimens on maternal hemodynamic parameters in early- and late-onset preeclampsia. Obstetrics and Gynecology. 2023; (1): 55-66 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.290.
- Ziganshina M.M., Muminova K.T., Khasbiullina N.R., Khodzhaeva Z.S., Yarotskaya E.L., Sukhikh G.T. Characterization of vascular patterns associated with endothelial glycocalyx damage in early- and late-onset preeclampsia. Biomedicines. 2022; 10(11): 2790. https://dx.doi.org/10.3390/biomedicines10112790.
- Matsubara K., Higaki T., Matsubara Y., Nawa A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int. J. Mol. Sci. 2015; 16(3): 4600-14. https://dx.doi.org/10.3390/ijms16034600.
- Chappell D.L., Xiao X., Radziszewski W., Laterza O.F. Development and validation of a LC/MS/MS method for 6-keto PGF1α, a metabolite of prostacyclin (PGI₂). J. Pharm. Biomed. Anal. 2011; 56(3): 600-3. https://dx.doi.org/10.1016/j.jpba.2011.06.019
- Гуманова Н.Г. Оксид азота и его циркулирующие метаболиты NOx, их роль в функционировании человеческого организма и прогнозе риска сердечно-сосудистой смерти (часть II). Профилактическая медицина. 2021; 24(10): 119-25. [Gubanova N.G. Nitrogen oxide and its circulating NOx metabolites, their role in human body functioning and cardiovascular death risk prediction (part II). Profilakticheskaya meditsina. 2021; 24(10): 119-25. (in Russian)]. https://dx.doi.org/10.17116/profmed202124101119.
- Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am. J. Physiol. Heart Circ Physiol. 2007; 292(3): 1209-24. https://dx.doi.org/10.1152/ajpheart.01047.2006.
- Dymara-Konopka W., Laskowska M. The role of nitric oxide, ADMA, and homocysteine in the etiopathogenesis of preeclampsia – review. Int. J. Mol. Sci. 2019; 20(11): 2757. https://dx.doi.org/10.3390/ijms20112757.
- Knott A.B., Bossy-Wetzel E. Impact of nitric oxide on metabolism in health and age-related disease. Diabetes Obes. Metab. 2010; 12 Suppl 2(0 2): 126-33. https://dx.doi.org/10.1111/j.1463-1326.2010.01267.x.
- Муминова К.Т., Ходжаева З.С., Яроцкая Е.Л., Зиганшина М.М. Анализ факторов, отражающих развитие стерильного воспаления, на фоне различных схем гипотензивной терапии у беременных с преэклампсией. Медицинская иммунология. 2023; 25(5): 1183-90. [Muminova K.T., Khodzhaeva Z.S., Yarotskaya E.L., Ziganshina M.M. Analysis of factors associated with sterile inflammation in women with pe receiving different antihypertensive treatment strategies. Medical Immunology (Russia). 2023; 25(5): 1183-90. (in Russian)]. https://dx.doi.org/10.15789/ 1563-0625-AOF-2809.
- da Silva G.M., da Silva M.C., Nascimento D.V.G., Lima Silva E.M., Gouvêa F.F.F., de França Lopes L.G. et al. Nitric oxide as a central molecule in hypertension: focus on the vasorelaxant activity of new nitric oxide donors. Biology (Basel). 2021; 10(10): 1041. https://dx.doi.org/10.3390/biology10101041.
- Hemmrich K., Suschek C.V., Lerzynski G., Kolb-Bachofen V. iNOS activity is essential for endothelial stress gene expression protecting against oxidative damage. J. Appl. Physiol (1985). 2003; 95(5): 1937-46. https://dx.doi.org/10.1152/japplphysiol.00419.2003.
- Ahmad A., Dempsey S.K., Daneva Z., Azam M., Li N., Li P.L., Ritter J.K. Role of nitric oxide in the cardiovascular and renal systems. Int. J. Mol. Sci. 2018; 19(9): 2605. https://dx.doi.org/10.3390/ijms19092605.
Received 05.10.2023
Accepted 24.10.2023
About the Authors
Marina M. Ziganshina, PhD (Bio), Leading Researcher at the Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-11-83, mmz@mail.ru, https://orcid.org/0000-0003-1578-8403,117997, Russia, Moscow, Ac. Oparina str., 4.
Kamilla T. Muminova, PhD, Researcher at the High Risk Pregnancy Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-06-74, kamika91@mail.ru, https://orcid.org/0000-0003-2708-4366, 117997, Russia, Moscow, Aс. Oparina str., 4.
Zulfiya S. Khodzhaeva, Dr. Med. Sci., Professor, Deputy Director of Obstetrics Institute, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-07-88, zkhodjaeva@mail.ru, https://orcid.org/0000-0001-8159-3714,
117997, Russia, Moscow, Ac. Oparina str., 4.
Igor I. Baranov, Dr. Med. Sci., Professor, Head of the Department of Scientific and Educational Programs, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, i_baranov@oparina4.ru, https://orcid.org/0000-0002-9813-2823,
117997, Russia, Moscow, Аc. Oparina str., 4.
Gennady T. Sukhikh, Dr. Med. Sci., Professor, Academician of the RAS, Director of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-18-00; sekretariat@oparina4.ru, https://orcid.org/0000-0002-7712-1260,
117997, Russia, Moscow, Ac. Oparina str., 4.



