Characteristics of autoantibodies associated with recurrent pregnancy loss
Objective. To investigate the spectrum of autoantibodies and their correlation with markers of endothelial damage in women with recurrent pregnancy loss.Chepanov S.V., Krivonos M.I., Arzhanova O.N., Shlaсhtenko T.N., Saidov N.Kh., Kornyushina E.A., Chudotvorov K.N., Sedihin V.Yu., Selkov S.A.
Material and methods. The study comprised 561 women of reproductive age, who were tested for peripheral blood serum levels of autoantibodies and markers of endothelial dysfunction.
Results. APS-associated autoantibodies were found in 31.7% of patients with recurrent pregnancy loss. Among the identified autoantibodies, the most prevalent were lupus anticoagulant and antibodies to beta-2 glycoprotein-I. Peripheral blood levels of endothelial damage markers in women with recurrent pregnancy loss were higher than in women with normal pregnancies.
Conclusion. Women with recurrent pregnancy loss have high detection rates of APS-associated autoantibodies. Vascular endothelial cell dysfunction mediated by APS-associated autoantibodies increases the risk of thrombosis.
Keywords
Pregnancy loss is one of the most important issues encountered by obstetricians and comprising 15–20% of pregnancy outcomes [1]. The most important cases of reproductive loss are disorders involving immune mechanisms. One example of such autoimmune disorders is the antiphospholipid syndrome (APS) that is associated with the persistent presence of antiphospholipid antibodies and the thrombophilic complications [2, 3]. The incidence of APS among patients with recurrent pregnancy loss reaches 27–42% [4].
Organ-specific autoantibodies can bind to negatively charged phospholipids on the cell membranes of platelets and endothelial cells [5], which may lead to thrombophilic complications resulting in preterm birth and miscarriage [6]. Of the diversity of existing autoantibodies, only three types are pathognomonic for APS: lupus anticoagulant, anticardiolipin and anti-b2-glycoprotein I [7]. However, there is also a fairly large range of antiphospholipid antibodies but are not included in the diagnostic criteria. These are antibodies to phosphatidylserine, phosphatidic acid, phosphatidylinositol, protein C, annexin 5, prothrombin, and others. Their role detection rates are controversial [8].
The pathogenesis of APS involves antibody-mediated thrombophilia. [9]. Activation of autoantibodies may be triggered by various factors including infections, malignancies, and medicinal substances. The autoantibodies bind to annexin 5, prothrombin, proteins C and S, interfering with the coagulation cascade [10], and potentiate platelet aggregation. Triggering the activation of endothelial cells may result in the development of endothelial dysfunction, which is one of the main pathogenic factors for the development of APS [11, 12]. Mediators secreted by damaged endothelial cells can serve as additional factors that aggravate the severity of the disease.
This study aimed to investigate the spectrum of APS-associated peripheral blood autoantibodies in women with recurrent pregnancy loss.
Material and methods
A total of 561 women of reproductive age was examined at the D.O. Ott Research Institute of Obstetrics, Gynecology, and Reproductology. The women were divided into two groups: group 1 (n = 76) included somatically healthy women with normal pregnancies, and group 2 (n = 485) comprised women, who were diagnosed with recurrent pregnancy loss. The age of women ranged from 25 to 47 (mean 32 ± 5.5) years. The inclusion criteria for the group I were the first trimester of pregnancy and the absence of pregnancy complications. The criteria for inclusion in group II were the first trimester of pregnancy and a history of threatened miscarriage or pregnancy loss. All women underwent clinical and laboratory examination, including complete blood count, urinalysis, biochemical, coagulation tests, and bacteriological studies. Exclusion criteria in both groups were diabetes mellitus, endocrine disorders, genetic factors of recurrent miscarriage, and uterine malformations.
Laboratory testing included measurement of serum levels of autoantibodies (IgG/IgM) to cardiolipin, phosphatidylserine, phosphatidylinositol, phosphatidic acid, beta2-glycoprotein-I, annexin 5, and prothrombin using enzyme-linked immunosorbent assay (ELISA) kits by Orgentec Diagnostika GmbH (Germany). The content of endothelial dysfunction markers (soluble intercellular adhesion molecule-1 (sICAM-I), endothelin-1, thrombomodulin, and von Willebrand factor) were determined by ELISA using sICAM-I BD (USA), ENDOTHELIN (1–21 ) (Biomedika, Austria), Thrombomodulin (USCN Life Science, USA) and Technozym vWF:Ag ELISA (Technoclone GmbH, Austria) kits, respectively. During testing, standard samples were used to build calibration curves and control samples. Testing results were registered instrumentally by measuring the optical density on an ELx808 microplate ELISA analyzer manufactured by BioTek Instruments Inc. (USA) at a wavelength of 450 nm. Testing results were evaluated by the manufacturer‘s recommendations. The lupus anticoagulant was determined on an ACL Elit PRO coagulometer (Instrumentation Laboratory, Spain) using reagents from Siemens (Germany).
Statistical analysis was performed with STATISTICA Windows 7.0 software using nonparametric statistics (Mann-Whitney test). Differences between the groups were considered significant at p <0.01 and p <0.001. The relation between variables was assessed with a nonparametric Spearman correlation coefficient, and the strength of the correlation was defined as follows: over 0.7 - stronger, from 0.3 to 0.7 - moderate, less than 0.3 - weak.
Results
Autoantibodies were found in 31.7% of patients with recurrent pregnancy loss (n = 485), while no autoantibodies were detected in the women with normal pregnancies (n = 76). Among patients with recurrent pregnancy loss, antibodies to cardiolipin were found in 14.3% of patients (n = 154), while an isolated increase in antibodies to cardiolipin was observed in 27.3% of cases; in 72.7% this was combined with other types of autoantibodies. Antibodies to phosphatidylserine, phosphatidylinositol and phosphatidic acid were detected in 7.1% of patients, there was no isolated increase in these autoantibodies, and in all cases, there was a combination with other types of autoantibodies. Antibodies to beta-2 glycoprotein-I were detected in 37.1% of patients both in isolation (56.1%) and combined (43.9%). Antibodies to annexin 5 were detected in 29.9% of patients both in isolation (71.7%) and combined with other antibodies (28.3%). Lupus anticoagulant was detected in 42.8% of patients, including 75.7% isolated and 24.3% combined (Fig. 1).
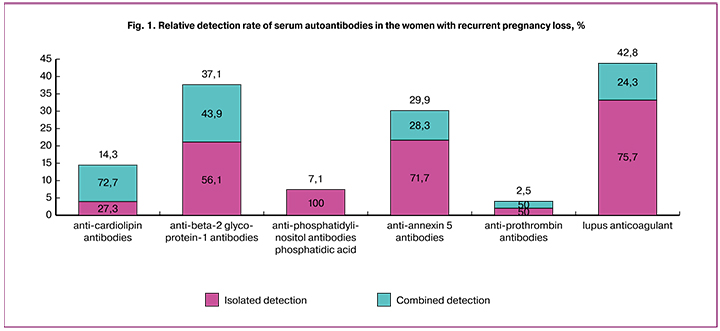
Combinations of autoantibodies were assessed depending on their titers. Women with recurrent pregnancy loss (n = 63) were subdivided into three groups with low (10–20 U/ml), medium (20–40 U/ml) and high titer (more than 40 U/ml) of autoantibodies to cardiolipin and beta-2 glycoprotein-I. Low, medium and high titers of autoantibodies were detected in 66.6%, 20.7%, and 12.7% of patients, respectively. It was noted that 30.1% of women with low autoantibody titers, along with antibodies to cardiolipin and beta-2 glycoprotein-I, also had antibodies to annexin 5, prothrombin, phosphatidylserine, phosphatidylinositol, phosphatidyl acid, and lupus anticoagulant; among patients with medium and high titers of autoantibodies these figures were 38.5% and 75.0%, respectively.
An assessment of endothelial dysfunction markers showed that blood levels of thrombomodulin and von Willebrand factor in women with recurrent pregnancy loss were higher than in women with normal pregnancies. Among women with recurrent pregnancy loss, levels of these markers were higher in those who had compared with those who had not had autoantibodies (Fig. 2). However, the Spearman correlation between antibodies to beta-2 glycoprotein-I and thrombomodulin was weak (r = 0.47, p < 0.05).
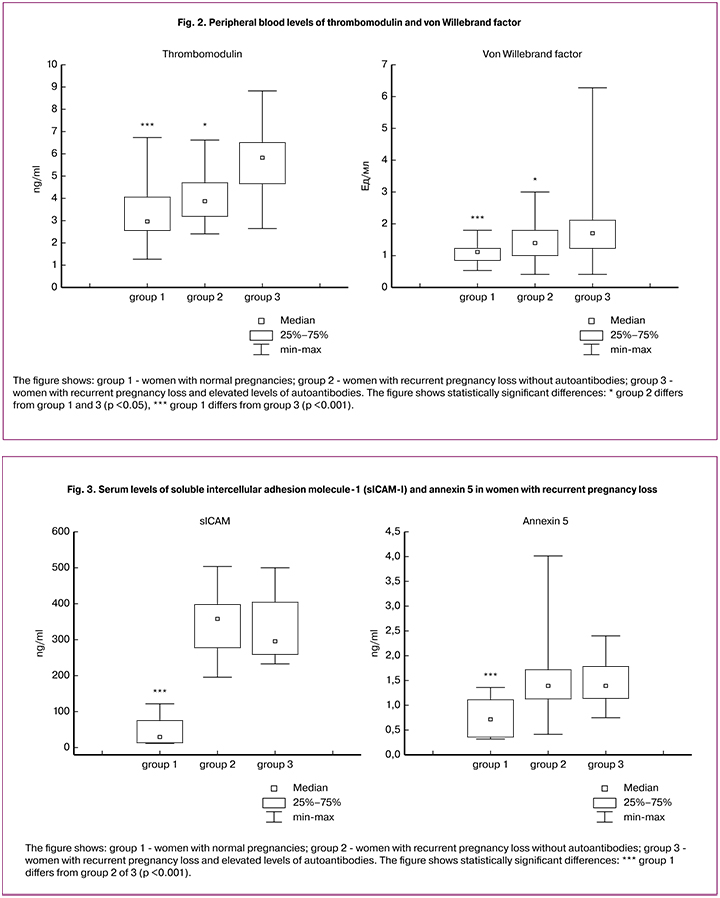
The peripheral blood level of soluble intercellular adhesion molecule-1 (sICAM-I) and serum annexin 5 in women with recurrent pregnancy loss was higher than in women with normal pregnancies. No significant differences were found between groups of women with recurrent pregnancy loss with and without autoantibodies in terms of sICAM-I and serum annexin 5 levels (Fig. 3). The blood levels of endothelin-1 did not differ significantly between groups of women with recurrent pregnancy loss and women with normal pregnancies.
Discussion
APS, as a condition with a different spectrum of clinical manifestations, has been studied for over 35 years. But despite the diversity of accumulated evidence on the alleged role of antiphospholipid antibodies obtained in experimental models [13] and clinical studies [14], there is still no convincing answer to the question of the relationship between them and miscarriage [15].
Our study showed that no autoantibodies were detected in women with normal pregnancies. At the same time, they were found in 31.7% of women with recurrent pregnancy loss, which shows their obvious involvement in the condition leading to pregnancy loss. Among the identified autoantibodies, the most prevalent were lupus anticoagulant and antibodies to beta-2 glycoprotein-I. In low and medium titers, antiphospholipid antibodies were determined mainly in isolation, whereas their high titers were found in combination with other types of autoantibodies in 75% of cases, which may indicate the activity of the autoimmune process as a whole.
Therefore, patients with recurrent pregnancy loss and other forms of autoimmune abnormalities require an in-depth examination. According to the literature, in women with a history of recurrent pregnancy loss and high positive antibody titers, 80% of next pregnancies culminate in a fetal demise [16, 17]. Patients with medium antiphospholipid antibody titers and planning pregnancy require prophylactic antithrombotic therapy. The clinical significance of low antibody titers is dubious and considered only as a risk factor in thrombophilic conditions [18], probably associated with transient instability of cell membranes.
Vascular endothelial cell dysfunction mediated by antiphospholipid antibodies plays a key role in APS pathogenesis. [19]. Activation of endothelial cells by antiphospholipid antibodies increases the expression of adhesion molecules (E-selectin, ICAM-1, VCAM-1) and enhance the synthesis and secretion of pro-inflammatory cytokines and chemokines, such as interleukin -1β and interleukin-6 [11]. There is an increase in the tissue factor expression on endothelial cells, which is one of the main triggers of the coagulation cascade, starting the external coagulation pathway. Activated endothelial cells also exhibit large amounts of von Willebrand factor (which is also a marker of endothelial cell damage). Besides, endothelial cells undergo desquamation of thrombomodulin from their surface [20, 21]. These changes lead to an increase in blood coagulation potential, increasing the risk of thrombosis.
Our study showed that concentrations of endothelial damage markers, such as thrombomodulin, von Willebrand factor, soluble intercellular adhesion molecule-1 (sICAM-I), and serum annexin 5 in women with recurrent pregnancy loss are higher than in women with normal pregnancies. It is also shown that the level of thrombomodulin and von Willebrand factor is higher in women with identified antiphospholipid antibodies. A positive correlation was found between antibodies to beta-2 glycoprotein-I and thrombomodulin. This fact confirms that antiphospholipid antibodies bind to membrane phospholipids of endothelial cells and initiate a cascade of reactions resulting in endothelial damage, procoagulant activity, and an increased risk of thrombosis, which ultimately leads to reproductive losses.
Conclusion
The study findings suggest that autoantibodies play a key role in the abnormalities of the coagulation process associated with recurrent pregnancy loss. They imply the need for new diagnostic tools to identify autoimmune abnormalities and pharmacological agents aimed at preventing the pathological effects of autoantibodies leading to premature termination of pregnancy. Comprehensive management of these disorders may involve intravenous immunoglobulins, coagulation modifiers and anticoagulants (antiplatelet agents, low molecular weight heparins), and therapeutic agents targeting the microcirculation.
References
- Danza A., Ruiz-Irastorza G., Khamashta M. Antiphospohlipid syndrome in obstetrics. Best Pract. Res. Clin. Obstet. Gynaecol. 2012; 26(1): 65-76.
- Решетняк Т.М. Антифосфолипидный синдром: диагностика и клинические проявления (лекция). Научно-практическая ревматология. 2014; 52(1): 56-71. [Reshetnyak TM Antiphospholipid syndrome: diagnosis and clinical manifestations (lecture). Scientific and practical rheumatology. 2014; 52 (1): 56-71. (in Russian)]
- Сидельникова В.М. Подготовка и ведение беременности у женщин с привычным невынашиванием.3-е изд. М.: МЕДпресс-информ; 2013. [Sidelnikova V.M. Preparation and management of pregnancy in women with habitual miscarriage. 3rd ed. M .: MEDpress-inform; 2013. (in Russian)]
- Беляева М.А., Бобров С.А., Лапин С.В. Клинико-иммунологические взаимосвязи при привычном невынашивании беременности и методы их коррекции (обзор). Вестник Северо-Западного государственного медицинского университета им. И.И. Мечникова. 2015; 7(3): 118-23. [Belyaeva M.A., Bobrov S.A., Lapin S.V. Clinical and immunological relationships with recurrent miscarriage and methods for their correction (review). Bulletin of the North-West State Medical University. I.I. Mechnikov. 2015; 7 (3): 118-23. (in Russian)]
- Бицадзе В.О., Хизроева Д.Х., Макацария Н.А., Егорова Е.С., Баймурадова С.М., Машкова Т.Я. Антифосфолипидные антитела, их патогенетическое и диагностическое значение при акушерской патологии. Акушерство, гинекология и репродукция. 2014; 8(2): 39-60. [Bitsadze V.O., Khizroeva D.Kh., Makatsaria N.A., Egorova E.S., Baimuradova S.M., Mashkova T.Ya. Antiphospholipid antibodies, their pathogenetic and diagnostic value in obstetric pathology. Obstetrics, gynecology and reproduction. 2014; 8 (2): 39-60. (in Russian)]
- Шляхтенко Т.Н., Алябьева Е.А., Аржанова О.Н., Сельков С.А., Плужникова Т.А., Чепанов С.В. Антифосфолипидный синдром при невынашивании беременности. Журнал акушерства и женских болезней. 2015; 64(5): 69-76. [Shlyakhtenko, T.N., Alyabieva, E.A., Arzhanova, ON, Selkov, S.A., Pluzhnikova, T.A., Chepanov, S.V. Antiphospholipid syndrome in miscarriage. Journal of Obstetrics and Women’s Diseases. 2015; 64 (5): 69-76. (in Russian)]
- Swadzba J., Sydor W.J., Kolodziejczyk J., Musial J. Summary of the 9th meeting of the European Forum on Antiphospholipid Antibodies. Lupus. 2014; 23(4): 395-9.
- Bertolaccini M.L., Amengual O., Atsumi T., Binder W.L., de Laat B., Forastiero R. et al. “Non-criteria” aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA. Lupus. 2011; 20(2): 191-205.
- Kutteh W.H., Hinote C.D. Antiphospholipid antibody syndrome. Obstet. Gynecol. Clin. North Am. 2014; 41(1): 113-32.
- Meroni P.L., Borghi M.O., Raschi E., Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat. Rev. Rheumatol. 2011; 7(6): 330-9.
- Allen K.L., Fonseca F.V., Betapudi V., Willard B., Zhang J., McCrae K.R. A novel pathway for human endothelial cell activation by antiphospholipid/anti-β2 glycoprotein I antibodies. Blood. 2012; 119(3): 884-93.
- Giannakopoulos B., Krilis S.A. The pathogenesis of the antiphospholipid syndrome. N. Engl. J. Med. 2013; 368(11): 1033-44.
- Tong M., Viall C., Chamley L.W. Antiphospholipid antibodies and the placenta: a systematic review of their in vitro effects and modulation by treatment. Hum. Reprod. Update. 2014; 21(1): 97-118.
- Bertolaccini M.L., Amengual O., Andreoli L., Atsumi T., Chighizola C.B., Forastiero R. et al. 14th International congress on antiphospholipid antibodies task force. Report on obstetric antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun. Rev. 2014; 13(9):917-30.
- Clark C.A., Laskin C.A., Spitzer K.A. Anticardiolipin antibodies and recurrent early pregnancy loss: a century of equivocal evidence. Hum. Reprod. Update. 2012; 18(5): 474-84.
- Simchen M.J., Dulitzki M., Rofe G., Shani H., Langevitz P., Schiff E., Pauzner R. High positive antibody titers and adverse pregnancy outcome in women with antiphospholipid syndrome. Acta Obstet. Gynecol. Scand. 2011; 90(12):1428-33.
- Howard J.A. Recurrent pregnancy loss: causes, controversies, and treatment. New York: CRC Press; 2015.
- Song Y., Wang H.Y., Qiao J., Liu P., Chi H.B. Antiphospholipid antibody titers and clinical outcomes in patients with recurrent miscarriage and antiphospholipid antibody syndrome: a prospective study. Chin. Med. J. (Engl.). 2017; 130(3): 267-72.
- Corban M.T., Duarte-Garcia A., McBane R.D., Matteson E.L., Lerman L.O., Lerman A. Antiphospholipid syndrome: role of vascular endothelial cells and implications for risk stratification and targeted therapeutics. J. Am. Coll. Cardiol. 2017; 69(18): 2317-30.
- Meifang W.U., Suman K., Keith R. Antiphospholipid antibodies stimulate release of endothelial cell microparticles enriched in IL-1β. Blood. 2013; 122: 35. (55th Annual Meeting of the American Society of Hematology)
- Garcia D., Erkan D. Diagnosis and management of the antiphospholipid syndrome. N. Engl. J. Med. 2018; 378(21): 2010-21.
Received 15.06.2018
Accepted 22.06.2018
About the Authors
Chepanov, Sergey V., PhD at the Clinical Laboratory Diagnostics, Laboratory of Clinical Immunology, D.O. Ott Research Institute of Obstetrics, Gynecology, and Reproductology, Saint Petersburg, Russia. 199034 Russia, St. Petersburg, Mendeleyevskaya line 3. Tel.: +78123289850. E-mail: chepanovsv@gmail.comKrivonos, Marina I., obstetrician-gynecologist at the D.O. Ott Research Institute of Obstetrics, Gynecology, and Reproductology, Saint Petersburg, Russia.
199034, Russia, St. Petersburg, Mendeleyevskaya line 3. Tel.: +78123289831. E-mail: mmirashvili@ya.ru
Arzhanova, Olga N., MD, professor, honored physician of the RF, head of the Obstetric Department of Pathology of Pregnancy№11, D.O. Ott Research Institute of Obstetrics, Gynecology, and Reproductology, Saint Petersburg, Russia; professor at the Department of Obstetrics, Gynecology, and Reproductology, Saint Petersburg State University. 199034, Russia, St. Petersburg, Mendeleyevskaya line 3.Tel.: +78123253220. E-mail: arjanova_ olga@mail.ru
Shlachtenko, Tatiana N., PhD, immunologist at the Center for Diagnosis, Prevention and Treatment of Recurrent Pregnancy Loss at Maternity Hospital No. 1, St. Petersburg, Russia. 1199034 Russia, St. Petersburg, 12th line of Vasilievsky Island, 39. Tel.: +78123289850. E-mail: tatnicshl@mail.ru
Saidov, Navruz H., clinical pathologist, researcher at the Laboratory of Biochemistry and Immunology, Research Institute of Obstetrics, Gynecology and Perinatology.
734002, Tajikistan, Dushanbe, Mirzo Tursunzadeh str. 31. Tel.: +992372213656. E-mail: navruz.saidov.1983@mail.ru
Kornyushina, Ekaterina A., obstetrician-gynecologist at the Obstetric Department of Pathology of Pregnancy, senior Researcher at the D.O. Ott Research Institute of Obstetrics, Gynecology, and Reproductology, Saint Petersburg, Russia.
199034, Russia, St. Petersburg, Mendeleyevskaya line 3. Tel.: +78123289882. E-mail: hapacheva@yandex.ru
Chudotvorov, Kirill N., IV year student at the First Pavlov SMU of St. Petersburg.
197022, Russia, Saint Petersburg, L’va Tolstogo str. 6-8. Tel.: +78123387895. E-mail: chudotvorovkn@gmail.com
Sedihin, Vladislav Yu., clinical pathologist at the Biochemistry Laboratory, D.O. Ott Research Institute of Obstetrics, Gynecology, and Reproductology, Saint Petersburg, Russia. 199034, Russia, St. Petersburg, Mendeleyevskaya line 3. Tel.: +78123289850. E-mail: sedihin@mail.ru
Selkov, Sergey A., MD, professor, the honored scientist of the RF, head of the Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute of Obstetrics, Gynecology, and Reproductology. 199034, Russia, St. Petersburg, Mendeleyevskaya line 3. Tel.: +78123289850. E-mail: selkovsa@mail.ru
For citation: Chepanov S.V., Krivonos M.I., Arzhanova O.N., Shlaсhtenko T.N., Saidov N.Kh., Kornyushina E.A., Chudotvorov K.N., Sedihin V.Yu., Selkov S.A. Characteristics of autoantibodies associated with recurrent pregnancy loss. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (3): 72-7. (in Russian)
https://dx.doi.org/10.18565/aig.2019.3.72-77



