Micronutrient status of women with impaired reproductive function in the Northwestern region of Russia
Background: In the modern world, the global population experiences metabolic syndrome pandemic as a result of micronutrient deficiencies. Intake levels of vitamin D, folates and polyunsaturated fatty acids vary in different populations. This issue is understudied among women with reproductive losses and infertility. Objective: To assess the micronutrient status (25-hydroxyvitamin D [25(OH)D], folic acid, omega-3 PUFAs) in the cohort of women in the Northwestern region of Russia, who have various reproductive impairments in history and evaluate relationship between impairments and the parameters of immunological profile. Materials and methods: The study included 299 women who had infertility in history. Their anamnestic data were obtained from the database of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology (St. Petersburg) from 2017 to 2022. The patients were divided into 2 groups depending on their reproductive anamnesis. Group I (n=131) consisted of women with primary infertility. Group II (n=168) was comprised of women with secondary infertility, who had both emergency childbirth and one or more cases of reproductive loss (spontaneous abortion or missed miscarriage) in history. Further, at the stage of pregnancy planning (3 months before getting pregnant), different parameters of the micronutrient status in blood plasma and serum and immunological profile in the peripheral blood were evaluated in these groups of women. The micronutrient status was evaluated using the following techniques: chemiluminescent microparticle immunoassay was used to assess 25(OH)D circulating form of vitamin D levels in serum samples; chemiluminescent microparticle immunoassay (CMIA) was used to evaluate plasma homocysteine levels (the Laboratory of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology); and gas chromatography-mass spectrometry was used to perform the Omega-3 Index test (the laboratory “Hemotest”). The assessment of immunological profile was performed by measuring the functional activity and the level of natural killer (NK) cells (the percentage (%) of NK cells (CD3-CD(16+56)+, the percentage (%) of NKT (CD3+CD(16+56)+, spontaneous NK cell activation (CD107a), activation of NK cells (CD107a) by flow cytometry. Antiphospholipid antibodies – lupus anticoagulant, antibodies against cardiolipin, β2-glycoprotein, phosphatidylserine, phosphatidylic acid, phosphatidylinositol, annexin, prothrombin and the level of a human chorionic gonadotropin IgG (hCG IgG) antibodies were measured by ELISA (in the Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology). The following target values were determined for the patients of reproductive age: vitamin D≥30 ng/ml; homocysteine concentration <7 µmol/L; Omega-3 Index ≥ 8%. Results: In the group of women with primary infertility vitamin D level (n=55) was 31.90 (20.65; 40.75) ng/ml, whereas in the group with secondary infertility (n=74) it was 24.70 (18,00; 34,00) ng/ml. In both groups, median values of homocysteine were above target values and reached 7.7(6.0; 9.0) μmol/L (n=79) and 7.3(5.9; 3.6) μmol/L (n=100) in group I and group II, respectively. Omega-3 Index was 6,5 (5.1; 7.6)% in the group of women with primary infertility (n=14), and 5,2 (4,4; 5,9)% in the group of women with secondary infertility (n=42). However, there was no statistical difference between the groups (p<0.062). There was no statistically significant difference in the levels of vitamin D and homocysteine between the groups. Due to this, the subsequent analysis of these parameters was performed entirely regardless of the type of infertility in the general cohort of patients. Thus, in the general cohort of patients with infertility, vitamin D deficiency (<20 ng/ml) was in 38/129 (29,46%) women, insufficiency (20–30 ng/ml) was in 35/129 (27.13%), normal level was in 56/129 (43.41%) women. The target level of homocysteine (<7 μmol/L) was in 79/179 (44.13%) women, and non-target values (>7 μmol/L) were in 100/179 (55.87%) women. Omega-3 Index was critically low (<4%) in 5/56 (8.93%) women, insufficient (4–8%) in 46/56 (82.14%), and optimal (>8 %) in 5/56 (8.93%) women. Correlation analysis showed direct relationship between the mean values of vitamin D and Omega-3 Index (rs=0.5; p<0,01) and the level of homocysteine and human chorionic gonadotropin (hCG) antibodies (rs=0.5 p<0.01), as well as inverse relationships between vitamin D and immunological parameters: β2-glycoprotein antibodies (rs=-0.34; p=0,04), percentage (%) of NK (rs=-0.4; p=0.03); and Omega-3 Index and cardiolipin antibodies (rs=-0.47 p=0.03). There was no significant correlation between the other values. It has been shown, that the value of Omega-3 Index changes depending on the level of vitamin D (H=12.5; p=0.002). So, in women with vitamin D deficiency, the median level of Omega-3 Index was the lowest and reached 4.2 (3.8; 4.6) %, and was statistically significantly lower than in women with vitamin D insufficiency and reached 5.5 (4.6; 7.1)% (p=0.012), and it was lower than in patients with normal level of vitamin D, and this value was the highest and reached 5.85 (5.2; 6.9)% (p=0.002). In women with serum vitamin D level <20 ng/mL, there was a risk of identifying critically low level of Omega-3 level (OR=14.4 (1.23–168.51), p<0.05). Conclusion: According to the data in our study, the cohort of women with reproductive failures and infertility had a high prevalence of vitamin D deficiency, high level of homocysteine and low Omega-3 Index. Correlations between the micronutrient status and immunological profile were identified, which may be a factor that contribute to reproductive dysfunction.Bespalova O.N., Zhernakova T.S., Shengelia M.О., Zagaynova V.A., Pachulia O.V., Kogan I.Yu.
Keywords
It has been proven that micronutrients (vitamins and minerals) are absolutely essential for the normal metabolism and all vital functions of human beings. According to the WHO, the global population is experiencing metabolic pandemic in the XXI century [1]. More than 2 billion people suffer from “hidden hunger” and deficiency of vitamins and other micronutrients due to malnutrition, sedentary and unhealthy lifestyle and environmental factors. So, combined deficiency of three of more vitamins is observed in more than 70% of women in the Russian Federation [2].
It is well known that modern medicine pursues the trend towards personification of treatment methods and prevention of diseases, and this directly refers to pregravidary preparation. Adequate vitamins supply to the human body plays an important role in maintaining couples’ reproductive health for successful conception, the course of pregnancy and childbirth. There are no large scale, multicenter, epidemiological studies In Russia, evaluating the prevalence of micronutrient deficiencies and insufficiencies in different geographic latitudes. The level of micronutrients may vary considerably in different populations due to differences in climate zones, ethnicity, food culture and somatic diseases. The issue vitamin status correction among women with reproductive losses and infertility has been understudied.
Thus, the objective of our study was to assess the prevalence of micronutrient deficiency (vitamin D, Omega-3 polyunsaturated fatty acids (PUFAs) and homocysteine) and their relationship with immunological parameters (functional activity and the level of NK cells in the peripherial blood, cardiopin antibodies, β2-glycoprotein antibodies, lupus anticoagulant, antibodies against phosphatidylserine, phosphatidylinositol, phosphatidylic acid, annexin V, prothrombin, human chorionic gonadotropin antibodies (hCG) (IgG)) in women planning pregnancy, who had infertility in history in the Northwestern region of Russia.
Currently, vitamin D in the form of a fat-soluble steroid hormone 25-hydroxyvitamin D [25(OH)D is a nutrient, which is most commonly studied and discussed in the scientific world. 80% of vitamin D is endogenously produced in the skin in response to ultraviolet B (UV-B) exposure as a result of conversion of 7-dehydrocholesterol, and 20% enters the body from food. The presence of vitamin D receptors (VDRs) in ovarian tissue, endometrium, fallopian tubes, placenta, and pituitary gland suggests that vitamin D is an important regulator of women’s reproductive health [3]. Vitamin D has several autocrine and endocrine functions in the reproductive system including regulation of endometrial proliferation and gene expression involved in endometrial receptivity [4, 5]. Vitamin D plays an important role in the mechanism of immunomodulation at the mother-fetus interface. To a certain extent, vitamin D changes the correlation between Th1/Th2 and regulatory T-cells/Th17, as well as influences NK cell activity and production of cytokines for reduction of recurrent reproductive losses. Moreover, vitamin D modulates primordial follicle recruitment through regulation of anti-Müllerian hormone (AMH) production [6, 7].
The experts assess deficiency of vitamin D as a new pandemic in XXI century [8]. Vitamin D status is defined by assessment of circulating serum 25(ОН)D levels using chemiluminescent microparticle immunoassay.
Serum is classified according to clinically established levels of vitamin D deficiency (<20 ng/mL), insufficiency (20–30 ng/mL), and saturation (>30 ng/mL) [9]. The standard threshold values are based on bone health in the general population and are not applicable for the preconception period. According to foreign authors, 25(ОН)D level at the stage of pregnancy planning should reach 40–60 ng/mL for the couple, who faced infertility and miscarriage. This level should be maintained at the same level during all trimesters of pregnancy [10]. In our study, the level of 25(ОН)D <30 ng/mL was chosen as the criterion for insufficiency.
The other equally popular and best known nutrient for its protective effect in prevention of cardiovascular complications are Omega-3 PUFAs. Almost half a century has passed since PUFAs were discovered for the first time, and Omega-3 remains the subject of intense research. It has been proven that sufficient intake of Omega-3 PUFAs is associated with a low risk of anovulation, increases the chances for conceiving, reduces the occurrence of preeclampsia, positivele influences the development of the immune system, fetal brain, and the cognitive functions of the child in future. At the same time, according to the results of an independent survey in Russia, the percentage of pregnant women taking Omega-3 PUFAs does not exceed 1% [11]. The Omega-3 Index helps to measure the level of omega-3 polyunsaturated fatty acids in human body.
The Omega-3 Index is a total percentage of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) contents of total quantity of fatty acids in the erythrocyte membrane, which is identified by gas chromatography-mass spectrometry (GC-MS). According to global studies, the target range for the Omega-3 Index during pregnancy is 8-11%. The upper limit of the range is defined as 11%, since this value does not significantly reduce the level of arachidonic acid (Omega-6) [12, 13]. It has been proven that arachidonic acid deficiency is associated with a higher risk of neonatal diseases and mortality in preterm infants [14]. Thus, it is necessary to maintain a balance between Omega-3 and Omega-6, and the optimal ratio should be 1:5 to 1:10. In this study, the target Omega-3 Index was ≥8%.
The third parameter, which is widely used in the practice of the obstetricians, is homocysteine. It reflects folate status in patients.
Homocysteine is a sulfur-containing amino acid formed during the metabolism of the essential amino acid – methionine – obtained from the daily diet. Hyperhomocysteinemia is a multifactorial pathological condition caused by genetic abnormalities (methylenetetrahydrofolate reductase (MTHFR) gene mutation), dietary factors (insufficient intake of folic acid, vitamins B6 and B12) and other extragenital pathologies. It has been shown that hyperhomocysteinemia is associated with pregnancy complications: recurrent miscarriage, preeclampsia, preterm births, placental abruption, fetal growth restriction and gestational diabetes [15]. According to general population studies, the value of low homocysteine level is 5 µmol/L, and the values exceeding 15 µmol/L are identified as hyperhomocysteinemia. In normal pregnancy, homocysteine level is lower than in the absence of pregnancy. This is due to hemodilution and partially due to fetal uptake of homocysteine. The normal values of homocysteine during pregnancy are: 3.9–7.3 µmol/L before 16 weeks; 3.5–5.3 µmol/L between 20–24 weeks and 3.3–7.5 µmol/L after 36 weeks of pregnancy [16]. In 2021, the results of the prospective cohort study were published, which showed reliable association between homocysteine level in the follicular fluid and embryo quality; homocysteine level <9.8 µmol/L was associated with good quality embryos [17].
Moreover, it was noted, that serum homocysteine level was statistically significantly higher in women with recurrent reproductive losses versus healthy women in the control group [18]. In a recent study, the researchers from USA demonstrated that women with reproductive losses in history and the median values of plasma homocysteine concentration 8.0 µmol/L are at higher risk of recurrent pregnancy loss versus the median values 6.0 µmol/L in female population in the USA (RR 1.43; CI 1.08–1.89). It should be noted that sampling of women was at the stage of pregnancy planning, and these women took 400 mcg folic acid per day [19]. Given the results of foreign researchers, in our study the target cut-off value of homocysteine at the stage of pregravidary preparation was 7 µmol/L.
Materials and methods
The study included 299 women who had infertility in history. Their anamnestic data were obtained from the database of the Department of Immunology Intercellular Communication and the Department of Assisted Reproductive Technologies, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology (St. Petersburg) from 2017 to 2022. The patients were divided into 2 groups depending on their reproductive anamnesis. Group I (n=131) consisted of women with primary infertility. Group II (n=168) was comprised of women with secondary infertility, who had both emergency childbirth and one or more cases of reproductive loss (spontaneous or missed miscarriage) in history.
In the general cohort of women with reproductive impairments (n=299), the mean age was 34.1 (4.4) years; body mass index (BMI) was 22.3 (4.1) kg/m2. Gynecologic pathology in patients with reproductive losses in history (primary or secondary infertility) was the following: pelvic inflammatory disease in 94/299 (31,4%) women, extragenital endometriosis and adenomyosis in 91/299 (30,4%); polycystic ovary syndrome with/without anovulation in 44/299 (14,4%); glandular fibrous endometrial polyps in 40/299 (13,3%) women.
In the group of women with secondary infertility, the incidence of emergency childbirth was in 20/168 (11.9%) women with emergency births in history and in 148/168 (88.1%) women with reproductive losses in history, including 116/148 (78.4%) with missed miscarriage, and 57/148 (38,5%) women with spontaneous abortion before 12 weeks of pregnancy.
Further, at the stage of pregnancy planning (3 months before getting pregnant), different parameters of the micronutrient status in blood plasma and serum and immunological profile in the peripheral blood were evaluated in these groups of women.
The micronutrient status was evaluated using chemiluminescent microparticle immunoassay to assess 25(OH)D circulating form of vitamin D levels in serum samples; chemiluminescent microparticle immunoassay to evaluate plasma homocysteine levels (in the Laboratory of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology); and gas chromatography-mass spectrometry to perform the Omega-3 Index test (in the laboratory “Hemotest”).
The assessment of immunological profile was performed by measuring the functional activity and the level of natural killer (NK) cells (the percentage (%) of NK cells (CD3-CD (16+56)+, % of NKT (CD3+CD(16+56)+, spontaneous NK cell activation (CD107a), activation of NK cells (CD107a) by flow cytometry. Antiphospholipid antibodies – lupus anticoagulant, antibodies against cardiolipin, β2-glycoprotein, phosphatidylserine, phosphatidylic acid, phosphatidylinositol, annexin, prothrombin and сhorionic gonadotropin IgG (hCG IgG) were measured by ELISA (in the Department of Immunology and Intercellular Interactions, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology).
The following target values were determined for the patients of reproductive age: vitamin D≥30 ng/ml; homocysteine <7 <7 µmol/L; Omega-3 Index ≥ 8%.
Additionally, the patients were examined in accordance with the Order No. 107n of 30.08.2012 of the Ministry of Health of the Russian Federation and the Procedure No. 1130 of 20.10.2020. for providing medical care in “Obstetrics and Gynecology” profile.
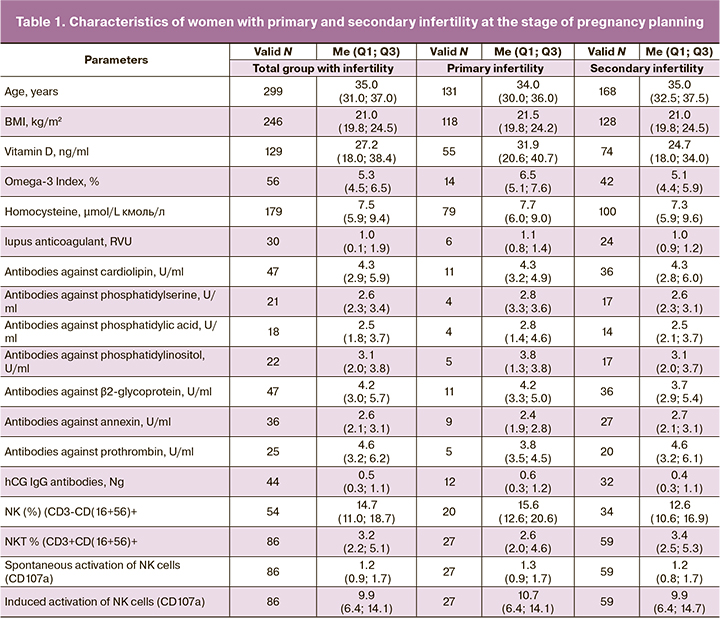
Characteristics of the parameters in patients with infertility are shown in Table 1.
Statistical analysis
Statistical data processing was performed using software program Statistica 10 (StatSoft, Inc.). Shapiro–Wilk test was used to check the normal distribution of data. Mann–Whitney U-test was used for pairwise comparison of the studied parameters. The Kruskal–Wallis H test and Bonferroni correction were used to test multiple comparisons between three samples. Continuous variables were presented as medians and interquartile range Me (Q1; Q3). Correlation analysis was performed using the Spearman's rank correlation coefficient (rs). The strength of relationship was assessed by the Chaddock scale: very weak (0–0.3), week (0.3–0.5), moderate (0.5–0.7), strong (0.7–0.9), very strong (0.9–1).
Pearson's chi-square test (χ²) was used to compare qualitative variables in the groups, and Yates correction was used for small samples. Odds ratio (OR) was used to measure relationship, and the upper and loer limits if significance were calculated with 95% confidence interval (95% CI). Statistical significance was at р<0.05.
Results
In the group of women with primary infertility (n=55), the level of vitamin D was 31.90 (20,65; 40,75) ng/mL. According to the reference values, the median had a normal distribution; while in the group with secondary infertility (n=74), the level of vitamin D was 24.70 (18.00; 34.00) ng/ml and indicated insufficiency. In both groups, the median values of homocysteine levels exceeded the target values – 7.7 (6,0;9,0) µmol/L (n=79) and 7.3 (5.9;3.6) µmol/L (n=100) in group 1 and 2, respectively. Omega-3 Index was 6.5 (5.1; 7.6) % in the group of primary infertility (n=14) and 5.2 (4.4; 5.9) % in the group of secondary infertility (n=42). However, there was no statistically significant difference between the groups (p<0.062). Also, there were no statistically significant difference between the levels of vitamin D in the groups. Due to this, the subsequent analysis of these parameters was performed entirely regardless of the type of infertility in the general cohort of patients.
Thus, in the general cohort of women with infertility, vitamin D deficiency (<20 ng/mL) was in 38/129 (29.46%) women, insufficiency (20–30 ng/mL) was in 35/129 (27.13%), and normal level was in 56/129 (43.41%) women. The study showed, that vitamin D deficiency (<20 ng/mL) was most often observed in women with extragenital endometriosis versus women without this pathology – in 15/31 (48,39%) and 25/97 (25,77%) women (χ²=8,516, p=0,014), respectively. The target level of homocysteine (<7 μmol/L) was in 79/179 (44.13%)women, and non-target values (>7 μmol/L) were in 100/179 (55.87%) women. Omega-3 Index was critically low ( 4%) in 5/56 (8.93%) women, insufficient (4–8%) in 46/56 (82.14%), and optimal (8 %) in 5/56 (8.93%) women (Table 2).
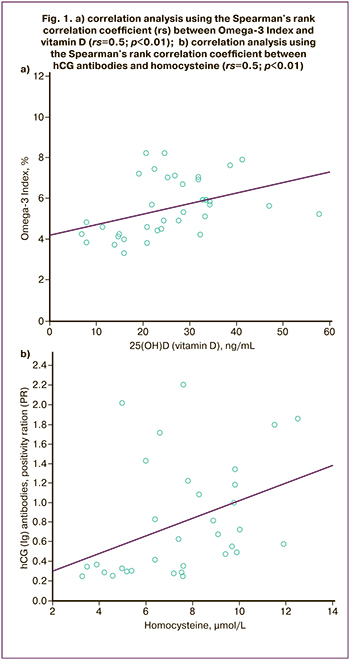
Correlation analysis showed direct average relationship between the values of vitamin D and Omega-3 Index (rs=0.5; p<0.01), and the level of homocysteine and human chorionic gonadotropin (hCG) antibodies (rs=0.5; p<0.01) (Fig.1), as well as inverse relationships between vitamin D and immunological parameters: β2-glycoprotein antibodies (rs=-0.34; p=0,04), NK% (rs=-0.4; p=0.03); and Omega-3 Index and cardiolipin antibodies (rs=-0.47; p=0.03). There was no significant correlation between the other values.
The study showed, that the value of Omega-3 Index changes depending on the level of vitamin D (H=12.5; p=0.002). So, in women with vitamin D deficiency, the median level of Omega-3 Index was the lowest and reached 4.2 (3.8; 4.6)%, and was statistically significantly lower than in women with vitamin D insufficiency and reached 5.5 (4.6;7.1)% (p=0.012), and it was lower than in patients with normal level of vitamin D, and this value was the highest and reached 5.85 (5.2;6.9)% (p=0.002). In women with serum vitamin D level < 20 ng/mL there is a risk of identifying critically low level of Omega-3 level (OR=14.4 (1.23–168.51), p<0.05).
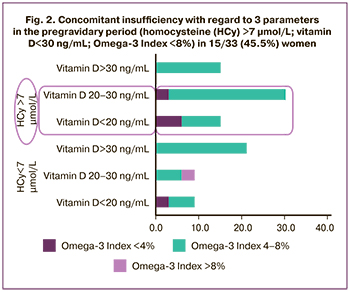
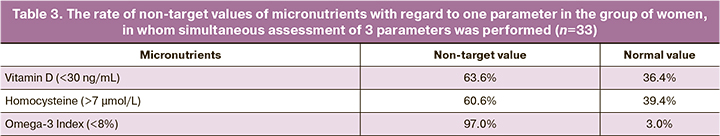
Simultaneous assessment of the levels of vitamin D, homocysteine and Omega-3 Index was performed in 33 out of 299 patients. Concomitant insufficiency with regard to 3 parameters (vitamin D 30ng/mL; Omega-3 Index 8%; homocysteine 7 μmol/L) was found in 15/33 (45,5%) women (Fig. 2); with regard to 2 parameters was in 20/299 (60.6%) cases. Non-target values with regared to one parameter were found: vitamin D (<30 ng/mL) in 21/33 (63,6%) women; homocysteine (>7 μmol/L) in 21/33 (60,6%) women; Omega-3 Index (<8%) in – у 32/33 (97.0%) women (Table 3).
Discussion
The results obtained by us showed a high level of vitamin D deficiency (≤30 ngг/mL) in 56.5% of women, essential fatty acid deficiency (≤8%) in 91%, a high level of homocysteine (>7 μmol/L) in 55.87% of women planning pregnancy, who had infertility and recurrent reproductive losses in history in the Northwestern region of Russia. Concomitant insufficiency with regard to 3 parameters was in 45% of cases.
It should be noted that among women with burdened reproductive anamnesis, pronounced deficiency of vitamin D (<20 ng/mL) was in 29.46% of women. On the other hand, the prevalence of vitamin D deficiency was lower in our study compared to the major part of female population in the Russian Federation, in whom (according to the data of the Russian study, 2021) vitamin D deficiency was 52.65% [20]. This was most likely due to higher intake of supplements containing vitamin D by the group of women planning pregnancy.
Also, according to the data obtained by us, vitamin D deficiency was most often associated with extragenital endometriosis – 43.39% (χ2=8.516, p=0.014). Immunological properties of vitamin D and VDR expression in reproductive tissues are brought up for discussion in many studies, but currently, there is no consensus regarding this issue [21]. In 2020, a systematic review suggested that due to multiple phenotypes in endometriosis, the relationship between vitamin D and the subtypes of endometriosis should be investigated for each subtype separately [22].
The non-target level of homocysteine >7 μmol/L was in more than half of women (55.87%) with reproductive losses and infertility. It is known that folic acid and B vitamins deficiency, and the defects in genes involved in folate cycle lead to excessive accumulation of homocysteine in the blood and impairment of methylation in the cell. Homocysteine has a pronounced toxic effect on the endothelium in blood vessels leading to impaired implantation and a number of obstetric complications [23].
Insufficient Omega-3 Index (<8%) is identified in 91% of women. The obtained data indicate a total deficiency of Omega-3 fatty acids among the patients with reproductive losses and infertility. Medical societies recommend to increase the intake of docosahexaenoic acid (Omega-3 fatty acid) up to a dose of 200 mg/day at the stage of pregnancy planning. Correlation between the intake and consumption levels is far from perfect [24, 25].
In the group with vitamin D deficiency, Omega-3 Index was the lowest and reached 4.2%. This is significantly lower than in the group with deficiency, and significantly lower than in the group with the normal level of vitamin D. Correlation analysis showed linear relationship of average strength between vitamin D and Omega-3 Index. This pattern can be explained by food composition. In particular, fish oil contains high doses of Omega-3 PUFAs and vitamin D, and this shows a good compatibility and utilization of these microelements.
Our study found average inverse relationship between micronutrients and immune parameters: vitamin D and β2-glycoprotein antibodies (rs=-0.34; p=0.04); vitamin D and % of NK cells (rs= -0.4; p=0.03); and Omega-3 Index and cardiolipin antibodies (rs=-0.47; p=0.03). The obtained results did not contradict to the data reported by foreign researchers. In January 2022, the researchers from the USA published a randomized double blind placebo control study. The purpose of this study was to assess, whether the use of vitamin D (2000 IU/day) and Omega-3 PUFAs (1000 mg/day) reduce the risk of autoimmune diseases development (n=25871 patients, including 13 085 women over the age of 55 years). The use of vitamin D during 5 years with or without Omega-3 PUFAs was associated with reduced risk of autoimmune diseases by 33%, while the use of Omega-3 with or without vitamin D reduced this risk by 15% versus without using supplements [26].
In patients with antophospholipid syndrome, vitamin D suppresses expression of antibodies against β2-glycoprotein, and therefore reduces the risk of thrombosis. Moreover, the studies in vitro proved that vitamin D inhibits expression of tissue factor induced by antibodies against β2-glycoprotein [27].
The researchers repeatedly reported on a possible regulatory role of vitamin D in relation to peripheral NK cells. Thus, significant differences were found between the levels of CD56+ blood NK cells and the levels of vitamin D <30ng/mL and >30 ng/Ml in patients with recurrent reproductive losses. With insufficient level of vitamin D, siginificantly increased cytotoxicity of peripherial NK cells was noted. It was successfully corrected by intake of vitamin D and had a dose-dependent effect [28].
The immunomodulatory properties of Omega-3 are widely known, and realized through the incorporation of fatty acids into membrane phospholipids and the synthesis of pro- and anti-inflammatory eicosanoids. Cell membrane containing a high level of Omega-3 PUFAs has a protective, anti-inflammatory and indirectly antioxidant function. In addition, the activity of fatty acids is mediated through peroxisome proliferator-activated receptors, the receptors associated with G-proteins, and CD1 receptors. It has been shown that activation of nuclear receptors reduces NK cell cytotoxicity due to low interferon-gamma (IFN-γ) gamma) secretion [29], and also inhibits the activity of macrophages, production of tumor necrosis factor, interleukins-1 and -6 [30].
In addition to the relationship between vitamin D, Omega-3, and immunological parameters, the study found a direct average relationship between homocysteine and hCG antibodies (rs=0.5; p<0.01).
According to some published data, hyperhomocysteinemia is a trigger for the development of autoimmune processes impairing the normal course of pregnancy [31].
Thus, the vitamins and micronutrients play an important role in functioning of both the innate and adaptive immunity. They are cofactors of various biochemical reactions. They prevent the development of fetal defects and pregnancy complications.
It should be noted that there was a number of limitations in our study: the sample was selected only among women with reproductive losses and infertility and did not include healthy fertile control group. Moreofver, the use of folic acid supplements, vitamin B6 and B12, which could change homocysteine level was not studied. A significant proportion of women could have taken folic acid, vitamin D, Omega-3 PUFAs, as well as prenatal multivitamins, and their itake could distor the results of our study.
Taking into account the obtained results and the specific features of patients with reproductive losses and infertility, high incidence of impairments of immunological profile in this group of patients, we suggest that further research should be devoted to thorough investigation of micronutrient status and immunological parameters in the randomized controlled trials.
Conclusion
According to the data obtained in our study, the cohort of women with reproductive failures and infertility had a high prevalence of vitamin D deficiency, high level of homocysteine and low Omega-3 Index. Correlations between the micronutrient status and immunological profile were identified, which may be a factor that contribute to reproductive dysfunction.
References
- https://www.who.int/ru/news-room/fact-sheets/detail/obesity-and-overweight
- Балан В.Е., Тихомирова Е.В., Овчинникова В.В. Микронутриентная поддержка женщин во время беременности. РМЖ. Мать и дитя. 2019; 2(4): 280-5. [Balan V.E., Tikhomirova E.V., Ovchinnikova V.V. Micronutrient supply during pregnancy. Russian Journal of Woman and Child Health. 2019; 2(4):280-5.(in Russian)].
- Беспалова О.Н., Баклейчева М.О., Ковалева И.В., Толибова Г.Х., Траль Т.Г., Коган И.Ю. Экспрессия витамина D и его рецепторов в ворсинчатом хорионе при неразвивающейся беременности. Акушерство и гинекология. 2019; 11: 89-96. https://dx.doi.org/10.18565/aig.2019.11.89-96. [Bespalova O.N., Bakleicheva M.O., Kovaleva I.V., Tolibova G.Kh., Tral T.G., Kogan I.Yu. Expression of vitamin d and its receptors in the villous chorion in missed miscarriage. Obstetrics and Gynecology. 2019; 11: 89-96. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.11.89-96.
- Ekapatria C., Hartanto B., Wiryawan P., Tono D., Maringan Diapari Lumban T., Meita D. et al. The effects of follicular fluid 25(OH)D concentration on intrafollicular estradiol level, oocyte quality, and fertilization rate in women who underwent IVF program. J. Obstet. Gynaecol. India. 2022; 72(Suppl. 1): 313-8. https://dx.doi.org/10.1007/s13224-021-01615-6.
- Cozzolino M. Is it realistic to consider vitamin D as a follicular and serum marker of human oocyte quality? J. Assist. Reprod. Genet. 2019; 36(1): 173-4.https://dx.doi.org/10.1007/s10815-018-1344-9.
- Gouvea T.M., Cota E., Souza L.A., Lima A.A. Correlation of serum anti-Mullerian hormone with hormonal and environmental parameters in Brazilian climacteric women. Sci. Rep. 2022; 12(1): 12065. https://dx.doi.org/10.1038/s41598-022-15429-7.
- Merhi Z.O., Seifer D.B., Weedon J., Adeyemi O., Holman S., Anastos K. et al. Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductive-aged women: Women’s Interagency HIV Study. Fertil. Steril. 2012; 98(1): 228-34. https://dx.doi.org/10.1016/j.fertnstert.2012.03.029.
- Kowalówka M., Główka A.K., Karaźniewicz-Łada M., Kosewski G. Clinical significance of analysis of vitamin D status in various diseases. Nutrients. 2020; 12(9): 2788. https://dx.doi.org/10.3390/nu12092788.
- Khan B., Shafiq H., Abbas S., Jabeen S., Khan S.A., Afsar T. et al. Vitamin D status and its correlation to depression. Ann. Gen. Psychiatry. 2022; 21(1): 32. https://dx.doi.org/10.1186/s12991-022-00406-1.
- Vanni V.S., Vigano' P., Somigliana E., Papaleo E., Paffoni A., Pagliardini L., Candiani M. Vitamin D and assisted reproduction technologies: current concepts. Reprod. Biol. Endocrinol. 2014; 12: 47. https://dx.doi.org/10.1186/1477-7827-12-47.
- Громова О.А., Торшин И.Ю. Витамины и минералы: между Сциллой и Харибдой: о мисконцепциях и других чудовищах. М.: МЦНМО; 2013. 764с. [Gromova O.A., Torshin I.Yu. Vitamins and minerals: between Scylla and Charybdis: about misconceptions and other monsters. Moscow; 2013. 764 p. (in Russian)].
- Von Schacky C. Omega-3 fatty acids in cardiovascular disease–an uphill battle. Prostaglandins Leukot. Essent. Fatty Acids. 2015; 92: 41-7. https://dx.doi.org/10.1016/j.plefa.2014.05.004.
- Von Schacky C. [Confusion about the effects of omega-3 fatty acids: Contemplation of study data taking the omega-3 index into consideration]. Internist (Berl.). 2019; 60(12): 1319-27. (in German). https://dx.doi.org/10.1007/s00108-019-00687-x.
- Fares S., Sethom M.M., Hammami M.B., Cheour M., Feki M., Hadj-Taieb S., Kacem S. Postnatal RBC arachidonic and docosahexaenoic acids deficiencies are associated with higher risk of neonatal morbidities and mortality in preterm infants. Prostaglandins Leukot. Essent. Fatty Acids. 2017; 126: 112-6.https://dx.doi.org/10.1016/j.plefa.2017.09.015.
- Баранов В.C., ред. Генетический паспорт – основа индивидуальной и предиктивной медицины. СПб.: Н-Л; 2022. 528с. [Baranov V.C., ed. The genetic passport is the basis of individual and predictive medicine. St.Petersburg: Liters; 2022. 528 p. (in Russian)].
- Dai C., Fei Y., Li J., Shi Y., Yang X. A novel review of homocysteine and pregnancy complications. Biomed. Res. Int. 2021; 2021: 6652231.https://dx.doi.org/10.1155/2021/6652231.
- Razi Y., Eftekhar M., Fesahat F., Dehghani Firouzabadi R., Razi N., Sabour M., Razi M.H. Concentrations of homocysteine in follicular fluid and embryo quality and oocyte maturity in infertile women: a prospective cohort. J. Obstet. Gynaecol. 2021; 41(4): 588-93. https://dx.doi.org/10.1080/01443615.2020.1785409.
- Li J., Feng D., He S., Wu Q., Su Z., Ye H. Meta-analysis: association of homocysteine with recurrent spontaneous abortion. Women Health. 2021; 61(7): 713-20. https://dx.doi.org/10.1080/03630242.2021.1957747.
- DeVilbiss E.A., Mumford S.L., Sjaarda L.A., Connell M.T., Kim K., Mills J.L. et al.Preconception folate status and reproductive outcomes among a prospective cohort of folate-replete women. Am. J. Obstet. Gynecol. 2019; 221(1): 51.e1-51.e10. https://dx.doi.org/10.1016/j.ajog.2019.02.039.
- Суплотова Л.А., Авдеева В.А., Пигарова Е.А., Рожинская Л.Я., Трошина Е.А. Дефицит витамина D в России: первые результаты регистрового неинтервенционного исследования частоты дефицита и недостаточности витамина D в различных географических регионах страны. Проблемы эндокринологии. 2021; 67(2): 84-92. https://dx.doi.org/10.14341/probl12736.. [Avdeeva V.A., Suplotova L.A., Pigarova E.A., Rozhinskaya L.Y., Troshina E.A.Vitamin D deficiency in Russia: the first results of a registered, non-interventional study of the frequency of vitamin D deficiency and insufficiency in various geographic regions of the country. Problems of Endocrinology. 2021; 67(2):84-92. (in Russian)]. https://dx.doi.org/10.14341/probl12736.
- Ярмолинская М.И., Денисова А.С., Толибова Г.Х., Беспалова О.Н., Траль Т.Г., Закураева К.А., Пьянкова В.О. Анализ экспрессии рецепторов витамина D у больных наружным генитальным эндометриозом. Акушерство и гинекология. 2021; 3: 117-23. https://dx.doi.org/10.18565/aig.2021.3.117-123. [Yarmolinskaya M.I., Denisova A.S., Tolibova G.Kh., Bespalova O.N., Tral T.G., Zakuraeva K.A., Pyankova V.O. Analysis of vitamin D receptor expression in patients with genital endometriosis. Obstetrics and Gynecology. 2021; 3:117-23. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.3.117-123.
- Kalaitzopoulos D.R., Lempesis I.G., Athanasaki F., Schizas D., Samartzis E.P., Kolibianakis E.M., Goulis D.G. Association between vitamin D and endometriosis: a systematic review. Hormones (Athens). 2020; 19(2): 109-21. https://dx.doi.org/10.1007/s42000-019-00166-w.
- Balint B., Jepchumba V.K., Guéant J.L., Guéant-Rodriguez R.M. Mechanisms of homocysteine-induced damage to the endothelial, medial and adventitial layers of the arterial wall. Biochimie. 2020; 173: 100-6. https://dx.doi.org/10.1016/j.biochi.2020.02.012.
- https://www.euro.who.int/__data/assets/pdf_file/0006/314493/Good-maternal-nutrition-The-best-start-in-life-rus.pdf
- МАРС. Междисциплинарная ассоциация специалистов репродуктивной медицины. Прегравидарная подготовка. Клинический протокол. Версия 2.0. М.: Редакция журнала StatusPraesens; 2020. 128с. [IARS. Interdisciplinary Association of Reproductive Medicine Specialists. Pregravidar preparation. Clinical protocol. Version 2.0. Moscow: Editorial office of the journal StatusPraesens; 2020. 128 p. (in Russian)]
- Hahn J., Cook N.R., Alexander E.K., Friedman S., Walter J., Bubes V. et al. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ. 2022; 376: e066452.
- Agmon-Levin N., Blank M., Zandman-Goddard G., Orbach H., Meroni P.L., Tincani A. et al. Vitamin D: an instrumental factor in the anti-phospholipid syndrome by inhibition of tissue factor expression. Ann. Rheum. Dis. 2011; 70(1): 145-50. https://dx.doi.org/10.1136/ard.2010.134817.
- Ota K., Dambaeva S., Han A.R., Beaman K., Gilman-Sachs A., Kwak-Kim J. Vitamin D deficiency may be a risk factor for recurrent pregnancy losses by increasing cellular immunity and autoimmunity. Hum. Reprod. 2014; 29(2): 208-19. https://dx.doi.org/10.1093/humrep/det424.
- Sfakianoudis K., Rapani A., Grigoriadis S., Pantou A., Maziotis E., Kokkini G. et al. The role of uterine natural killer cells on recurrent miscarriage and recurrent implantation failure: from pathophysiology to treatment. Biomedicines. 2021; 9(10):1425. https://dx.doi.org/10.3390/biomedicines9101425.
- El Fadl D.K.A., Ahmed M.A., Aly Y.A., Darweesh E.A.G., Sabri N.A. Impact of Docosahexaenoic acid supplementation on proinflammatory cytokines release and the development of Necrotizing enterocolitis in preterm Neonates: a randomized controlled study. Saudi Pharm. J. 2021; 29(11): 1314-22.https://dx.doi.org/10.1016/j.jsps.2021.09.012.
- Alijotas-Reig J., Esteve-Valverde E., Anunciación-Llunell A., Marques-Soares J., Pardos-Gea J., Miró-Mur F. Pathogenesis, diagnosis and management of obstetric antiphospholipid syndrome: a comprehensive review. J. Clin. Med. 2022; 11(3): 675. https://dx.doi.org/10.3390/jcm11030675.
Received 17.06.2022
Accepted 04.10.2022
About the Authors
Olesya N. Bespalova, Dr. Med. Sci., Deputy Director, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, shiggerra@mail.ru,https://orcid.org/0000-0002-6542-5953, 199034, Mendeleevskaya line, 3, St. Petersburg, Russia.
Tatiana S. Zhernakova, Postgraduate student, Junior Researcher, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, tatazhernakova@gmail.com, https://orcid.org/0000-0002-5131-4363, 199034, Mendeleevskaya line, 3, St. Petersburg, Russia.
Margarita O. Shengelia, Junior Researcher, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, bakleicheva@gmail.com,
https://orcid.org/0000-0002-0103-8583, 199034, Mendeleevskaya line, 3, St. Petersburg, Russia.
Valeriya A. Zagaynova, Postgraduate student at the Department of Assisted Reproductive Technologies, D.O. Ott Research Institute of Obstetrics,
Gynecology and Reproductology, zagaynovav.al.52@mail.ru, https://orcid.org/0000-0001-6971-7024, 199034, Mendeleevskaya line, 3, St. Petersburg, Russia.
Olga V. Pachulia, PhD, Researcher, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, for.olga.kosyakova@gmail.com,
https://orcid.org/0000-0003-4116-0222, 199034, Mendeleevskaya line, 3, St. Petersburg, Russia.
Igor Yu. Kogan, Corresponding Member of RAS, Dr. Med. Sci., Professor, Director of the D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
ovrt@ott.ru, https://orcid.org/0000-0002-7351-6900, 199034, Mendeleevskaya line, 3, St. Petersburg, Russia.
Authors’ contributions: Bespalova O.N. – the concept of the study, writing the text and editing the manuscript; Zhernakova T.S. – review of publications on the topic of the article, writing the text of the manuscript, material collection, statistical data processing; Shengelia M.О. – review of publications on the topic of the article, material collection; Zagaynova V.A., Pachulia O.V. – material collection; Kogan I.Yu. – the concept of the study and editing the manuscript.
Сonflicts of interest: The authors declare that they have no conflict of interests.
Funding: The study was carried out without any sponsorship. The article was prepared for publication within implementation of fundamental research topic of the State Assignment of the Ministry of Science and Higher Education of the Russian Federation No. 1021062512052-5-3.2.2 “Development of diagnostic criteria for predicting and overcoming reproductive losses.”
Ethical Approval: The study was approved by the Ethics Committee of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology.
Patients’Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Bespalova O.N., Zhernakova T.S., Shengelia M.О., Zagaynova V.A.,
Pachulia O.V., Kogan I.Yu. Micronutrient status of women with impaired reproductive
function in the Northwestern region of Russia.
Akusherstovo i Gynecologia/Obstetrics and Gynecology. 2022; 10: 93-102 (in Russian)
https://dx.doi.org/10.18565/aig.2022.10.93-102



