Fetal sex in the development of gestational diabetes mellitus and endothelial dysfunction
Objective. To evaluate the effect of the fetal sex on the levels of certain hormones, neurotransmitters, vasoconstrictors, as well as on the pro- and contrinsular factors, and to reveal the role of the fetal sex in the genesis of gestational diabetes mellitus and the development of endothelial dysfunction.Botasheva T.L., Palieva N.V., Khloponina A.V., Vasiljeva V.V., Zheleznyakova E.V., Zavodnov O.P., Gudz E.B.
Materials and methods. The study included 1101 pregnant women: 517 women with gestational diabetes mellitus and 584 women with normal gestation. Certain metabolism-associated hormones, neurotransmitters, vasoconstrictors, pro- and contrinsular factors were assessed in blood serum of these women with ELISA test.
Results. Male fetus was found to mediate the greatest changes in the levels of prolactin, endothelin-1, angiotensin II, epinephrine, glycosylation-end product receptors, insulin-like growth factor-1 and its transport proteins which contribute to metabolic disorders and the development of endothelial dysfunction in pregnant women.
Conclusion. The fetal sex is an important factor affecting the carbohydrates metabolism and the nature of the hormonal response of the mother’s organism during gestational changes. The fetal sex is a trigger in the development of gestational diabetes and endothelial dysfunction, which cause obstetric complications.
Keywords
Over the past 30 years, there has been a steady trend towards changes in the metabolic profile in pregnant women. This trend can be seen in changing metabolic processes and approaching pathological anabolism, as well as a decrease in adaptive resources, and the failure of compensation mechanisms, which are increasingly associated with gestational hyperglycemia [1–4].
According to the concept of N.L. Garmasheva and N.I. Konstantinova [5], the functional system mother-placenta-fetus (FSMPF) starts developing with the onset of pregnancy; fetal sex is an important characteristic of the fetal subsystem, however, it is not taken into account in this concept. Some studies show that fetal sex can influence adaptation processes in the FSMPF and contribute to the development of obstetric pathology [6, 7]. A number of chromosomal aberrations are known to be associated with fetal sex [8, 9]. Male fetal sex has been recognized as a risk factor for preterm birth [10], placental dysfunction [7, 11], fetal pyelectasis, and intestinal hyperechogenicity [12]. Female fetal sex contributes to toxicosis in the first half of pregnancy and preeclampsia. At the same time, severe forms of preeclampsia more often occur in male fetus pregnancies [13]. According to Scheiner E. et al. (2004) [14] gestational diabetes mellitus (GDM) is more common in male fetus pregnancies. But there is no certainty about all these facts and they require further study.
One of the main pathogenetic mechanisms of GDM development is neurohumoral dysfunction in the body of a pregnant woman. This dysfunction is manifested by relative hyperreactivity of the sympathoadrenal system. Such functioning of the sympathoadrenal system results in increased synthesis of a number of biologically active mediators (adrenaline (Adr), noradrenaline (NorA), and neuropeptide Y (NPY), etc.) [15] directly and through the endothelium (angiotensin II (At II), endothelin-1 (Et-1), etc.). They change vascular tone, causing vasoconstriction, and transforming tissue sensitivity to insulin and mitotic activity of cells through activation of the synthesis of insulin-like growth factors and their carrier proteins [15–20]. These dysfunctional processes affect all elements of the FSMPF and lead to hypoxia, oxidative stress, and the development of obstetric and fetal pathology [2, 4, 15–17]. However, there is very limited scientific data on the role of fetal sex in developing GDM.
The aim of the study is to determine the levels of certain hormones, neurotransmitters, vasoconstrictors, as well as on the pro- and contrinsular factors in the maternal organism depending on the fetal sex, and to reveal the role of the fetal sex in the genesis of gestational diabetes mellitus and the development of endothelial dysfunction.
Materials and Methods
The study was conducted in Rostov Research Institute of Obstetrics and Pediatrics, Rostov State Medical University, Rostov-on-Don, Russia, in 2013–2018. The research was approved by the Local Ethics Committee (Protocol No. 23/1 dated 25.04.2017). Informed consent was obtained from all pregnant women (according to «The rules for conducting high-quality clinical trials in the Russian Federation», dated 29.12.1998).
Group I included 517 women with gestational diabetes mellitus (GDM). Among them, there were 253 female fetus pregnancies (FFP) (group Ia) and 264 male fetus pregnancies (MFP) (group 1b). Group II consisted of 584 women with normal pregnancy (NP) and was divided into group IIa (280, FFP) and group IIb (304, MFP).
Patients from both groups met the same inclusion criteria: single pregnancy and the age of 18-28 years. When women were included in group I, the level of glucose was also taken into account, as it is diagnostic for GDM, according to the criteria established in the clinical guidelines of the Ministry of Health of the Russian Federation dated 17.12.2013 no. 15-4/10/2-9478 «Gestational diabetes mellitus: diagnosis, treatment, postpartum care». Additional inclusion criteria for group II were uncomplicated pregnancy, absence of endocrine pathology and extragenital disease in the stage of decompensation and exacerbation.
Exclusion criteria were multiple and induced pregnancies, chromosomal aberrations and congenital malformations of the fetus, congenital malformations in women, decompensation of extragenital diseases and endocrinopathies (except for GDM in group I), and lack of desire of women to participate in the study.
In order to determine the frequency of GDM detection depending on fetal sex, records of 14,256 pregnant women were analyzed. The patients were distributed in clinical groups using random number and coin methods [18]; the groups were quantitatively comparable. The required number of observations was calculated based on the criteria of the «General theory of statistics» [19]. During the study, we followed the conditions of sequence, multiplicity, and time of conducting the research.
In blood plasma, the basal glucose level was determined using the glucose kit (Bandox, England) by glucose oxidase method; immunoreactive insulin was identified using the enzyme-linked immunoassay method (DRG Insulin ELISA EIA–2935, Germany); then the insulin resistance index of HOMA-IR (fasting glucose (mmol/L)×immunoreactive insulin (mIU/ml)/22.5) was calculated. An index value of more than 2.77 showed the presence of insulin resistance.
Serum levels of certain hormones, neurotransmitters, vasoconstrictors were estimated using enzyme-linked immunoassay method, and pro- and contrinsular factors were determined using «Tecan Sunrise» photometer (Austria). We used the IBL set (Germany) to determine the levels of Adr and NorA (ng/ml), the IDS set (USA) to identify the level of insulin-like growth factor-1 (IGF-1) (mcg/L), the Mediagnost set (Germany) to determine the level of IGF binding protein (IGFBP-1) (ng/ml), R&D Systems kit (USA) to identify the level of receptor for glycosylation end product receptor (RAGE) (pg/ml), AccuBind INC kit (USA) to determine prolactin level (PRL) (ng/ ml), IMMUN DIAGNOSTIK kit (Europe) to identify the level of retinol binding protein (RBP4) (mcg/L), RayBio kit (Europe) to determine At II level (ng/ml), BIOMEDICA GRUPPE kit (Germany) to determine Et-1 level (mmol/ml), BACHEM GROUP kit (USA) to estimate NPY level (ng/ml).
Statistical analysis
Statistical data processing was performed using nonparametric Kruskal–Wallis criteria for independent samples and the Mann-Whitney U–test. The original values were processed using the application program packages MS OFFICE EXCEL 2010 and IBM SPSS 25.0.0.2. The differences in the indicators were considered statistically significant at the significance level of p˂0.05.
Results and Discussion
The assessment of the glycemic status in pregnant women from group I was carried out in the 1st and 2nd trimesters; 124 pregnant women (23.9%) were evaluated in the 1st trimester. Fasting glycemia values in the 1st and 2nd trimesters were significantly higher (p=0.01 and p=0.01, respectively) in group Ib (6.31 mmol/L [5.01–6.38] and 6.64 mmol/L [5.58–6.71], respectively) in comparison with group Ia (1st trimester – 5.48 mmol/L [4.81–5.60] and 2nd trimester – 5.51 mmol/L [5.21–6.03]). However, at the level of tendency, the values in MFPs were higher than in FFPs (1st trimester – 4.55 mmol/L [4.46–5.01] and 4.21 mmol/L [4.08–4.46] (p=0.20), 2nd trimester (4.68 mmol/L [4.29–4.81] and 4.05 mmol/L [3.72– 4.33] (p=0.68), respectively). When assessing the levels of insulin resistance (IR) in clinical groups, no statistically significant differences were found. However, in the 2nd trimester, intergroup comparison of pregnant women with GDM, IR levels were higher (FFP – 10.88 mIU/ml [9.23–12.04] and MFP – 13.62 mIU/ml [12.36–13.85]) than in the control group (FFP – 7.04 mIU/ml [6.45–8.31] and MFP – 7.62 mIU/ml [6.28–9.11]) (p=0.25, p=0.17, respectively). Insulin resistance in the group with GDM in the 2nd trimester was established in 136 patients (26.3%): there were 52 (20.5%) patients of group Ia and 84 (31.8%) women of group Ib; its index (3.58 [3.24–3.96]) was significantly higher in MFP (p=0.02) compared to FFP (2.93 [2.85–3.41]). In other words, it can be suggested that more remarkable shifts in carbohydrate metabolism are observed in MFP.
Over the past years, more and more attention has been paid to the study of central regulators of eating behavior in disorders of carbohydrate metabolism. One of the main structures regulating eating behavior is the melanocortin system, which is located in the hypothalamus arcuate nucleus. The neurons of the melanocortin system synthesize NPY [23]. NPY are classified as orexigenic neuropeptides. Inhibiting neurons that express type 4 melanocortin receptors, it promotes hyperphagia and reduces energy consumption [24], stimulates proliferation and fat deposition, and is an endogenous stabilizer of α-adrenergic receptors. The data in literature indicate that pregnancy promotes active secretion of this neuropeptide by changing the center-peripheral priorities, resulting in the formation of new hormone-producing «units» (decidual tissue, cytotrophoblast, placenta, and syncytioblast) [25]. Due to the gravidal alteration, there is an increased secretion of another important regulator of the energy balance, the feeling of satiety, fat and carbohydrate metabolism, PRL. High levels of this hormone during pregnancy contribute to leptin resistance, insulin resistance, and hyperinsulinemia. Moreover, PRL is responsible for modulating the maternal immunological response to the developing fetus and the amniotic structures that ensure its vital activity [26, 27].
The results of the study showed that the level of NPY is fairly constant during pregnancy and neither fetal sex nor the state of carbohydrate metabolism affect its production. However, they are crucial in the secretion of PRL. In the 1st trimester, a statistically significant predominance of its level in group I was found in MFP by 40.1%, in comparison with FFP; its level was higher in MFP in group I by 70.9% compared to the control group. All pregnant women were characterized by a statistically significant increase in its level before delivery (Table 1).
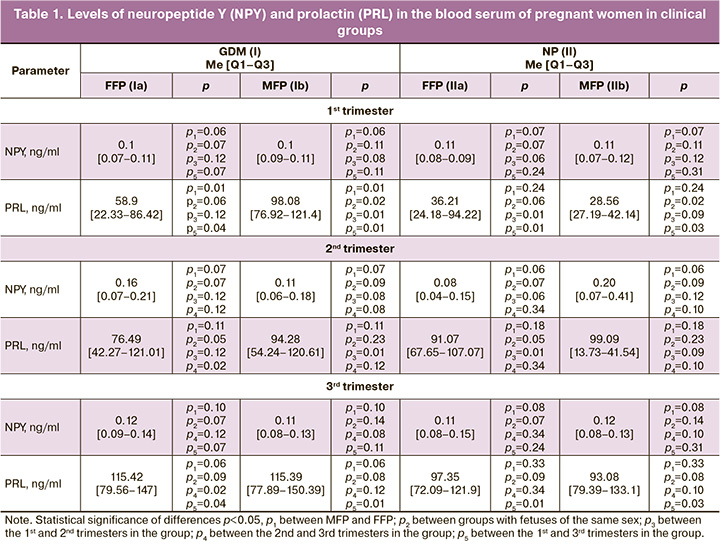
The placenta is not only a barrier organ, but also a gestational gland of internal secretion, where metabolic processes take place actively between the mother and the fetus. It has a different throughput for biological factors; the placenta with the uterine activity composes the utero-placental «pump» that regulates blood supply in the FSMPF [1, 3, 15]. The autonomy of the utero-placental-fetal blood flow is provided by changing the activity of specific substances, namely, vasoconstrictors and vasodilators. In GDM, when sympathoadrenal nervous regulation prevails, this balance shifts and vasoconstrictor synthesis prevails, eventually resulting in potentiation of dysregulation processes and leading to a complicated course of pregnancy [4, 15, 16].
Table 2 shows the levels of important vascular tone regulators (At II, Et-1, NorA, Adr) in the blood of the participants.
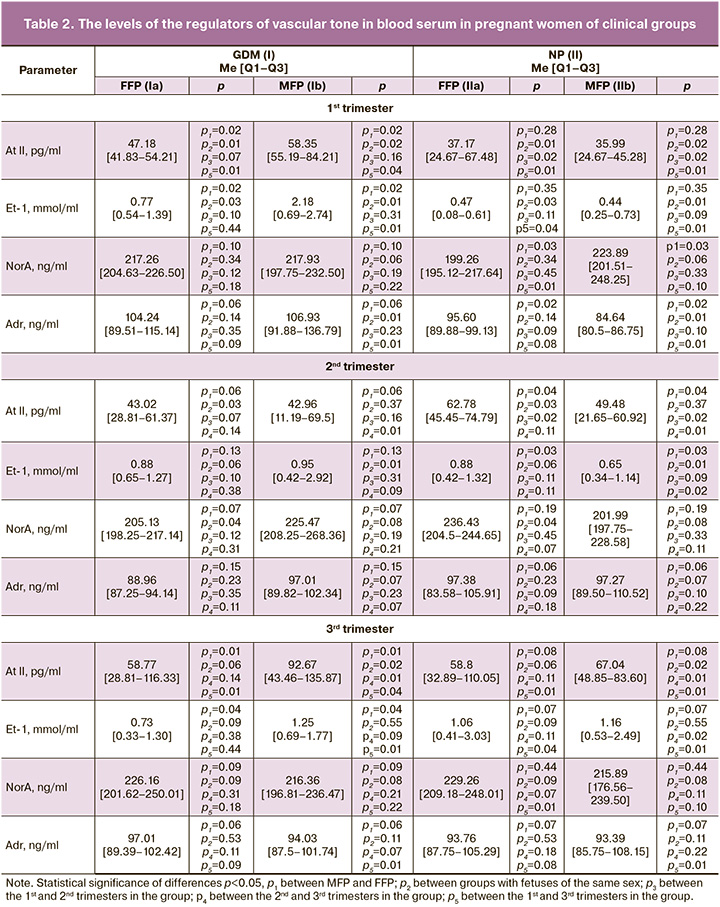
According to the data analysis, in the case of GDM, the level of At II was significantly higher in the 1st trimester in FFP and MFP (by 21.2% and 38.3%, respectively) and in the 3rd trimester in MFP (by 27.6%) compared to the NP group. Moreover, in MFP it was significantly higher in the 1st (by 38.3%) and 3rd (by 36.6%) trimesters than in FFP. In the 2nd trimester, the situation changed, and its level was statistically significantly higher in FFP in the case of NP (by 31.5%). Similar dynamics of indicators were found in the content of Et-1. In the 1st trimester, its level significantly prevailed in the group with GDM (in FFP by 38.9%, in MFP by 79.8%) in comparison with NP, and it was higher in MFP (by 64.7%), compared to FFP.
The NorA level significantly prevailed in the group with GDM, especially in the 2nd trimester. The Adr level in the 1st trimester was also significantly higher in the group with GDM (by 11.5% in group Ia and 20.8% in group Ib), compared to patients with NP.
The results show that the highest levels of At II, Et-1, and Adr are typical for pregnant women with GDM carrying boys. However, closer to the childbirth, their At II level increased, while their Et-1 and Adr levels decreased. In normal pregnancy, there was an inverse correlation. NorA values were highest and increased closer to delivery in the control group in FFP. This production of vasoconstrictors may be related to their biologically appropriate effects on vascular tone and carbohydrate metabolism during gestational restructuring. Relatively high levels of Et-1 may be associated with vascular endothelial damage, dysfunction, and impaired placental perfusion in GDM [16]. This can be confirmed by high values of another vasoconstrictor At II, which is known to be an activator of Et-1 expression and reduces the sensitivity of cells to insulin [28]. Swiderski S. et al. (2010) in their work showed that pregnant women with diabetes mellitus have a decrease in sensitivity to Et-1 receptors [17]. At the beginning of pregnancy, the predominant levels of Adr in MFP can be explained by a more severe stress of the mother’s body in the case of male fetus gestation; they can also be influenced by its contrinsular effects. Although NorA is an adrenal hormone, it acts more as a neurotransmitter and, due to its greater affinity for alpha-adrenergic receptors, it prepares the uterine muscles for childbirth. This fact can explain its higher levels in the control group of pregnant women who are carrying female fetuses, as well as the increase in its concentration before delivery. Thus, it is possible to trace the mechanisms of physiological preparation of the uterus for childbirth. Moreover, it is obvious that in the case of a male fetus, pregnant women show more severe signs of carbohydrate and endothelial dysfunction.
Metabolic changes during pregnancy are aimed at the formation of physiological insulin resistance [1, 3, 15]. They can trigger pathological insulin resistance if the pregnant woman has concomitant disorders (for example, obesity, polymorphism of genes responsible for the regulation of carbohydrate and fat metabolism, etc.). Contra- and proinsular factors are reported to determine the ability of insulin to implement its biological effects [2, 18, 19], which include IGF-1, IGFBP-1, RAGE, and RBP4. They are known to play a significant role in regulating carbohydrate metabolism in non-pregnant women, but there is little information regarding GDM, obstetric complications, and the effect of fetal sex on them.
We found that IGF-1 and RBP4 levels significantly prevailed in group I in MFP (by 44.9% and 21.5%), compared to FFP in the 2nd trimester. These periods were also characterized by a significant increase in IGF-1 (by 28.5%) and RBP4 (by 13.3%) in group Ib compared to the values in group IIb, as well as a significant increase in MFP by 60.4% and 16.3% closer to the childbirth, respectively (Table 3).
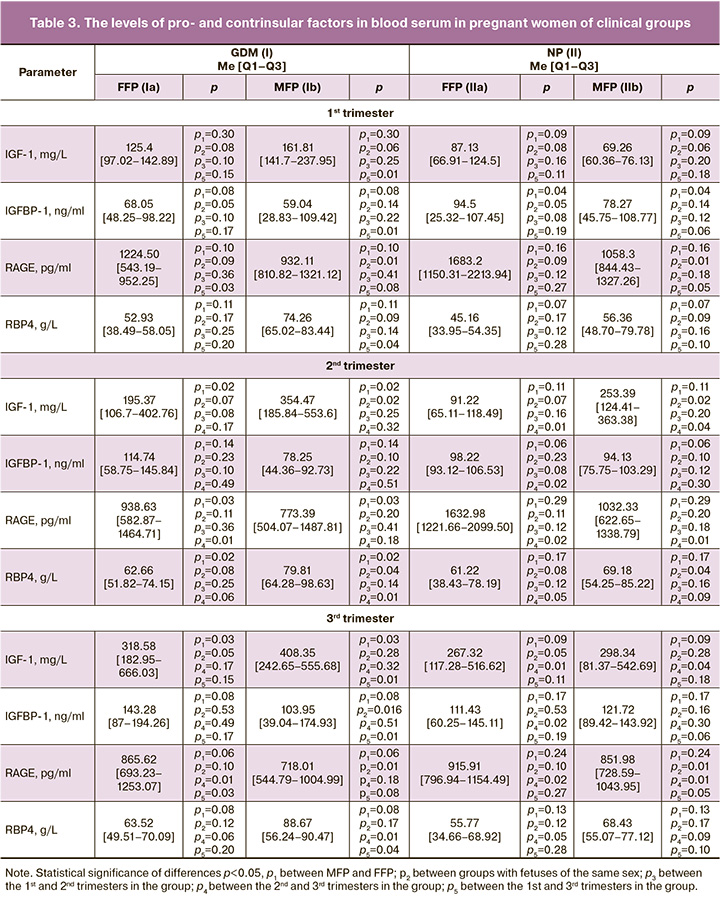
On the contrary, the RAGE levels in the 1st and 3rd trimesters were significantly higher in group IIb (by 11.9% and 15.7%, respectively) compared to group Ib and higher in group Ia in the 2nd trimester (by 17.6%) compared to group Ib. However, its maximum values during pregnancy were revealed in FFP when pregnancy was normal. The RAGE level decreased closer to the childbirth in groups Ia (by 29.3%), IIa (by 43.9%) and IIb (by 17.5%).
IGFBP-1 was significantly higher in the 1st trimester in group IIa (by 17.2%) versus group IIb and significantly increased closer to the childbirth in groups Ib (by 43.2%) and IIa (by 11.8%). Its maximum values were obtained in pregnant women of group Ia.
Higher levels of IGF-1 in pregnant women with GDM who are carrying boys can be explained by their endotheliopathy, which is indirectly connected with the prevailing levels of Et-1, At II, and Adr; they also contribute to a decrease in the sensitivity of insulin receptors. Present endotheliopathy, affecting the function of biosynthesis in the liver, impairs the formation of IGFBP-1, in particular, which inactivates 95% of IGF-1 on average [18]; inactivation in its turn increases the concentration of free (active) forms of IGF-1. One of the first events associated with excessive visceral fat accumulation is thought to be the development of inflammation accompanied by hormonal dysfunction of adipose tissue, including excessive RBP4 production. A decrease in the level of the insulin-dependent glucose transporter GLUT-4 is considered to be a direct cause of stimulation of RBP4 release by adipocytes into the bloodstream. Circulating RBP4 inhibits insulin-stimulated signaling pathways in skeletal muscle cells, leading to the development of insulin resistance. Population studies have shown that increased RBP4 secretion stimulates the expression of molecule adhesion in endothelial cells, contributing to the development of endotheliopathy [19]. Insulin resistance and endotheliopathy have been shown to be closely related to high levels of this protein. In particular, Du X. et al. (2019) showed that RBP4 can be considered as an early marker of GDM due to high correlations of the insulin resistance index and RBP4 in pregnant women with GDM; they also found its significant increase in pregnant women with GDM closer to the childbirth, in contrast to pregnant women with normal carbohydrate metabolism [20]. The results of our study are consistent with their findings. However, in addition to the existing information about this protein, it has also become known that one of the determining factors in the development of insulin resistance and endotheliopathy is the male sex of the fetus.
The lower levels of RAGE observed in MFP with GDM and its predominant decrease closer to the childbirth indicate the presence of oxidative stress, increased catabolism and inflammatory response in endothelial cells (which are associated with a higher content of the end products of protein glycosylation). These processes are likely to be related to the progression of endothelial dysfunction, which triggers impairment of placentogenesis. The obtained data on the content of RAGE are consistent with the data of Nakamura K. et al. (2007) and Matsui T. et al. (2017), who also showed and proved the association of expression of these receptors with the development of endotheliopathy in patients with GDM and other forms of diabetes, and also in patients with markers of inflammation [29, 30].
In the course of clinical evaluation of pregnancy, it was found that GDM prevailed in women carrying boys in 71.0% of cases, regardless of parity. Obstetric complications prevailed in women with GDM carrying male fetuses: placental dysfunction by 2.1 times (p=0.03), preterm birth by 1.5 times (p=0.01), gestational hypertension by 2.2 times (p=0.01), diabetic fetopathy by 1.8 times (p=0.01). Due to the higher level of obstetric complications, these pregnant women had operative deliveries 1.4 times more frequently (p=0.04).
Thus, the male sex of the fetus is one of the possible «participants» in biochemical processes that impair the carbohydrate balance and the function of the vascular wall endothelium.
Conclusion
Our study revealed a statistically significant effect of the fetal sex on the likelihood of developing disorders of carbohydrate metabolism and the formation of endothelial dysfunction in the mother’s body, which is confirmed by an increase in the levels of PRL, At II, Et-1, Adr, IGF-1, RBP4 and a decrease in the levels of IGFBP-1, RAGE. This biochemical replacement contributes to the aggravation of the already existing physiological insulin resistance and the development of GDM mainly in mothers carrying boys.
MFP can be classified as a risk group for the development of GDM and pregnancy complications (fetoplacental insufficiency, threatening preterm birth, gestational hypertension, diabetic fetopathy).
Our results determine the prospects for developing measures aimed at possible revision of clinical algorithms for medical diagnostic and preventive management of pregnant women with GDM, taking into account fetal sex.
References
- Капустин Р.В., Аржанова О.Н., Беспалова О.Н., Пакин В.С., Айламазян Э.К. Патологическая прибавка веса как фактор развития гестационного сахарного диабета: систематический обзор и мета-анализ. Акушерство и гинекология. 2016; 5: 12-9. [Kapustin R.V., Arzhanova O.N., Bespalova O.N., Pakin V.S., Ailamazyan E.K. Abnormal weight gain as a factor in the development of gestational diabetes mellitus: systematic review and meta-analysis. Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2016; 5: 12-9. (in Russian)]. http://dx.doi.org/10.18565/aig.2016.5.12-19
- Линде В.А., Палиева Н.В., Боташева Т.Л., Авруцкая В.В., Дударева М.В. Роль про- и контринсулярных факторов в формировании акушерской патологии. Акушерство и гинекология. 2017; 2: 32-8. [Linde V.A., Palieva N.V., Botasheva T.L., Avrutskaya V.V., Dudareva M.V. Role of pro-and contra-insular factors in the formation of obstetric pathology. Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2017; 2: 32-8. (in Russian)] https://dx.doi.org/10.18565/aig.2017.2.32-8.
- Радзинский В.Е., Боташева Т.Л., Котайш Г.А. ред. Ожирение. Диабет. Беременность. Версии и контраверсии. Клинические практики. Перспективы. Радзинский В.Е., Боташева Т.Л, Папышева О.В., Волкова Н.И., Палиева Н.В. и др. Москва: ГЭОТАР-Медиа. 2020; 528 с. [Radzinsky V.E., Botasheva T.L., Kotaysh G.A. ed. Obesity. Diabetes. Pregnancy. Versions and contraverses. Clinical practices. Prospects. Radzinsky V.E., Botasheva T.L., Papysheva O.V., Volkova N.I., Palieva N.V. et al. Moscow: GEOTAR-Media. 2020; 528 р. (in Russian)].
- Палиева Н.В., Боташева Т.Л., Линде В.А., Авруцкая В.В., Железнякова Е.В. Особенности некоторых вазоактивных гормонов и сосудистых факторов у женщин с метаболическим синдромом и их влияние на формирование акушерских осложнений. Акушерство и гинекология. 2017; 6: 48-54. [Palieva N.V., Botasheva T.L., Linde V.A., Avrutskaya V.V., Zheleznyakova E.V. Features of some vasoactive hormones and vascular factors in women with metabolic syndrome and their influence on the development formation of obstetric complications. Akusherstvo i ginekologija / Obstetrics and gynecology. 2017; 6: 48-54. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.6.48-54.
- Гармашева Н.Л., Константинова Н.Н. Патофизиологические основы охраны внутриутробного развития человека. Л.: Медицина. 1985; 159 с. [Garmasheva N.L., Konstantinova N.N. Pathophysiological basis for the protection of intrauterine development. Leningrad: Medicine. 1985; 159 р. (in Russian)].
- Боташева Т.Л., Васильева В.В., Хлопонина А.В., Заводнов О.П., Каушанская Л.В., Железнякова Е.В. Сезонная периодичность мелатонинового обмена и гормонального статуса беременных в зависимости от пола плода. Медицинский вестник Юга России. 2018; 9(3): 70-6. [Botasheva T.L., Khloponina A.V., Vasil’eva V.V., Zavodnov O.P., Kaushanskaya L.V., Zheleznyakova E.V. Seasonal periodicity of melatonin exchange and hormonal status of pregnant women in dependence on fetus sex. Medicinskij vestnik Yuga Rossii / Medical Herald of the South of Russia. 2018; 9(3): 70-6. (in Russian)]. https://dx.doi.org/10.21886/2219-8075-2018-9-3-70-76.
- Боташева Т.Л., Радзинский В.Е., Хлопонина А.В., Васильева В.В., Заводнов О.П., Железнякова Е.В. и др. Пол плода в экспрессии ангиогенных факторов и поддержании цитокинового баланса в материнском организме при физиологической беременности и плацентарной недостаточности. Медицинский вестник Северного Кавказа. 2019; 14(2): 325-9. [Botasheva T.L., Radzinsky V.E., Khloponina A.V., Vasil’eva V.V., Zavodnov O.P., Zheleznyakova E.V. et al. Role of fetus sex in expression of angiogenic factors and supporting of citokine balance in maternal organism in physiological pregnancy and in placentary insufficiency. Medicinskij vestnik Severnogo Kavkaza / Medical Bulletin of the North Caucasus. 2019; 14(2): 325-9. (in Russian)]. https://doi.org/10.14300/mnnc.2019.14079.
- Larsen S.O., Wojdemann K.R., Shalmi A.C., Sundberg K., Christiansen M., Tabor A. Gender impact on first trimester markers in Down syndrome screening. PrenatDiagn. 2002; 22: 1207-8. https://doi.org/10.1002/pd.493.
- Knippel A.J. Role of fetal sex in amniotic fluid alphafetoprotein screening. Prenatal Diagnosis. 2002; 22(10): 941-5. https://doi.org/10.1002/pd.408
- Di Renzo G.C., Rosati A., Sarti R.D., Cruciani L., Cutuli A.M. Does fetal sex affect pregnancy outcome? Gend Med. 2007; l 4(1): 19-30.
- Gonzalez T.L., Sun T., Koeppel A.F., Lee B., Wang E.T., Farber C.R. et al. Sex differences in the late first trimester human placenta transcriptome. Biology of Sex Differences. 2018; 9(1): 4. https://doi.org/10.1186/s13293-018-0165-y
- Wax J.R., Cartin A., Pinette M.G., Blackstone J. Does the frequency of soft sonographic aneuploidy markers vary by fetal sex? J. Ultrasound Med. 2005; 24(8): 1059-1063. http://dx.doi.org/10.7863/jum.2005.24.8.1059
- Del Mar Melero-Montes М., Jick Н. Hyperemesis Gravidarum and the Sex of the Offspring. Epidemiology. 2000; 12(1): 123-4.
- Sheiner E., Levy A., Katz M., Hershkovitz R., Leron E., Mazor M. Gender does matter in perinatal medicine. Fetal Diagn Ther. 2004; 19(4): 366-9. http://dx.doi.org/10.1159/000077967
- Палиева Н.В., Боташева Т.Л., Радзинский В.Е., Черноситов А.В. Особенности метаболизма при физиологической и осложненной беременности с позиций стереофункциональной и хронофизиологической организации системы «мать-плацента-плод»: монография. Ростов н/Д: РГЭУ (РИНХ). 2016; 178 с. [Palieva N.V., Botasheva T.L., Radzinsky V.E., Chernositov A.V. Features of metabolism in physiological and complicated pregnancies in dependence on the stereofunctional and chronophysiological organization of the mother-placenta-fetus system: monograph. Rostov-on-Don: RSEU (RINH). 2016; 178 p.].
- George E.M., Granger J.P. Endothelin: key mediator of hypertension in preeclampsia. Am. J. Hypertens. 2011; 24(9): 964–9.
- Swiderski S., Celewicz Z., Miazgowski T., Ogonowski J. Maternal endothelin-1 and cyclic guanosine monophosphate concentrations in pregnancies complicated by pregravid and gestational diabetes mellitus. Gynecol. Obstet. Invest. 2010; 69: 46-50.
- Lappas M., Jinks D., Shub A. , Willcox J.C., Georgiou H.M., Permezel M. Postpartum IGF-I and IGFBP-2 levels are prospectively associated with the development of type 2 diabetes in women with previous gestational diabetes mellitus. Diabetes & Metabolism. 2016; 42: 442-7.
- Majerczyk M., Olszanecka-Glinianowicz M., Puzianowska-Kuźnicka M., Chudek J. Retinol-binding Protein 4 (RBP4) as the Causative Factor and Marker of Vascular Injury Related to Insulin Resistance. Postepy Hig Med Dosw (Online). 2016, Dec 21; 70(0): 1267-1275.
- Du X., Dong Y., Xiao L., Hui Liu G., Qin W., Yu H. Association between retinol-binding protein 4 concentrations and gestational diabetes mellitus (A1GDM and A2GDM) in different pregnancy and postpartum periods. Ann Transl Med. 2019, Sep; 7(18): 479. http://dx.doi.org/10.21037/atm.2019.08.45.
- Двойрин В.В., Клименков А.А. Методика контролируемых клинических испытаний. Москва. 2004; 143 с. [Dvoirin V.V., Klimenkov A.A. Methodology of controlled clinical trials. Moscow. 2004; 143 p.(in Russian)].
- Боярский А.Я., Громыко Л.Г. Общая теория статистики. М.: Московский университет. 1985; 376 с. [Boyarsky A.Ya., Gromyko L.G. General theory of statistics. Moscow: Moscow University, 1985. 376 p. (in Russian)].
- Mountjoy K.G. Proopiomelanocortin (POMC) neurones, POMC-derived peptides, melanocortin receptors and obesity: how understanding of this system has changed over the last decade. J. Neuroendocrinol. 2015; 27(6): 406-18. http://dx.doi.org/10.1111/jne.12285.
- Sternson S.M., Atasoy D. Agouti-related protein neuron circuits that regulate appetite. Neuroendocrinology. 2014; 100(2/3): 95-102. http://dx.doi.org/10.1159/000369072.
- Макарова Е.Н., Романова И.В., Бажан Н.М. Регуляция потребления пищи в период беременности и лактации у мышей со сниженной активностью меланокортиновой системы. Вавиловский журнал генетики и селекции. 2016; 20(2): 138-44. doi: 10.18699/VJ16.124. [Makarova E.N., Romanova I.V., Bazhan N.M. Regulation of food intake during pregnancy and lactation in mice with reduced activity of the melanocortin system. Vavilov Journal of Genetics and Breeding. 2016; 20 (2): 138-44. (in Russian)]. http://dx.doi.org/10.18699 /VJ16.124.
- Ling C., Billig H. PRL receptor-mediated effects in female mouse adipocytes: PRL induces suppressors of cytokine signaling expression and suppresses insulin-induced leptin production in adipocytes in vitro. Endocrinology. 2001; 142 (11): 480-90.
- Tuzcu A., Bahceci M., Dursun M. et al. Insulin sensitivity and hyperprolactinemia. J Endocrinol Invest. 2003; 26 (4): 341-6.
- Reinhold S.W., Uihlein D.C., Böger C.A., Kloiber S., Frölich K., Bergler T., Banas B., Schweda F., Krämer B.K. Renin, Endothelial NO Synthase and Endothelin Gene Expression in the 2kidney-1clip Goldblatt Model of Long-Term Renovascular Hypertension. Eur J Med Res. 2009; 14(12): 520-5. http://dx.doi.org/10.1186/2047-783x-14-12-520.
- Nakamura K., Yamagishi S.I., Adachi H., Kurita Y., Takanori N., Matsui T., Imaizumi Y.T. Serum Levels of sRAGE, the Soluble Form of Receptor for Advanced Glycation End Products, Are Associated with Inflammatory Markers in Patients with Type 2 Diabetes. Mol Med. 2007, Mar-Apr; 13(3-4): 185-9. http://dx.doi.org/10.2119/2006-00090.Nakamura.
- Matsui T., Higashimoto Y., Nishino Y., Nakamura N., Fukami K., Yamagishi S.I., Matsui T., et al. RAGE-Aptamer Blocks the Development and Progression of Experimental Diabetic Nephropathy. Diabetes. 2017, Jun; 66(6): 1683-95. http://dx.doi.org/10.2337/db16-1281.
Received 12.03.2020
Accepted 25.06.2020
About the Authors
Tatyana L. Botasheva, MD, PhD, Professor, chief researcher, Department of Biomedical Problems in Obstetrics, Gynecology and Pediatrics, Federal State Budget Establishment of High Education “Rostov State Medical University” of the Ministry of Health of the Russian Federation.Tel.: + 7(906)424-81-03. E-mail: t_botasheva@mail.ru. ORCID: 0000-0001-5136-1752. 344012, Russia, Rostov-on-Don, Mechnikova str., 43.
Natalia V. Palieva, MD, PhD, chief research officer at the Obstetrics and Gynecology Department, Federal State Budget Establishment of High Education “Rostov State Medical University” of the Ministry of Health of the Russian Federation. Tel.: +7(989)727-96-45. E-mail: nat-palieva@yandex.ru.
ORCID: 0000-0003-2278-5198. 344012, Russia, Rostov-on-Don, Mechnikova str., 43.
Anna V. Khloponina, PhD, senior research fellow at the Obstetrics and Gynecology Department, Federal State Budget Establishment of High Education “Rostov State Medical University” of the Ministry of Health of the Russian Federation. Tel.: +7(918)556-00-32. E-mail: annakhloponina@yandex.ru.
ORCID: 0000-0002-2056-5231. 344012, Russia, Rostov-on-Don, Mechnikova str., 43.
Valentina V. Vasil’eva, Doctor of Biology, Associate Professor, leading researcher, Department of Biomedical Problems in Obstetrics, Gynecology and Pediatrics, Federal State Budget Establishment of High Education “Rostov State Medical University” of the Ministry of Health of the Russian Federation.
Tel.: +7(909)408-01-59. E-mail: v.vasiljeva1965@mail.ru. ORCID: 0000-0001-5948-6605. 344012, Russia, Rostov-on-Don, Mechnikova str., 43.
Elena V. Zheleznyakova, PhD, research associate, Department of Biomedical Problems in Obstetrics, Gynecology and Pediatrics, Federal State Budget Establishment of High Education “Rostov State Medical University” of the Ministry of Health of the Russian Federation. Tel.: +7(951)845-94-51. E-mail: elena.Gel.1961@yandex.ru.
ORCID: 0000-0003-4496-6387. 344012, Russia, Rostov-on-Don, Mechnikova str., 43.
Oleg P. Zavodnov, PhD in Biology, researcher, Department of Biomedical Problems in Obstetrics, Gynecology and Pediatrics, Federal State Budget Establishment of High Education “Rostov State Medical University” of the Ministry of Health of the Russian Federation. Tel.: +7(903)401-50-09. E-mail: ozz2007@mail.ru.
ORCID: 0000-0002-9555-2267. 344012, Russia, Rostov-on-Don, Mechnikova str., 43.
Ekaterina B. Gudz, PhD, research fellow at the Obstetrics and Gynecology Department, Federal State Budget Establishment of High Education “Rostov State Medical University” of the Ministry of Health of the Russian Federation. Tel.: +7(918)898-47-88. E-mail: ya-kate4ka@yandex.ru. ORCID: 0000-0002-7886-6061.
344012, Russia, Rostov-on-Don, Mechnikova str., 43.
For citation: Botasheva T.L., Palieva N.V., Khloponina A.V., Vasiljeva V.V., Zheleznyakova E.V., Zavodnov O.P., Gudz E.B. Fetal sex in the development of gestational diabetes mellitus and endothelial dysfunction.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 9: 56-64 (in Russian)
https://dx.doi.org/10.18565/aig.2020.9.56-64



