The dynamics of renin, angiotensin (1-7) and angiotensin II in severe and moderate preeclampsia
Objective. To investigate changes in plasma levels of renin, angiotensin II, and angiotensin (1–7) in patients with different PE severity.Khlestova G.V., Nizyaeva N.V., Romanov A.Yu., Baev O.R., Ivanets T.Yu., Lapshina I.I., Mullabaeva S.M., Shuklina D.A.
Material and methods. The study comprised 58 pregnant patients of reproductive age at 28–40 weeks’ gestation. Of the 29 patients in the study group, 11 were diagnosed with severe PE, and the remaining 18 had moderate PE. Twenty-nine women with normal pregnancy were enrolled in the control group. Plasma markers were determined by quantitative enzyme immunoassay using Renin (active) ELISA kits (IBL International GMBH, Germany), Angiotensin 1–7 (Ang 1–7) (Cloud-Clone Corp., USA), and Human ANGII EIA (RayBiotech, Inc., USA).
Results. The level of angiotensin II in severe PE (24.0 ± 5.0 pg/ml) was significantly higher than in moderate PE (15.3 + 1.2 pg/ml; p = 0.046) and in normal pregnancy (14, 7 ± 1.9 pg / ml; p = 0.023). The level of angiotensin (1–7) in severe PE (424.0 ± 16.6 pg/ml) was higher than in moderate PE (361.1 ± 26.7 pg/ml; p = 0.051) and in normal pregnancy (390.7 ± 13.86 pg/ml); p = 0.095. In severe PE, the angiotensin (1–7) / angiotensin II ratio was significantly lower than in normal pregnancy (21.8 ± 3.1 vs. 29.4 ± 2.6 pg/ml, p = 0.049). There were no significant differences in renin concentrations between the study groups (p> 0.05).
Conclusion. Severe pre-eclampsia is associated with a pronounced increase in the production of the vasoconstrictor angiotensin II concomitantly with an insufficient increase in the production of angiotensin (1–7). The absence of differences in plasma renin concentrations suggests the need for further studies to clarify the underlying mechanisms and causes of elevated plasma angiotensin II levels in patients with severe and moderate preeclampsia.
Keywords
Preeclampsia (PE) is a multisystem pathological condition that develops during pregnancy and clinically manifests after 20-week’ gestation. PE not only complicates from 2% to 8% of pregnancies [1] but also leads to adverse maternal and fetal outcomes [2]. Increased blood pressure is one of the leading factors for the development of PE. One of the most significant regulators of blood pressure and systemic vascular resistance in the human body is the renin-angiotensin system.
Renin cleaves angiotensinogen at its N terminus, generating the inactive decapeptide, angiotensin I (1–10). Angiotensin I is in turn cleaved by angiotensin-converting enzyme (ACE) to produce the octapeptide angiotensin II (1–8), which produces a pronounced vasoconstrictor effect [3].
Although angiotensin II is the most biologically active product of the renin-angiotensin system, there is evidence suggesting that other metabolites of angiotensin I may also affect blood pressure. Thus, angiotensin (1–7) can be formed from both angiotensin (1–10) and angiotensin II as a result of exposure to some endo- and carboxypeptidases. Unlike angiotensin II, angiotensin (1–7) has pronounced vasodilating properties [4].
An imbalance of the renin-angiotensin-aldosterone system (RAAS) is associated with the most common diseases of civilization, such as cardiovascular diseases, diabetes, kidney diseases, preeclampsia, osteoporosis and neurodegenerative diseases [5].
Evaluation of RAAS activity in pregnant women is of great importance for investigating the pathogenesis of such common pregnancy complications as PE and gestational arterial hypertension. It has been shown that during PE, mRNA expression and ACE protein synthesis in placental tissue is significantly higher than in healthy pregnant women. The RAAS imbalance can explain the changes in the blood plasma electrolyte composition in PE [6]. There is also evidence that the angiotensin receptor (AT1 receptor) activates multiple intracellular signaling pathways involved in the development of arterial hypertension, endothelial dysfunction and the vascular wall remodeling [7]. The AT1-binding substance and autoantibodies to the AT1 receptor also have been shown to have a role in the development of arterial hypertension, including gestational arterial hypertension [8-10]. However, there is a considerable lack of research addressing the role of angiotensin (1–7) and II in PE, including insufficient data on the nature of changes in their products in patients with different PE severity.
This study aimed to investigate changes in plasma levels of renin, angiotensin II, and angiotensin (1-7) in patients with different PE severity.
Material and methods
The study comprised 58 pregnant patients of reproductive age at 28–40 weeks’ gestation. Of the 29 patients in the study group, 11 were diagnosed with severe PE, and the remaining 18 had moderate PE. Twenty-nine women with normal pregnancy were enrolled in the control group using a matched pairs selection method based on age and gestational age.
The groups did not differ in demographic characteristics and anthropometric data, except the body mass index.
Inclusion criteria for study patients were based on the clinical guidelines “Hypertensive disorders during pregnancy, during childbirth and the postpartum period. Pre-eclampsia. Eclampsia”, 2016 [11]. For the control group, the inclusion criterion was the physiological course of current pregnancy. All patients signed informed consent to take part in the study.
Exclusion criteria of the study were as follows: severe extragenital pathology, a history of internal organ transplantation, a history of malignancies, diabetes mellitus, severe fetal disorders, and congenital fetal malformations.
Plasma markers were determined by quantitative enzyme immunoassay using Renin (active) ELISA kits (IBL International GMBH, Germany), Angiotensin 1–7 (Ang 1–7) (Cloud-Clone Corp., USA), and Human ANGII EIA ( RayBiotech, Inc., USA) according to the manufacturer’s instructions.
Statistical analysis and data visualization were performed using Statistica 10 (StatSoft, USA) and Exel 2016 (Microsoft, USA) software. Differences between groups were analyzed using either Student’s t-test for parametric or Mann-Whitney U test for non-parametric data. ANOVA was used to test quantitative parameters between more than 2 groups, and categorical variables were compared with the Pearson χ2 test. Differences between the groups were considered statistically significant at p < 0.05.
Results and discussion
There were no statistically significant differences in age (32.5 ± 3.1 and 31.7 ± 4.3 years, respectively, p = 0.571) between patients with PE and control patients (Table 1). At the same time, patients with PE had higher body mass index compared with the patients in the control group (31.1 ± 7.1 and 25.7 ± 0.7 kg/m2, respectively, p = 0.003). In patients with PE, delivery occurred on average 3.1weeks earlier (35.5 ± 3.2 weeks) than in the control group (38.7 ± 1.6 weeks), p <0.001. All women with severe PE underwent a cesarean section. The cesarean section rates among the patients with moderate PE and the control group were 55.6% and 37.9%, respectively (p = 0.001 for the trend).

As we demonstrated in our previous study, a normal pregnancy is accompanied by an increase in the level of angiotensin II and a decrease in the level of angiotensin (1–7), while the renin level does not change [12]. In this study, no significant differences in renin concentration were found in the comparison groups, although there was a tendency for its increase in the group with severe PE. Renin concentrations in the group with severe PE, moderate PE, and in the control group were 29.9 ± 15.0 pg/ml, 16.2 ± 13.7 pg/ml, and 21.8 ± 5.2 pg/ml, respectively (p > 0.05).
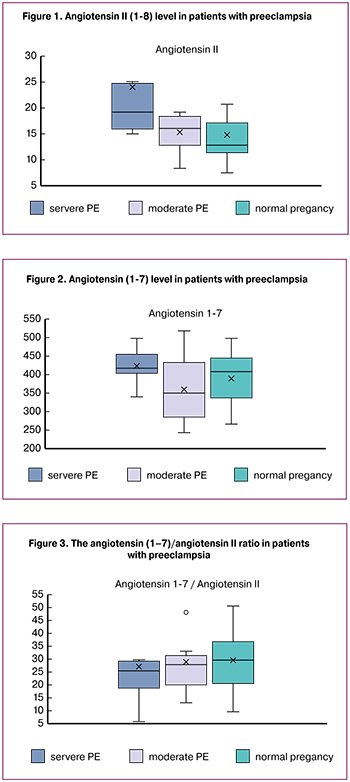 The level of angiotensin II in severe PE (24.0 ± 5.0 pg/ml) was significantly higher than in moderate PE (15.3 + 1.2 pg/ml; p = 0.046) and in normal pregnancy (14, 7 ± 1.9 pg / ml; p = 0.023) (Fig. 1), which is in line with the data of Gao et al. They reported that in PE patients, the concentrations of angiotensin II in maternal blood, umbilical cord blood, and placental tissues were significantly higher than in women with normal pregnancy [13]. Higher plasma levels of angiotensin II in patients with severe PE may play an important role in increasing blood pressure.
The level of angiotensin II in severe PE (24.0 ± 5.0 pg/ml) was significantly higher than in moderate PE (15.3 + 1.2 pg/ml; p = 0.046) and in normal pregnancy (14, 7 ± 1.9 pg / ml; p = 0.023) (Fig. 1), which is in line with the data of Gao et al. They reported that in PE patients, the concentrations of angiotensin II in maternal blood, umbilical cord blood, and placental tissues were significantly higher than in women with normal pregnancy [13]. Higher plasma levels of angiotensin II in patients with severe PE may play an important role in increasing blood pressure.
Merill et al. reported that in women with PE the level of angiotensin (1–7) was significantly decreased compared with normal pregnant subjects [14]. Our findings showed a tendency for its decrease in women with moderate PE (Fig. 2). However, an increase in plasma angiotensin concentration was found in patients with severe PE (1–7). The level of angiotensin (1–7) in severe PE (424.0 ± 16.6 pg/ml) was higher than in moderate PE (361.1 ± 26.7 pg / ml; p = 0.051) and in normal pregnancy (390.7 ± 13.86 pg/ml); p = 0.095.
Since the angiotensin (1–7) effect on the vascular bed is opposite to the vasoconstrictor effect of angiotensin II, its increase can be viewed as a defensive reaction. Therefore, the suppression of angiotensin (1–7) may cause the progression of hypertension in PE.
To test this assumption, we investigated the maternal angiotensin (1–7) / angiotensin II ratio in PE patients (Fig. 3) and found that its values in patients with severe PE, moderate PE, and normal pregnancy were 21.8 ± 3.1, 26.5 ± 4.9, and 29.4 ± 2.6, respectively. Although there were no significant differences between the moderate PE and the control group (p > 0.05), in severe PE, the angiotensin (1–7) / angiotensin II ratio was significantly lower than in normal pregnancy (p = 0.049). This result confirms dysregulation of the renin-angiotensin system in severe PE as a result of an insufficient increase in angiotensin (1–7) level in response to the excessive pathological production of angiotensin II.
Conclusion
Severe PE is associated with a pronounced increase in the production of angiotensin II concomitantly with an insufficient increase in the production of angiotensin (1–7). A decrease in the angiotensin (1–7) / angiotensin II ratio in severe PE reflects an impaired regulation of the renin-angiotensin system as a result of insufficient increase in the level of angiotensin (1–7) in response to the excessive pathological production of angiotensin II, which is an important pathogenic link in development this complication. Analysis of angiotensin II and angiotensin (1–7) regulatory mechanisms suggest the need for further studies in this area.
References
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009; 33(3): 130-7.
- Хлестова Г.В., Карапетян А.О., Шакая М.Н., Романов А.Ю., Баев О.Р. Материнские и перинатальные исходы при ранней и поздней преэклампсии. Акушерство и гинекология. 2017; 6: 41-7. [Khlestova G.V., Karapetyan A.O., Shakaya M.N., Romanov A.Yu., Baev O.R. Maternal and perinatal outcomes with early and late preeclampsia. Obstetrics and gynecology. 2017; 6: 41-7. (in Russian)]
- Беловол А.Н., Князькова И.И., Цыганков А.И. Гендерные особенности ренин-ангиотензин- альдостероновой системы: клиническое значение при артериальной гипертензии. Гендерна Медицина. 2014; 4: 18-23. [Belovol A.N., Knyazkova I.I., Tsygankov A.I. Gender features of the renin-angiotensin-aldosterone system: clinical significance in hypertension. Gender Medicine. 2014; 4: 18-23. (in Russian)]
- Gironacci M.M. Angiotensin-(1–7): beyond its central effects on blood pressure. Ther. Adv. Cardiovasc. Dis. 2015; 9(4): 209-16.
- Chaszczewska-Markowska M., Sagan M., Bogunia-Kubik K. The renin-angiotensin-aldosterone system (RAAS) physiology and molecular mechanisms of functioning. Postepy Hig. Med. Dosw. ()nline). 2016; 70: 917-27.
- Красный А.М., Хлестова Г.В., Романов А.Ю., Щипицына В.С., Баев О.Р. Влияние магнезиальной терапии на ионный гомеостаз у женщин с преэклампсией. Вопросы гинекологии, акушерства и перинатологии. 2016; 15(6): 12-6. [Krasniy A.M., Khlestova G.V., Romanov A.Yu., Shchipitsyna VS., Baev OR The effect of magnesian therapy on ionic homeostasis in women with preeclampsia. Gynecology, obstetrics and perinatology issues. 2016; 15 (6): 12-6. (in Russian)]
- Kawai T., Forrester S.J., O’Brien S., Baggett A., Rizzo V., Eguchi S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol. Res. 2017; 125(Pt A): 4-13.
- Kobayashi R., Wakui H., Azushima K., Uneda K., Haku S., Ohki K. et al. An angiotensin II type 1 receptor binding molecule has a critical role in hypertension in a chronic kidney disease model. Kidney Int. 2017; 91(5): 1115-25.
- Herse F., LaMarca B. Angiotensin II type 1 receptor autoantibody (AT1-AA)-mediated pregnancy hypertension. Am. J. Reprod. Immunol. 2013; 69(4): 413-8.
- Yang X., Wang F., Lau W.B., Zhang S., Zhang S., Liu H. et al. Autoantibodies isolated from preeclamptic patients induce endothelial dysfunction via interaction with the angiotensin II AT1 receptor. Cardiovasc. Toxicol. 2014; 14(1): 21-9.
- Гипертензивные расстройства во время беременности, в родах и послеродовом периоде. Преэклампсия. Эклампсия. Клинические рекомендации (протокол лечения). М.; 2016. [Hypertensive disorders during pregnancy, during childbirth and the postpartum period. Pre-eclampsia. Eclampsia. Clinical recommendations (treatment protocol). M.; 2016. (in Russian)]
- Хлестова Г.В., Романов А.Ю., Низяева Н.В., Карапетян А.О., Баев О.Р., Иванец Т.Ю. Динамика ренина, ангиотензина II и ангиотензина (1-7) при беременности и предрасположенность к гипертензивным осложнениям. Бюллетень экспериментальной биологии и медицины. 2018; 165(4): 425-7. [Khlestova G.V., Romanov A.Yu., Nizyaeva N.V., Karapetyan A.O., Baev O.R., Ivanets T.Yu. Dynamics of renin, angiotensin II and angiotensin (1-7) during pregnancy and susceptibility to hypertensive complications. Bulletin of experimental biology and medicine. 2018; 165 (4): 425-7. (in Russian)]
- Gao Q., Tang J., Li N., Zhou X., Li Y., Liu Y. et al. A novel mechanism of angiotensin II-regulated placental vascular tone in the development of hypertension in preeclampsia. Oncotarget. 2017; 8(19):30734-41.
- Merrill D.C., Karoly M., Chen K., Ferrario C.M., Brosnihan K.B. Angiotensin-(1-7) in normal and preeclamptic pregnancy. Endocrine. 2002; 18(3): 239-45.
Received 13.04.2018
Accepted 20.04.2018
About the Authors
Khlestova, Galina V., PhD, student at the V National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. KulakovMinistry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4.Tel.: +79647838076. E-mail: g_khlestova@oparina4.ru
Romanov, Andrey Yu., clinical resident at the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov
Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4Tel.: +79031589400. E-mail: romanov1553@yandex.ru
Nizyaeva, Natalia V., PhD, senior researcher at the Pathology Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79262482893. E-mail: niziaeva@gmail.com
Baev, Oleg R.,MD, professor, head of the Maternity Department, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia; Professor at the Department of Obstetrics, Gynecology, Perinatology, and Reproductology, I.M. Sechenov First MSMU of Minzdrav of Russia (Sechenov University).
117997, Russia, Moscow, Ac. Oparina str. 4. Tel: +74954381188. E-mail: o_baev@oparina4.ru
Ivanets, Tatiana Yu., PhD, head of Clinical and Diagnostic Laboratory, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: t_ivanets@oparina4.ru
Mullabaeva, Svetlana M., head of the Laboratory for Collection and Storage of Biomaterials, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: s_mullabaeva@oparina4.ru
Lapshina, Irina I., PhD (bio.sci.), senior researcher at the Clinical and Diagnostic Laboratory, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: lapshinira09@mail.ru
Shuklina, Dariya A., clinical resident, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry
of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: shuk2323@mail.ru
For citation: Khlestova G.V., Nizyaeva N.V., Romanov A.Yu., Baev O.R., Ivanets T.Yu., Lapshina I.I., Mullabaeva S.M., Shuklina D.A. The dynamics of renin, angiotensin (1-7) and angiotensin ii in severe and moderate preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (1): 62-6. (in Russian)
https://dx.doi.org/10.18565/aig.2019.1.62-66



