Possibilities of predicting preterm birth using mitochondrial DNA and VDAC1 protein
Objective. To study the prognostic role of determining the content of mitochondrial DNA in the blood and voltage-dependent anion channel 1 (VDAC1) in the placenta during preterm birth. Materials and methods. The study included 142 pregnant women. They were divided into three groups: group 1 consisted of 43 patients who had a spontaneous preterm birth; group 2 included 47 women who had a preterm birth and premature rupture of membranes (PROM) and group 3 consisted of 52 women who had threatened preterm labor followed by term birth. The quantitative assessment of mtDNA copy number in peripheral blood plasma (reverse transcription quantitative real-time PCR (RT-qPCR)) as well as determining the level of VDAC1 in the placenta were carried out using western blotting. Results. The study of the VDAC1 protein content in the placenta showed its statistically significantly high content in patients who had a preterm birth and PROM at 22–27 6/7 and 28–33 6/7 weeks’ gestation compared to the patients who had a term birth and a spontaneous preterm birth at 22–27 6/7 weeks’ gestation (p<0.05). Patients with normal pregnancy showed an increase in the level of mtDNA in the peripheral blood plasma with the gestational age, reaching the maximum values by 37–40 weeks. Patients who had preterm labor with PROM showed significantly higher mtDNA levels at 22–27 6/7 and 28–33 6/7 weeks than those who had a normal pregnancy, but it was lower than in patients with term birth. In case of spontaneous preterm birth, the level of mtDNA at 22–27 6/7 and 34–36 6/7 weeks’ gestation was statistically significantly higher, compared with one in normal pregnancy and term birth. Determining the level of mtDNA copy number using ROC analysis with high sensitivity (77%) and specificity (93%) makes it possible to predict the risk of preterm birth. Conclusion. Statistically significant differences in the content of VDAC1 protein in the placenta and the level of mtDNA copy number in the peripheral blood plasma in spontaneous preterm birth and in preterm birth with PROM in contrast to the normal course of pregnancy suggest that they contribute to the development of these complications of pregnancy. The identification of these markers can facilitate timely diagnosis and initiation of personalized complex therapy aimed at prolonging pregnancy.Tyutyunnik V.L., Kan N.E., Vysokikh M.Yu., Kokoeva D.N., Donnikov A.E., Saribekova A.G., Medzhidova M.К.
Keywords
Currently, preterm birth is an important interdisciplinary issue that is of great importance not only for practical obstetricians, but for neonatal services as well [1–3]. The incidence of preterm birth in developed countries varies from 7% to 9%, and in developing countries it reaches 20%, and there is no tendency to its decrease [4–6]. Children born prematurely have severe health consequences such as neurological, metabolic, and cognitive disorders; therefore, it is important and necessary to find the causes and methods of prevention of this pregnancy complication [7–9].
To date, the pathogenesis of preterm birth has not been fully studied, and the predisposition to its development may be due to various risk factors [10–13]. Taking into consideration the concept of the role of aseptic inflammation in the genesis of preterm birth, a number of scientists have given special attention to the so-called damage associated molecular patterns (DAMPs) [14–16]. The effector action of these patterns, which have a pro-inflammatory effect, serves as a trigger that causes the induction of preterm birth [17, 18]. The mechanism of this phenomenon remains understudied, and therefore the scientists are particularly interested in studying the correlations between the levels of mitochondrial DAMPs (mtDNA, membrane proteins) and risk of preterm birth [10, 14, 19]. According to different studies, mtDAMPs also include TFAM (mitochondrial transcription factor A), multifunctional mitochondrial protein OPA1, which is responsible for mitochondrial fusion and VDAC1 (mitochondrial membrane channel). The latter is the main channel of the mitochondrial outer membrane; it provides the diffusion of adenosine triphosphate (ATP) from mitochondria and plays a key role in mitochondrial-mediated apoptosis, whose dysregulation leads to various pathological conditions [1, 4, 9, 15, 18, 20]. Therefore, the study of mitochondrial DNA (mtDNA) and VDAC1 is considered to be promising for the purpose of clarifying the pathogenetic mechanisms and predicting preterm birth.
The objective of the research is to study the prognostic role of determining the content of mtDNA in the blood and voltage-dependent anion channel 1 (VDAC1) in the placenta during preterm birth.
Materials and Methods
The study included 142 pregnant women. Taking into consideration the different genesis of spontaneous preterm birth (SPB) and preterm birth with premature rupture of membranes (PROM), the women were divided into groups depending on the pathogenetic forms [2, 12, 18]. Group 1 consisted of 43 pregnant women who had SPB; group 2 included 47 cases of preterm birth with PROM. Moreover, group 3 included 52 patients with threatening preterm labor followed by full-term delivery.
The criterion for inclusion in the main group of the study was a singleton pregnancy that occurred in the natural cycle. Pregnant women who were 22 to 36.6 weeks pregnant, who had preterm birth, and who gave their voluntary informed consent to participate in the study were also eligible for inclusion. The exclusion criteria for all groups were abdominal delivery, severe extragenital diseases, obstetric pathology requiring elective preterm delivery, fetal growth retardation, fetal malformations and multiple pregnancies. There was a study of clinical and anamnestic data, characteristics of the course and outcomes of pregnancies, as well as a study of the condition of fetuses and newborns.
The following methods were used in addition to the standard ones: the quantitative assessment of the level of mtDNA copy number in peripheral blood plasma (reverse transcription quantitative real-time PCR (RT-qPCR)) as well as determining the content of structural and functional protein VDAC1 in the samples of the placenta (western blotting).
Statistical analysis
SPSS Statistics 23.0 software was used for statistical analysis of the data. The normally distributed quantitative data were presented as M±SD, where M is the mean value and SD is the standard deviation. The rest of the data is presented in the form of Me (Q1; Q3), where Me is the median, (Q1; Q3) is the interquartile interval. The nonparametric Mann-Whitney test for independent populations was used for paired comparison of groups. The proportions were compared using the criterion χ2. The Kruskal–Wallis test and multiple comparison of groups with the Dunn’s test were used. The predictive value was assessed and the ROC analysis models were constructed. To establish the cut-off threshold, the mtDNA level values were reviewed from the maximum to the minimum value. The groups were divided for each value, sensitivity and specificity were determined. The criterion for selecting the cut-off threshold was the maximum total sensitivity and specificity of the model. A direct assessment of the effectiveness of the classification and the model quality was obtained by calculating AUC using trapezoidal method. The null hypothesis was rejected taking into account the correction for the multiplicity of the comparison [9]. The results were considered to be significant at the level of p<0.05.
Results and Discussion
All pregnant women included in the study were evaluated for clinical and anamnestic characteristics. In the groups of SPB and preterm birth with PROM, there were mainly women of advanced reproductive age; this fact confirms the idea of the relationship between the incidence of preterm birth and the age of a woman [1, 11, 14]. The groups of patients did not show statistically significant differences in the diseases of the cardiovascular, respiratory, endocrine, digestive systems, and ENT organs. The comparative analysis of diseases of the urinary system revealed the presence of chronic cystitis in 13/47 (27.6%) patients (p=0.0051), which was statistically significantly more frequent in the patients who had preterm birth with PROM. Among the gynecological diseases, chronic endometritis prevailed in 3/47 (6.4%) and salpingoophoritis in 11/47 (23.4%) patients (p<0.05) in the group who had preterm birth with PROM.
The history of patients revealed a statistically significant increase in the frequency of intrauterine instrumental interventions in the groups of SPB and preterm birth with PROM, namely, in 6/43 (27.9%) and 17/47 (36.2%) patients; and preterm birth in 14/43 (32.6%) and 16/47 (34.0%) of cases, respectively (p<0.01). This indicates the certain role of a remarkable obstetric and gynecological history in preterm birth [10, 12, 19]. The clinical characteristics and anamnestic data of the pregnant women in the study are presented in Table 1.
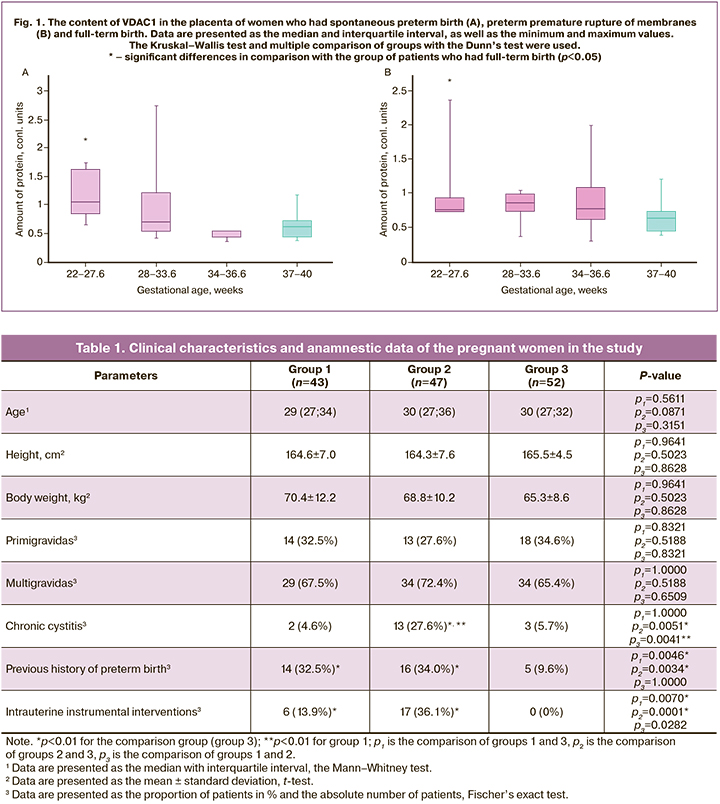
According to many researchers [2, 5, 12, 14], the frequency of preterm birth is high in case of complicated pregnancy; therefore, the course of pregnancy was analyzed in this study. In the first trimester, pregnancy-related nausea was statistically significantly more frequent in patients with PROM, namely 8/47 (17.0%), and the rate of acute respiratory viral infection was higher in patients with SPB, 5/43 (11.6%), (p<0.01). In the third trimester, there were no statistical differences among the study groups. Patients with abdominal delivery were not included in the study, and all babies were born through the natural birth canal. The postpartum period in patients with PROM was characterized by complications: anemia in 12/47 (25.5%) and endometritis in 1/47 (2.1%) of patients.
Preterm birth is known to be associated with a high frequency of adverse perinatal outcomes; therefore, the study of the early neonatal period was of particular interest. All children were born alive, but in the groups with SPB and preterm birth with PROM, indicators of weight and length were lower, as well as the Apgar score, which was due to earlier delivery. Among the neonatal diseases in newborns in groups of SPB and preterm birth with PROM, respiratory distress syndrome occurred statistically more frequently, in 33/43 (76.7%) and 37/47 (78.7%) cases, congenital pneumonia in 6/43 (13.9%) and 9/47 (19.1%) cases, intraventricular hemorrhages in 30/43 (69.7%) and 29/47 (61.7%) (p<0.01), respectively. The obtained results are consistent with the literature data on the association of preterm birth with high morbidity in newborns [4, 11, 14].
Preterm birth may be caused by different pathogenetic mechanisms; gestational age can also influence its etiology. The question of early and preclinical diagnosis remains understudied, and an active search for predictors of preterm birth at the molecular and cellular levels still continues [1, 2, 8, 19, 20]. At the next stage of our research, we studied the copy number of mtDNA in the blood plasma and the content of the VDAC1 protein in the placenta.
The analysis of the content of the VDAC1 protein in the placenta in preterm birth revealed statistically significant differences at 22–27.6 weeks gestation, both in SPB – 0.43 (0.18; 0.96) and in preterm birth with PROM – 0.75 (0.72; 0.90), compared to the patients who had full-term birth – 0.63 (0.46; 0.82). In SPB at 28–33.6 weeks gestation, its content was 0.69 (0.54; 1.20), at 34–36.6 gestation 0.85 (0.73; 0.97); compared to 0.63 (0.46; 0.82) in patients with full-time birth. The patients who had preterm birth with PROM at 28–33.6 weeks gestation demonstrated the VDAC1 content at the level of 0.85 (0.73; 0.97), at 34–36.6 weeks it was 0.77 (0.61; 1.07), and in case of full-term birth it was 0.63 (0.46; 0.82). The analysis of its content at 34–36.6 weeks gestation showed a statistically significantly low level in the group with SPB, compared to those with PROM (p=0.027); no other statistically significant differences were obtained. The data are shown in Figures 1A and 1B.
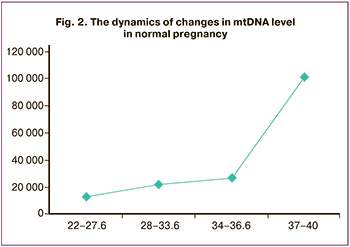 Taking into consideration the obtained differences in the VDAC1 content, the next stage was to study mtDNA in the peripheral blood of pregnant women. In order to clarify the reference values of mtDNA, a group of patients with normal pregnancy was additionally analyzed.
Taking into consideration the obtained differences in the VDAC1 content, the next stage was to study mtDNA in the peripheral blood of pregnant women. In order to clarify the reference values of mtDNA, a group of patients with normal pregnancy was additionally analyzed.
Normal pregnancy was characterized by an increase in the level of mtDNA which correlated with the gestation, its maximum values were noted at 37–40 weeks gestation (Fig. 2).
The analysis of the mtDNA level in SPB at 22–27.6 and 34–36.6 weeks gestation revealed its higher level (p<0.05), in comparison with the normal pregnancy. At 28–33.6 weeks gestation, a similar trend was observed, but no statistically significant differences were found. Differences were also found in preterm birth with PROM: the level of mtDNA at 22–27 and 28–33 weeks gestation was statistically significantly higher than in the normal pregnancy (p<0.05), but there was a statistically significant decrease in comparison with full-term birth. The analysis of the mtDNA level at 34–37 weeks gestation showed that its level in preterm birth with PROM was also higher than in the normal pregnancy, but no statistically significant differences were found (Fig. 3A and 3B).
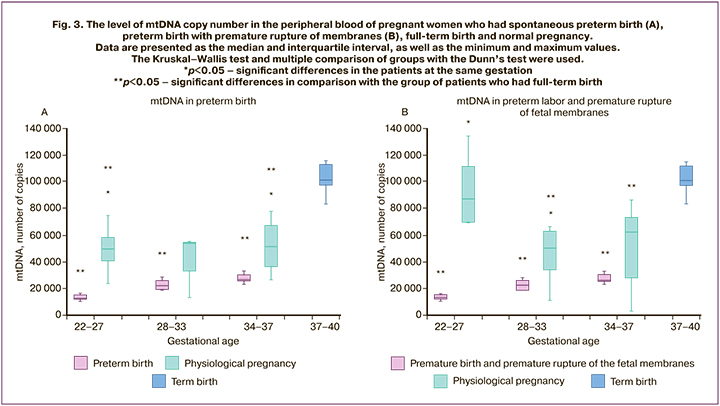
To assess the diagnostic effectiveness of determining the level of mtDNA in preterm birth, ROC analysis was performed (Fig. 4). As a predicted outcome, we chose the fact of the onset of preterm bitrh within 7 days from the moment of taking the biomaterial.
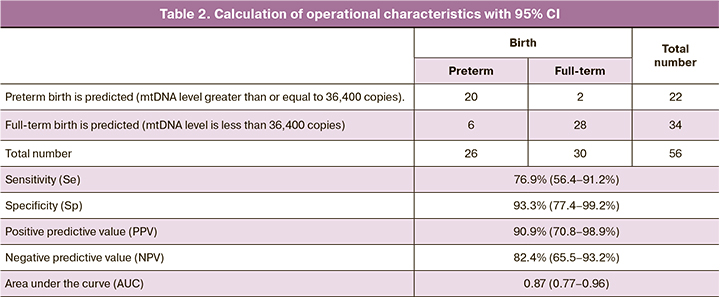
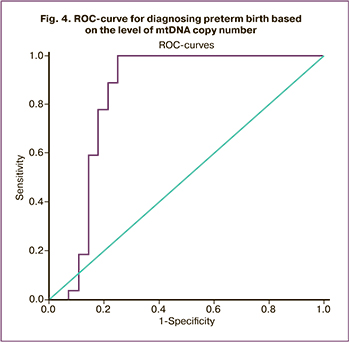 The area under the curve AUC=0.87 (0.77–0.96), p=0.00002. According to the expert scale, this evaluation model is defined as ‘excellent’. When choosing the threshold level, the criterion of maximum total sensitivity and specificity was used. The cut-off level was determined to be 36,400 copies of mtDNA (4.6 decimal logarithm) with a sensitivity of 77% (56.4–91.2) and a specificity of 93% (77.4–99.2). Positive predictive value (PPV) was 90.9 (70.8–98.9), and negative predictive value (NPV) was 82.4 (65.5–93.2). The data are presented in Table 2.
The area under the curve AUC=0.87 (0.77–0.96), p=0.00002. According to the expert scale, this evaluation model is defined as ‘excellent’. When choosing the threshold level, the criterion of maximum total sensitivity and specificity was used. The cut-off level was determined to be 36,400 copies of mtDNA (4.6 decimal logarithm) with a sensitivity of 77% (56.4–91.2) and a specificity of 93% (77.4–99.2). Positive predictive value (PPV) was 90.9 (70.8–98.9), and negative predictive value (NPV) was 82.4 (65.5–93.2). The data are presented in Table 2.
Thus, the number of mtDNA level greater than 36,400 copies in a patient can predict preterm birth in the next 7 days. Determining this marker can contribute to the timely diagnosis and initiation of personalized complex therapy aimed at prolonging pregnancy.
Mitochondrial dysfunction of various etiology, which leads to impaired balance of redox reactions in cells and tissues, is considered to be one of the main factors of pathogenetic changes in the fetoplacental complex, causing such pregnancy complication as preterm birth [5, 15, 20]. Mitochondria are one of the central mediators of inflammation. In order to study the relationship between the differences in the functional manifestation of mitochondrial activity in SPB and preterm birth with PROM, as well as in full-term birth with a possible change in the number of mitochondria in the placenta, we studied the content of the VDAC1 protein, which is a marker of the outer membranes of mitochondria. The obtained differences in the content of VDAC1 in the placenta confirmed its involvement in the development of this complication and served as the basis for the study of mtDNA, which also refers to mitochondrial DAMPs.
It should be noted that the dynamics of the increase in the level of mtDNA in preterm birth had a different character, which influenced the mechanisms of the induction processes in SPB and preterm birth with PROM. The established statistically significantly high levels of mtDNA in SPB and preterm birth with PROM, which reach the level of full-term birth, reflect their potential role in the development of this complication; this conclusion is consistent with the data of R. Romero et al. [18]. So, it is possible to determine this marker for predicting preterm birth and provide timely treatment and preventive measures.
Conclusion
Thus, mitochondrial DAMPs can serve as predictors of spontaneous preterm birth and preterm premature rupture of the membranes. The identification of these markers can facilitate timely diagnosis and initiation of personalized complex therapy aimed at prolonging pregnancy.
References
- Romero R., Dey S.K., Fisher S.J. Preterm labor: one syndrome, many causes. Science. 2014; 345(6198): 760-5. https://dx.doi.org/10.1126/science.1251816.
- Радзинский В.Е., Оразмурадов А.А., Савенкова И.В., Дамирова К.Ф., Хаддад Х. Преждевременные роды – нерешенная проблема XXI века. Кубанский научный медицинский вестник. 2020; 27(4): 27-37. [Radzinsky V.E., Orazmuradov A.A., Savenkova I.V., Damirova K.F., Haddad H. Preterm labor – an unsolved problem of the XXI century. Kuban Scientific Medical Bulletin. 2020; 27 (4): 27-37. (in Russian)].
- Areia A.L., Moura P., Mota-Pinto A.; PROSPERO Nº CRD42018089859. The role of innate immunity in spontaneous preterm labor: A systematic review. J. Reprod. Immunol. 2019; 136: 102616. https://dx.doi.org/10.1016/j.jri.2019.102616.
- Daskalakis G., Goya M., Pergialiotis V., Cabero L., Kyvernitakis I., Antsaklis A., Arabin B. Prevention of spontaneous preterm birth. Arch. Gynecol. Obstet. 2019; 299(5): 1261-73. https://dx.doi.org/10.1007/s00404-019-05095-y.
- Белоусова В.С., Стрижаков А.Н., Свитич О.А., Тимохина Е.В., Кукина П.И., Богомазова И.М., Пицхелаури Е.Г. Преждевременные роды: при-чины, патогенез, тактика. Акушерство и гинекология. 2020; 2: 82-7. [Belousova V.S., Strizhakov A.N., Svitich O.A., Timokhina E.V., Kukina P.I., Bogomazova I.M., Pitskhelauri E.G. Premature birth: causes, pathogenesis, tactics. Akusherstvo i ginekologiya / Obstetrics and Gynecology, 2020; 2: 82-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.2.82-87.
- Lee A.C., Blencowe H., Lawn J.E. Small babies, big numbers: global estimates of preterm birth. Lancet Glob. Health. 2019; 7(1): e2-3. https://dx.doi.org/10.1016/S2214-109X(18)30484-4.
- Abdel Ghany E.A., Alsharany W., Ali A.A., Youness E.R., Hussein J.S. Anti-oxidant profiles and markers of oxidative stress in preterm neonates. Paediatr. Int. Child Health. 2016; 36(2): 134-40. https://dx.doi.org/10.1179/2046905515Y.0000000017.
- Becker D.A., Szychowski J.M., Kuper S.G., Jauk V.C., Wang M.J., Harper L.M. Labor curve analysis of medically indicated early preterm induction of labor. Obstet. Gynecol. 2019; 134(4): 759-64. https://dx.doi.org/10.1097/AOG.0000000000003467.
- Moore T.A., Ahmad I.M., Zimmerman M.C. Oxidative stress and preterm birth: an integrative review. Biol. Res. Nurs. 2018; 20(5): 497-512. https://dx.doi.org/10.1177/1099800418791028.
- Тютюнник В.Л., Курчакова Т.А., Кан Н.Е., Непша О.С., Донников А.Е., Меджидова М.К., Кокоева Д.Н. Локальные факторы врожденного имму-нитета в прогнозировании преждевременных родов. Акушерство и гинекология. 2016; 10: 59-63. [Tyutyunnik V.L., Kurchakova T.A., Kan N.E., Nepsha O.S., Donnikov A.E., Medzhidova M.K., Kokoeva D.N. Local factors of innate immunity in the prediction of preterm birth. Akusherstvo i ginekologiya/ Obstetrics and Gynecology. 2016; 10: 59-63. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.10.59-63.
- Шадеева Ю.А., Гурьева В.А., Николаева М.Г., Евтушенко Н.В. Прогнозирование риска внутриутробной инфекции плода при сверх-ранних и ранних преждевременных родах, индуцированных разрывом околоплодных оболочек. Акушерство, гинекология и репродукция. 2020; 14(4): 490-501. [Shadeeva Yu.A., Gurieva V.A., Nikolaeva M.G., Evtushenko N.V. Predicting the risk of intrauterine infection of the fetus in early and early preterm labor induced by rupture of the amniotic membranes. Obstetrics, gynecology and reproduction. 2020; 14 (4): 490-501. (in Russian)].
- Muñoz-Pérez V.M., Ortiz M.I., Cariño-Cortés R., Fernández-Martínez E., Rocha-Zavaleta L., Bautista-Ávila M. Preterm birth, inflammation and infection: new alternative strategies for their prevention. Curr. Pharm. Biotechnol. 2019; 20(5): 354-65. https://dx.doi.org/10.2174/1389201020666190408112013.
- Wiegman C.H., Michaeloudes C., Haji G., Narang P., Clarke C.J., Russell K.E. et al. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015; 136(3): 769-80. https://dx.doi.org/10.1016/j.jaci.2015.01.046.
- Dutta E.H., Behnia F., Boldogh I., Saade G.R., Taylor B.D., Kacerovský M., Menon R. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol. Hum. Reprod. 2016; 22(2): 143-57. https://dx.doi.org/10.1093/molehr/gav074.
- Müller-Rischart A.K., Pilsl A., Beaudette P., Patra M., Hadian K., Funke M. et al. The E3 ligase parkin maintains mitochondrial integrity by increasing linear ubiquitination of NEMO. Mol. Cell. 2013; 49(5): 908-21. https://dx.doi.org/10.1016/j.molcel.2013.01.036.
- Wu F., Tian J., Lin Y. Oxidative stress in placenta: health and diseases. Biomed. Res. Int. 2015; 2015: 293271. https://dx.doi.org/10.1155/2015/293271.
- Jauniaux E., Burton G.J. The role of oxidative stress in placental-related diseases of pregnancy. J. Gynecol. Obstet. Biol. Reprod. (Paris). 2016; 45(8): 775-85. https://dx.doi.org/10.1016/j.jgyn.2016.02.012.
- Romero R., Chaiworapongsa T., Alpay Savasan Z., Xu Y., Hussein Y., Dong Z.et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J. Matern. Fetal Neonatal Med 2011; 24(12): 1444-55. https://dx.doi.org/10.3109/14767058.2011.591460.
- Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front. Immunol. 2014; 5: 567. https://dx.doi.org/10.3389/fimmu.2014.00567.
- Ni H.M., Williams J.A., Ding W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015; 4: 6-13. https://dx.doi.org/10.1016/j.redox.2014.11.006.
Received 04.02.2021
Accepted 26.02.2021
About the Authors
Victor L. Tyutyunnik, professor, M.D., Ph.D., Leading Researcher of Research and Development Service, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia; Head doctor of the Perinatal Center European Medical Center. Tel.: +7(903)969-50-41. E-mail: tioutiounnik@mail.ru. ORCID: 0000-0002-5830-5099. Researcher ID: B-2364-2015, SPIN-код: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500.117997, Russia, Moscow, Ac. Oparina str., 4; 125040, Russia, Moscow, Pravda str. 15/1.
Natalia E. Kan, professor, M.D., Ph.D., Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(926)220-86-55. E-mail: kan-med@mail.ru. ORCID: 0000-0001-5087-5946. Researcher ID: B-2370-2015, SPIN-код: 5378-8437,
Authors ID: 624900, Scopus Author ID: 57008835600. 117997, Russia, Moscow, Ac. Oparina str., 4.
Mikhail Yu. Vysokikh, Ph.D., Head of Mitochondrial Medicine Research Group, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-76-33 (ex. 1472). E-mail: m_vysokikh@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Diana N. Kokoeva, postgraduate student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(929)663-93-73. E-mail: dikokoeva@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Andrey E. Donnikov, Ph.D., Head of the Laboratory of Molecular Genetic Methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(903)684-52-47. E-mail: donnikov@dna-technology.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Alena G. Saribekova, postgraduate student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology named after of Ministry of Healthcare of Russian Federation (117997, Moscow, Ac. Oparina str. 4). Tel.: +7(926)551-78-24. E-mail: a_aruschanova@oparina4.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
Marzhanat К. Medzhidova, Ph,D., doctoral student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia. Tel.: +7(926)381-17-10. E-mail: marzhana-m@yandex.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Tyutyunnik V.L., Kan N.E., Vysokikh M.Yu., Kokoeva D.N., Donnikov A.E., Saribekova A.G., Medzhidova M.К. Possibilities of predicting preterm birth using mitochondrial DNA and VDAC1 protein.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2021; 3: 58-65 (in Russian)
https://dx.doi.org/10.18565/aig.2021.3.58-65



